Academic Editor: Soo-Jin Choi
Background: Clostridium perfringens and Shiga toxin (Stx)-producing Escherichia coli (STEC) are common causes of food poisoning. We previously demonstrated the efficacy of Stx2B-C-CPE, a fusion protein of the C-terminal region of C. perfringens enterotoxin (C-CPE) and Shiga toxin 2 B subunit (Stx2B), as a bivalent vaccine against C. perfringens and STEC infections. Methods: Here, we applied an E. coli expression system and Triton X-114 phase separation to prepare tag- and endotoxin-free Stx2B-C-CPE for use in vaccine formulations. Results: As we anticipated, endotoxin removal from the purified antigen reduced both Stx2B- and C-CPE-specific IgG antibody responses in subcutaneously immunized mice, suggesting that endotoxin contamination influences the immunological assessment of Stx2B-C-CPE. However, the combined use of aluminum and Alcaligenes lipid A adjuvants improved IgG antibody responses to the injected antigen, thus indicating the suitability of purified Stx2B-C-CPE for vaccine formulation. Conclusions: Our current findings provide important knowledge regarding the design of an effective commercial Stx2B-C-CPE vaccine.
Although Clostridium perfringens and Shiga toxin (Stx)-producing Escherichia coli (STEC, also known as enterohemorrhagic E. coli [EHEC]) are common causes of food poisoning, a commercial vaccine to combat these infections is unavailable. Given that C. perfringens enterotoxin (CPE) and Stx2 independently cause severe systemic symptoms and death, we previously developed a fusion protein (Stx2B-C-CPE) from the C-terminal region of CPE (C-CPE) and Stx2 B subunit (Stx2B), which are non-toxic sites for binding to host receptors, as a bivalent vaccine against C. perfringens and STEC infections [1].
Many recombinant proteins that are studied in laboratories, including vaccine
antigens such as Stx2B-C-CPE, are produced in E. coli and therefore
contaminated with bacterial components, such as lipopolysaccharide (LPS). One of
the group of bacterial toxins known as endotoxins, LPS is recognized by Toll-like
receptor (TLR) 4 in host cells including immune cells and typically induces
inflammatory responses through the activation of various transcription factors,
such as nuclear factor-kappa B (NF-
However, after contaminating endotoxin is removed, highly purified protein vaccines frequently have low immunogenicity, which necessitates the use of adjuvants [6]. For example, aluminum salts (alum) are frequently used as vaccine adjuvants to enhance antibody production in animals and humans [6]. LPS has also been applied as a vaccine adjuvant, but its activity must be controlled quantitatively and/or qualitatively so that host immune responses are activated appropriately [3]. In this regard, we previously reported that LPS from lymphoid tissue–resident Alcaligenes and its active component lipid A moderately stimulated dendritic cells (DCs) in a TLR4-dependent manner and acted as effective adjuvants to enhance antigen-specific immune responses, including antibody production and Th17 responses, without excessive inflammation [7, 8, 9].
Although we previously used histidine-tagged recombinant Stx2B-C-CPE protein as a vaccine antigen due to the convenience of its preparation [1], here we established a method suitable for commercial production of Stx2B-C-CPE vaccine protein that is free of antigen modification and endotoxin contamination. In addition, we assessed the immunogenicity of endotoxin-free Stx2B-C-CPE when used with an optimized adjuvant regimen to immunize mice.
A fragment of C-CPE (GenBank accession no. M98037.1) was cloned into pET16b
(Novagen, Darmstadt, Germany) to prepare pET16b-C-CPE, as described previously
[10] to be used for ELISA. A fragment of Stx2B (GenBank accession no.
NP_050540.1) was cloned into pCold I (Takara Bio, Shiga, Japan), as described
previously [1] to be used for ELISA. The Stx2B-C-CPE fragment was obtained from
pCold I-Stx2B-C-CPE [1] and cloned into pET9a (Takara Bio). Briefly, Stx2B-C-CPE
DNA was amplified through polymerase chain reaction (PCR) by using specific
primers (forward primer, 5
Recombinant histidine-tagged C-CPE and Stx2B proteins were prepared as described
previously [1]. To obtain recombinant non-tagged Stx2B-C-CPE protein, the
pET9a-Stx2B-C-CPE plasmid was transformed into E. coli strain BL21(DE3)
(Toyobo, Osaka, Japan) and cultured in Terrific broth (Invitrogen, Carlsbad, CA,
USA) supplemented with 50
Endotoxin was removed by using Acrodisc units containing Mustang E membrane
(Pall, Port Washington, NY, USA), which is a positively charged polyethersulfone
membrane with high binding capacity for endotoxins, in accordance with the
manufacturer’s instructions or by using Triton X-114 phase separation, which is a
liquid-liquid extraction method [11]. For the phase separation technique, 500
Female BALB/c mice (age, 7 wk) were purchased from CLEA Japan (Tokyo, Japan) and used for experiments after pre-breeding for 1 wk. All experiments were approved by the Animal Care and Use Committee of the National Institutes of Biomedical Innovation, Health and Nutrition (Approval No. DSR01-1R2) and conducted in accordance with their guidelines. Mice were subcutaneously immunized with PBS (vehicle control), Stx2B-C-CPE, endotoxin-free Stx2B-C-CPE without or with aluminum adjuvant (BIKEN, Osaka, Japan), endotoxin-free Stx2B-C-CPE without or with Alcaligenes lipid A (Peptide Institute, Osaka, Japan) [9], or endotoxin-free Stx2B-C-CPE without or with both aluminum adjuvant and Alcaligenes lipid A once weekly for 2 consecutive weeks. Serum was collected 1 week after the last immunization. The immunization schedule followed the previous study [1].
We coated 96-well immunoplates (Thermo Fisher Scientific, Waltham, MA, USA) with
Stx2B or C-CPE (0.5
Statistical significance was evaluated through one-way ANOVA for comparison of multiple groups and the unpaired t-test for two groups by using Prism 7 (GraphPad Software, La Jolla, CA, USA). A p value less than 0.05 was considered to be significant.
We previously reported the fusion protein Stx2B-C-CPE as a bivalent vaccine to induce protective immunity against Clostridium perfringens and Shiga toxin (Stx)-producing Escherichia coli (STEC) infections and thus prevent food poisoning [1]. To establish an antigen preparation method for commercial vaccine production, we cloned a target fusion gene without any tag or other protein-modifying sequences into pET9a vector (Supplementary Fig. 1) and then examined the experimental conditions for protein expression. A strong signal due to Stx2B-C-CPE was detected at 24 h rather than 6 h in the cell pellet of E. coli strain BL21 (DE3) cultured in Terrific broth at both 37 °C and 25 °C (Fig. 1A). Because this fusion protein retains the signal peptide present in native Stx2B, Stx2B-C-CPE was secreted from E. coli cells and detected in the culture supernatant (Fig. 1A). The amount of impurities, such as the bands at 28–98 kDa, appeared to be less when the cells were grown at 25 °C than at 37 °C (Fig. 1A). We therefore considered that the supernatant obtained after culturing at 25 °C for 24 h is suitable for the next step in our protein purification process.
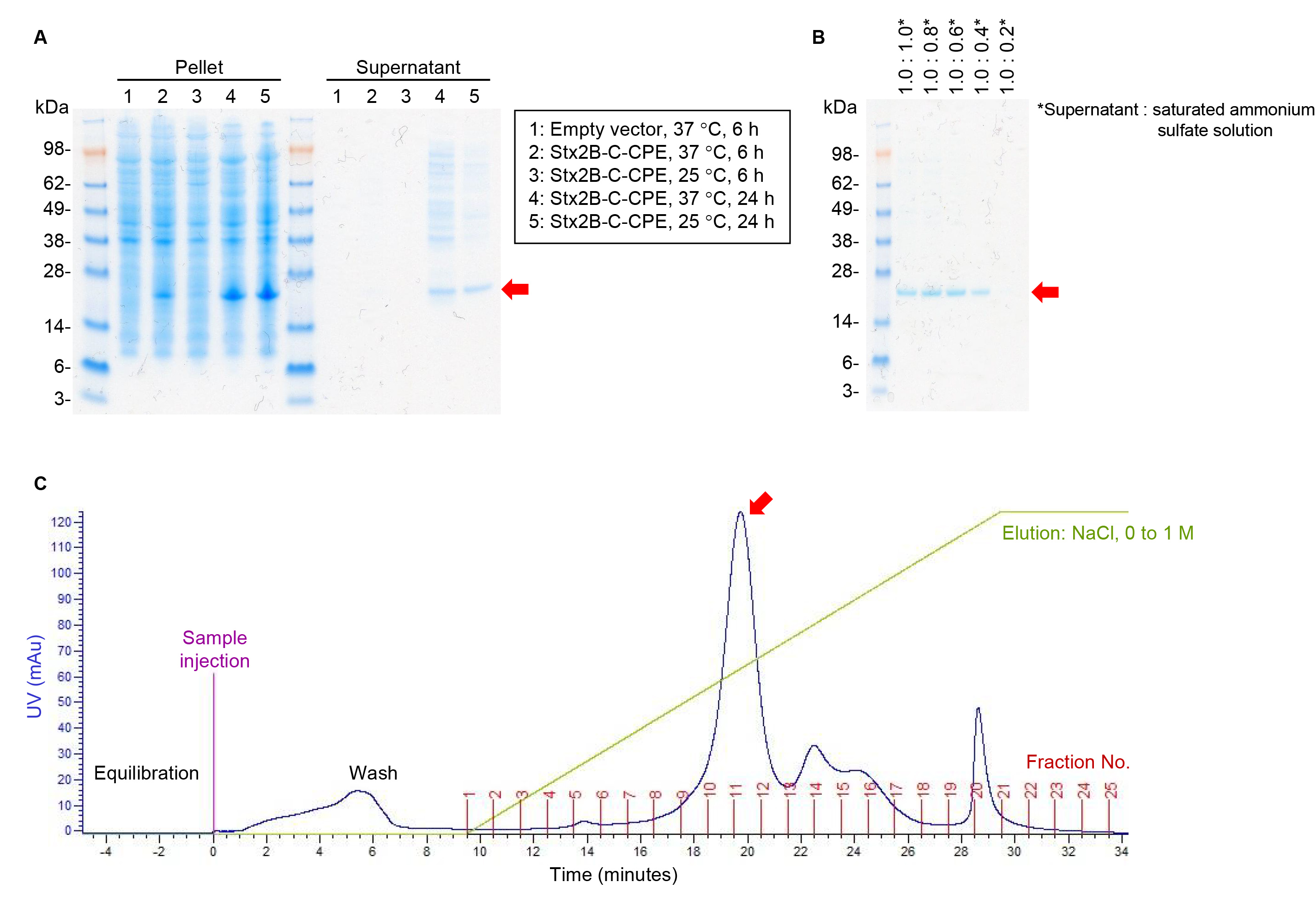 Fig. 1.
Fig. 1.Stx2B-C-CPE antigen preparation. (A) Expression of Stx2B-C-CPE.
Stx2B-C-CPE was expressed by using the pET9a system, E. coli B21(DE3)
cells, and Terrific broth (Invitrogen). After incubation for 6 h at 37
°C or for 24 h at 25 °C, Stx2B-C-CPE was detected in the
centrifuged bacterial pellets (15
Because of the large volume of the culture supernatant, we next addressed conditions for concentrating the protein. Addition of the saturated ammonium sulfate solution resulted in Stx2B-C-CPE protein precipitation, and a concentration of 37.5% (supernatant: saturated ammonium sulfate solution, 1:0.6) appeared to be the minimum for efficient protein recovery (Fig. 1B). Next, Stx2B-C-CPE was purified by using anion-exchange chromatography with a NaCl concentration gradient. Stx2B-C-CPE was eluted in fractions 10 and 11 (Fig. 1C). Thus, we were able to optimize the experimental conditions for the expression and purification of tag-free Stx2B-C-CPE.
Because we used an E. coli expression system to prepare Stx2B-C-CPE, contamination of the preparation by bacteria-derived components such as LPS (i.e., endotoxin) is likely. In fact, the Stx2B-C-CPE solution showed high endotoxin activity (Fig. 2A). However, when we used a filtration system to remove the contaminating endotoxin, the amount of Stx2B-C-CPE protein was greatly decreased also, probably because it was adsorbed on the membrane (Supplementary Fig. 2). These findings indicate that the filtration system we used is unsuitable for removing endotoxin from preparations of Stx2B-C-CPE.
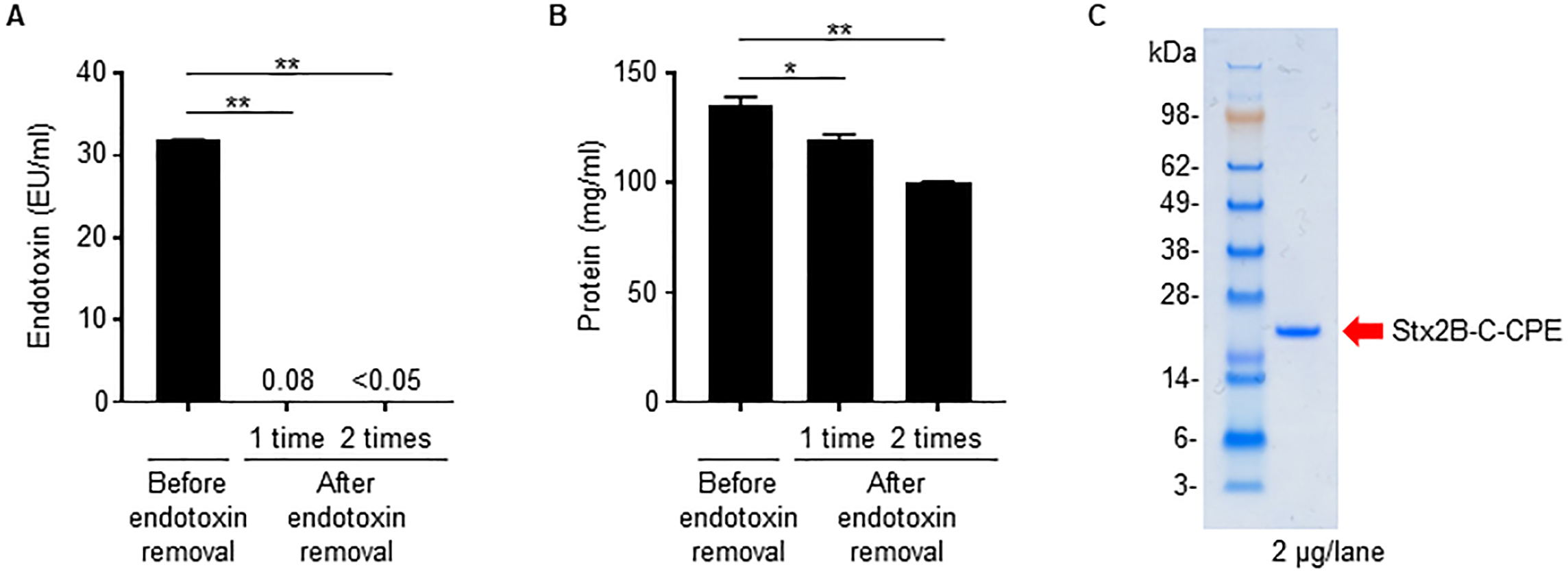 Fig. 2.
Fig. 2.Removal of contaminating endotoxin from Stx2B-C-CPE. (A)
Endotoxin activity in the Stx2B-C-CPE preparation. Endotoxin was extracted from
Stx2B-C-CPE by using Triton X-114 phase separation, and the supernatant was
collected as the endotoxin-removed sample fraction. This procedure was repeated
twice. The endotoxin activity in the Stx2B-C-CPE preparation before and after
endotoxin removal was measured by using ToxinSensor chromogenic LAL endotoxin
assay kit (GenScript). The endotoxin-free reference value is
We then tried Triton X-114 phase separation [11] as a means to remove contaminating endotoxin from preparations of Stx2B-C-CPE. Two rounds of Triton X-114 treatment reduced the endotoxin activity in Stx2B-C-CPE solution to below the limit of detection and below the industry standard of 0.05 EU/mL (Fig. 2A), whereas, although the protein was slightly reduced, Stx2B-C-CPE protein recovery remained high and in excess of 80% (Fig. 2B). Finally, the endotoxin-depleted Stx2B-C-CPE showed a highly purified single band by electrophoresis (Fig. 2C).
We therefore summarize our proposed optimal procedure for preparation of endotoxin-free Stx2B-C-CPE vaccine antigen (Fig. 3) as follows: Step 1, E. coli strain BL21(DE3) cells transformed with pET9a containing Stx2B-C-CPE are cultured in Terrific broth at 25 °C for 24 h; Step 2, the culture supernatant is mixed with saturated ammonium sulfate solution at a ratio of 1:0.6, and the protein is precipitated by centrifugation; Step 3, the protein is purified by using anion exchange chromatography; and Step 4, endotoxin and other contaminants are depleted by using Triton X-114 phase separation.
 Fig. 3.
Fig. 3.Summary of preparative procedure for endotoxin-free Stx2B-C-CPE antigen.
Although LPS is recognized as one of the bacterial toxins known as endotoxins, it has potential to be applied as an immunological modulator, including vaccine adjuvant [3]. Therefore, we examined the effect of removing endotoxin on the immunogenicity of Stx2B-C-CPE. When mice were subcutaneously immunized with endotoxin-contaminated Stx2B-C-CPE, endotoxin-free Stx2B-C-CPE, or PBS (vehicle control) according to the experimental schedule shown in Fig. 4A, administration of endotoxin-contaminated Stx2B-C-CPE induced high levels of both C-CPE-specific and Stx2B-specific serum IgG antibodies (Fig. 4B). In contrast, the induction levels of these antibodies were dramatically decreased in mice that received endotoxin-free Stx2B-C-CPE (Fig. 4B). These results indicate that the contaminating endotoxin enhanced the production of Stx2B-C-CPE-specific antibodies by acting as an adjuvant. Consequently, the presence of endotoxin in antigen preparations may give rise to the misconception that the protein antigens themselves are highly immunogenic. Therefore, the immunological properties of bacterially expressed recombinant antigens should be assessed very carefully.
 Fig. 4.
Fig. 4.Endotoxin contamination and immunogenicity of Stx2B-C-CPE. (A)
Immunization schedule. (B) Induction of IgG production by immunization with
Stx2B-C-CPE. Female Balb/c mice (age, 8 wk) were subcutaneously immunized with
100
Endotoxin removal is critical from the viewpoint of safety and quality control, and simultaneously high immunogenicity is required for the induction of appropriate immune responses to achieve protective immunity. Therefore, to apply endotoxin-free Stx2B-C-CPE as a vaccine antigen, it may need to be injected in combination with an adjuvant to enhance its immunogenicity for the induction of antigen-specific antibody production. To address this issue, we used aluminum, an adjuvant in several human vaccines, and lipid A, the active component of LPS. However, both compounds were only weak antigens when used as the sole adjuvant, slightly enhancing Stx2B-specific but not C-CPE-specific IgG production (Fig. 5). In contrast, combined use of aluminum and lipid A adjuvants with endotoxin-free Stx2B-C-CPE strongly promoted both C-CPE- and Stx2B-specific IgG production in immunized mice (Fig. 5). Thus, the immunogenicity of endotoxin-free Stx2B-C-CPE was significantly improved through the use of vaccine adjuvants.
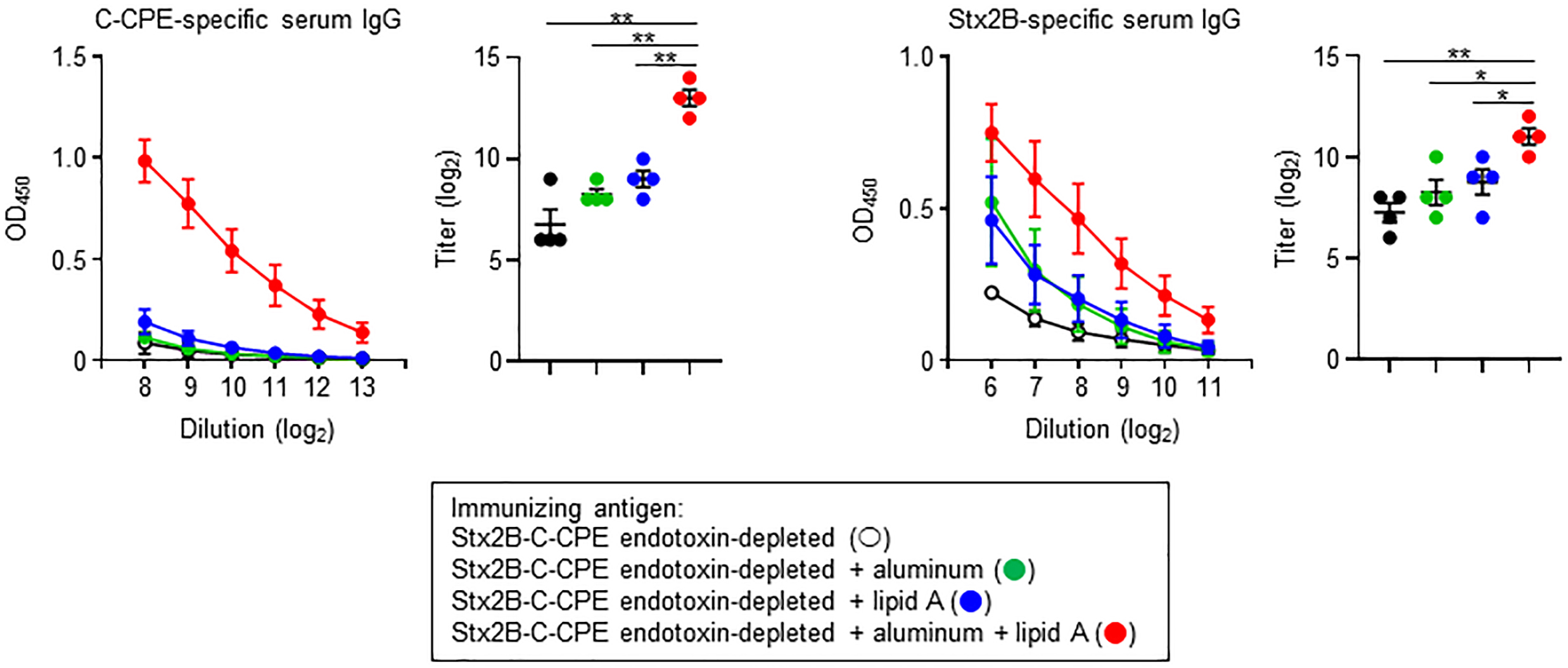 Fig. 5.
Fig. 5.Improved IgG production after combining aluminum and lipid A
adjuvants with endotoxin-free Stx2B-C-CPE for immunization. Female Balb/c mice
(age, 8 wk) were subcutaneously immunized with 10
Most commercially available human vaccines must be evaluated for bacterial endotoxin contamination in accordance with batch release guidelines, such as Official Control Authority Batch Release (OCABR) guidelines. For safety reasons, most vaccines including recombinant protein antigens should ideally be free of endotoxin. Furthermore, endotoxin limits for preclinical animal models have been derived according to the threshold pyrogenic human dose of 5 endotoxin units (EU) per kilogram [5]. In this context, we established a method for preparing a non-tagged endotoxin-free Stx2B-C-CPE recombinant protein for vaccine formulation. When combined with aluminum and Alcaligenes lipid A adjuvants, Stx2B-C-CPE showed immunogenicity sufficient to induce both Stx2B- and C-CPE-specific IgG antibody production in mice. These results suggest the non-tagged endotoxin-free Stx2B-C-CPE as a potential bivalent vaccine antigen to prevent systemic toxicity due to C. perfringens and STEC infections.
The secretion of recombinant proteins into the culture supernatant facilitates their downstream processing and considerably reduces production costs [12, 13]. Importantly, cell disruption is unnecessary for harvesting secreted proteins, the number of subsequent processing steps can be minimized, and the secreted proteins spontaneously form their correct final conformation, including appropriate formation of disulfide bonds [12]. In addition, the secretion of recombinant proteins can prevent their accumulation as insoluble cytoplasmic inclusion bodies and avoid unexpected toxic effects on the bacterial host cells of the expression system [12].
Regarding the expression temperature we chose for Stx2B-C-CPE, 37 °C is a suitable temperature for culturing E. coli but can lead to overgrowth and the formation of inclusion bodies due to incorrect folding of the expressed protein [14]. Therefore, decreasing the culture temperature is a common approach to obtain soluble proteins and avoid these issues [15, 16]. Indeed, incubating at 25 °C instead of 37 °C maintained the amount of soluble Stx2B-C-CPE and decreased the impurities in the culture supernatant. In addition to promoting the solubility of the expressed protein, a low culture temperature prevents protein degradation due to decreased protease activity [14]. For these reasons, we think that a method in which Stx2B-C-CPE is expressed at low temperature (25 °C) and purified from the culture supernatant is appropriate and beneficial.
Triton X-114 phase separation exploits the property that amphiphilic molecules are recovered in the detergent phase [17]; therefore, hydrophilic Stx2B-C-CPE is found exclusively in the aqueous phase, and amphiphilic LPS is separated. Triton X-114 phase separation might also be used to remove other amphiphilic components. For example, the bacterial membrane contains amphiphilic phospholipids, cholesterol, and glycolipids; these lipids have the potential to be immunomodulators, and some of them actually act as natural adjuvants to promote adaptive immune responses [18, 19]. Indeed, endotoxin-free Stx2B-C-CPE plus lipid A failed to induce noteworthy Stx2B- and C-CPE-specific immune responses in immunized mice. This finding suggests that Triton X-114 phase separation likely removed not only LPS but also other immune-stimulating molecules. The activities of contaminating components might lead to the misconception that the antigenicity of Stx2B-C-CPE was higher than it actually was; in addition, these contaminants could be risk factors for causing side effects such as inflammation. Therefore, Triton X-114 phase separation seems to have eliminated several potential problems, thus leading to the opportunity for the formulation of a safe Stx2B-C-CPE vaccine.
One particularly interesting finding in this study is the synergistic or additive effect of the two adjuvants. Although each compound lacked noteworthy adjuvant activity when used on its own, the combined use of aluminum and Alcaligenes lipid A with Stx2B-C-CPE during immunization enhanced both Stx2B- and C-CPE-specific IgG antibody production. Alcaligenes lipid A activates antigen-presenting cells, such as DCs, through TLR4 to upregulate the expression of major histocompatibility complex (MHC) class II molecules and costimulatory molecules, such as CD80 and CD86 [8]. This mechanism suggests that Alcaligenes lipid A promoted antigen presentation and the activation of T cells, which may have stimulated B cell responses, including IgG antibody production, to Stx2B-C-CPE. Like lipid A, aluminum also enhances MHC class II–mediated antigen presentation to T cells. However, aluminum seems to be a poor activator of DCs and promotes uptake of the antigen due to its precipitation on aluminum particles [20]. Furthermore, Alcaligenes lipid A selectively promotes antigen-specific Th17 responses through the production of cytokines, including IL-6, from DCs [21]. In contrast, aluminum induces Th2 responses including IL-4 production, which boosts humoral immunity and IgG antibody production [22]. Therefore, Alcaligenes lipid A and aluminum likely exert different effects on host immune cells, particularly DCs and T cells, and thus potentially cooperatively enhance antigen-specific IgG antibody production. Other combinations of vaccine antigens and adjuvants have also been reported [20]. For example, the combination of monophosphonyl lipid A (MPL) and QS21, a purified form of saponin, enhanced cellular and humoral immune responses to a recombinant HIV protein [23], and aluminum and MPL are used concurrently for the human papillomavirus virus-like particle vaccine [24]. Therefore, we here propose a vaccination strategy for the bivalent vaccine antigen candidate Stx2B-C-CPE that uses aluminum and Alcaligenes lipid A in combination as adjuvants; our adjuvant strategy may be helpful for successful vaccine development.
To develop an effective and safe vaccine formulation of a candidate bivalent antigen, we developed a method for preparing non-tagged endotoxin-free Stx2B-C-CPE recombinant protein and a strategy for its immunization with combined use of aluminum and lipid A adjuvants. In addition, this study highlights the detrimental effects of natural contaminants, including endotoxin, on the assessment of the immunogenicity and potential safety risks of vaccines.
C-CPE, C-terminal region of Clostridium perfringens enterotoxin; CPE, Clostridium perfringens enterotoxin; DC, dendritic cell; EU, endotoxin units; IgG, immunoglobulin G; IL, interleukin; LPS, lipopolysaccharide; MHC, major histocompatibility complex; MPL, monophosphonyl lipid A; PBS, phosphate-buffered saline; STEC, Shiga toxin–producing Escherichia coli; Stx, Shiga toxin; Stx2B, Shiga toxin 2 B subunit; Th2, type 2 T helper; Th17, type 17 T helper; TLR, Toll-like receptor.
All data generated or analyzed during this study are included in this published article.
KH and JK designed the research study. KH, YT, and MF performed the experiments. AS, AH, NH, TN, HE, MK, HK, SY, and KF provided materials and helpful discussion. KH analyzed the data. KH and JK wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We thank the members of our laboratories for helpful discussion.
This research was funded by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)/Japan Society for the Promotion of Science (KAKENHI), grant numbers 22K15004, 22H03953, 22K19936, 18H05280, 15H05836, 16H01885, 19KK0145, 20H00404, 20H05675, 18H04620, 20H04776, and 20K05749; the Japan Agency for Medical Research and Development (AMED), grant numbers JP223fa727001h0001, JP22fk0108145h0003, JP223fa627003, JP19fk0108112j0001, JP19im0210623h0001, JP20fk0108122h0001, JP21am0401029h0001, JP21ae0121040, and JP223fa727001s0301; the Grant for Joint Research Project of the Institute of Medical Science, the University of Tokyo; and the Nippon Foundation - Osaka University Project for Infectious Disease Prevention.
The authors of this manuscript have the following potential conflicts of interest: T. Noguchi and H. Ebina are employees of BIKEN (Osaka, Japan). Other authors declare no competing interests.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
