-
- Academic Editor
-
-
-
Ankyrin G (ANK3), belonging to the ankyrin family, contributes to cellular structural integrity by linking the cytoskeleton to the plasma membrane. Abnormal ANK3 expression has been reported across several human malignancies, yet the regulatory mechanisms involved are still poorly understood. The process of dividing introns into several steps is referred to as recursive splicing (RS). RS can control the quality of transcripts produced by regulating the retention of the RS-exon. Hundreds of annotated RS-exons in human mRNAs are attributed to the inhibition of RS by the exon junction complex (EJC).
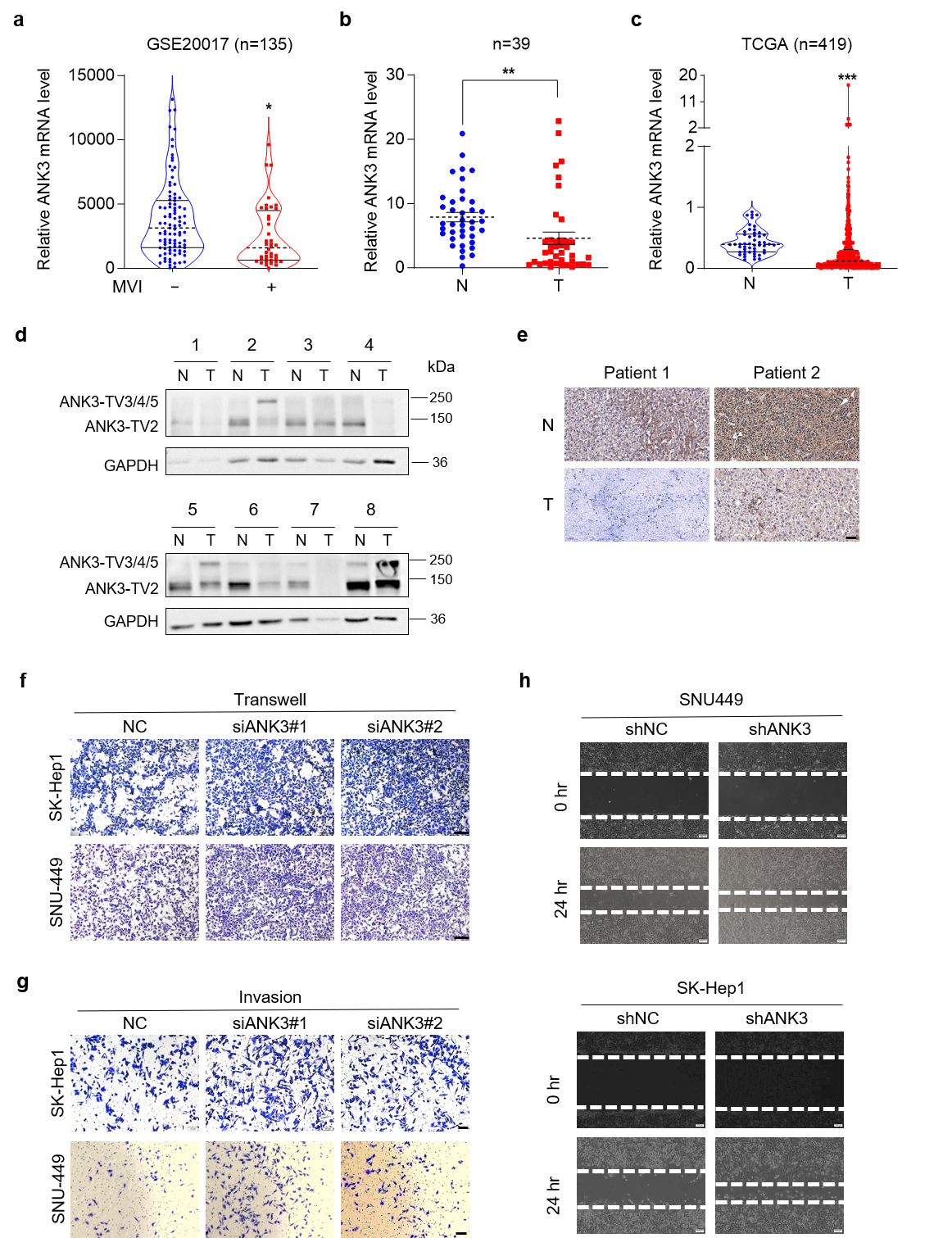
In this study, we demonstrated that ANK3 is reduced in hepatocellular carcinoma (HCC) and suppresses HCC metastasis. We then analyzed the multiple splicing methods of ANK3, confirming that RS exists in ANK3 transcript variant 4 (ANK3-TV4) and that RS was weakened in HCC.
Mechanistically, ANK3 inhibited HCC metastasis, which may be partly attributed to inhibition of the Wnt pathway. ANK3 binds to E-cadherin via its N-terminal ankyrin repeat domain to regulate E-cadherin expression. ANK3 knockdown activates the Wnt pathway, downregulates E-cadherin expression, and promotes its degradation. Conversely, ANK3-TV4 overexpression inhibited the Wnt signaling pathway, upregulated E-cadherin protein expression, and inhibited E-cadherin degradation. RBM8A, a core EJC factor, regulates the RS of ANK3-TV4.
Knockdown of RBM8A promoted RS of ANK3-TV4 and upregulated its expression. We investigate the role of RS in HCC, providing a novel therapeutic perspective and identifying potential targets for intervention.