Academic Editors: Antonio Barbieri and Francesca Bruzzese
Background: Cancer-associated fibroblasts (CAFs) are of considerable
importance in tumor progression by interacting with the tumor microenvironment.
However, the hidden mechanism explaining how tumor cells interact with CAFs in
the tumor mechanical microenvironment remains largely unknown. Methods:
We highlighted exosomes as the mediator modulating the interaction between liver
cancer cells and CAFs under mechanical conditions. The normal hepatic stellate
cells LX2 were exposed to the medium or exosomes from the HepG2 cells with or
without fluid shear stress subjection, and the CAFs activation markers were
checked. To further explore the potential role of PI3K, which is active in liver
fibrosis, the PI3K inhibitor was used. Results: The specific markers of
CAFs, FAP, and
The tumor microenvironment (TME) is composed of the surrounding fibroblasts,
immune cells, cytokines, chemokines, as well as the extracellular matrix (ECM)
[1, 2, 3]. Cancer-associated fibroblasts (CAFs), the amplest fibroblasts in the
cancer stroma, is a continuously activated subpopulation of fibroblasts, playing
a crucial role in promoting tumor progression [4, 5]. CAFs exhibit a high degree
of heterogeneity because of the diversity of sources and expressed different
specific markers for identification. In the liver and pancreas, the static
stellate cells activation is the main source of CAFs [6]. Thereinto,
There are various biological and physical abnormalities in the TME. Among these,
biomechanical properties alterations of the TME are a new hallmark of cancer [15, 16]. Here, in the tumor biomechanical environment, due to the recruitment of
leaky blood vessels and deficiency of functioning lymphatic vessels, there’s a
rise in the interstitial fluid pressure (IFP), causing an abnormal interstitial
flow which induces elevated fluid shear stress (FSS) [15]. 0.1–2 dyn/cm
Exosomes, a kind of extracellular vehicle with a diameter between 30 nm and 160 nm, are widely involved in cell-cell communication [20]. Exosomes secreted from cancer cells could be absorbed by fibroblasts and reshape the biological functions of the recipient cells, finally resulting in the CAFs activation [21, 22]. Therefore, the altered bioactive components, which are contained in the exosomes released from FSS-induced liver cancer cells, may activate CAFs.
IGF2, playing a critical role in regulating cell proliferation and migration in solid tumors, is found to be upregulated in a variety of human malignancies, including liver cancer, and is associated with poor prognosis [23]. In liver cancer, though the loss of imprinting, loss of heterozygosity, or reactivation of IGF2 transcription [24] could partially explain the upregulation of IGF2 in cancer, the detailed functions and mechanisms about IGF2 release in the TME still remain unknown. Whether the aberrant expression of IGF2 in exosomes derived from FSS-induced liver cancer cells could mediate the interaction of tumor cells and CAFs needs to be elucidated.
Here, we hypothesize that the medium from liver cancer cells with fluid shear stress subjection is more capable of activating fibroblasts through the bioactive substance in exosomes. Therefore, we investigated the activation of normal hepatic stellate cells LX2 treated with the medium or exosomes from liver cancer HepG2 cells in the absence or the presence of fluid shear stress. Furthermore, we explored the molecular process of IGF2 in exomes activating the recipient fibroblasts.
The human liver cancer cell lines HepG2, QGY-7703, and hepatic stellate cells
LX2 (Institute of Biochemistry and Cell Biology, Shanghai, China) were cultured
in an incubator with 5% CO
The fluid shear stress loading system and HepG2 cell loading conditions were described previously [18, 19]. Briefly, a parallel plate flow chamber with a peristaltic pump system (Masterflex model 7518-10, Cole-Parmer Instrument Company, Vernon Hills, IL, USA) makes up the shear flow loading system, which can produce a directed steady flow for this study. The loading shear stress is determined using the formula below.
where
The shear flow was determined according to the velocity of the circulation
medium using the chamber parameters (H = 0.3 mm, W = 95 mm) and the viscosity of
the circulating medium (0.83 mPa∙s). Liver cancer cells were seeded on
a 25
To ascertain the effects of soluble factors secreted by liver cancer cells on
LX2 activation, we collected different culture mediums of HepG2 cells. Firstly,
static condition medium (CM) means the medium extracted from the HepG2 cells
after cell starvation overnight without exposure to fluid shear stress, and then
the medium was collected and centrifuged at 3000 g (Centrifuge 5804R; Eppendorf,
Hamburg, Germany) at 4
The detailed information about antibodies used for Western blot analysis and immunofluorescence staining are shown in Supplementary Table 1.
When the cell growth density reached 80%, the cells were lysed, then the total
protein was collected and quantified. Afterward, protein electrophoresis was
performed using SDS-PAGE. Following that, transmembrane was conducted to transfer
proteins to polyvinylidene difluoride (PVDF) membranes. After that, the membranes
were blocked for 3 h in TBST buffer containing 5% BSA at room temperature. The
membranes were then treated with primary antibodies overnight at 4
LX2 cells were treated with CM and FM, respectively for 5 days. And then, the
cells were fixed with 4% paraformald ehyde for 10 min, blocked, and permeabilized with
5% BSA (Solarbio, Beijing, China)/0.2% Triton X-100/PBS at room temperature for
60 min. Next, cells were incubated with primary antibodies (FAP, 1:100, diluted
in PBS) overnight at 4
The method performing qPCR was described previously [19]. The primer sequences
were as follows: FAP, 5’-CCAGCAATGATAGCCTCAAG-3’ (forward) and
5’-GACCGAAACATTCTGGACTC-3’ (reverse); ACTA2, 5’- TCCTTCATCGGGATGGAGTC-3’
(forward) and 5’-GGCAATGCCAGGGTACATAG-3’ (reverse); PDGFR-
The proliferation ability of LX2 was tested using the MTT assay. Firstly, LX2
cells were grown in CM and FM until 5 days, respectively. The media was then
removed, and each well of LX2 cells was treated with 20
Exosomes were isolated utilizing a combination of centrifugation,
ultracentrifugation, and filtration as previous study [25]. Briefly, LX2 culture
medium, HepG2 static condition medium, and HepG2 fluid shear stress-induced
medium were collected. Then exosomes were isolated by gradient centrifugation as:
300 g
Exosomes suspended in PBS were dropped on the golden grid. Then, exosomes were negatively stained by 2% phosphotungstic acid for 3 min, following air-drying for 15min. Finally, TEM (H-600IV, Japan) at 80 kV was used to observe the exosomes.
After being diluted in PBS, exosomes were analyzed using the Zeta View (Particle Metrix GmbH, Meerbusch, Germany). To get reliable results, exosome samples were diluted by PBS, ending with approximately 20–100 particles in a field of view.
The PKH26 (Thermo Fisher Scientific, Waltham, USA) is a kind of red lipophilic dye that could be integrated into the exosome membrane. In this study, freshly isolated exosomes were stained in PKH26 and PKH linker mixture in the dark. Then the labeled exosomes were washed with PBS and purified as the step of 2.9. Finally, labeled exosomes were incubated with cells to verify the exosome uptake.
To determine the role of exosomes in activating LX2 cells, exosomes derived from
HepG2 static condition medium (CM) and fluid shear stress-induced medium (FM)
were isolated and used to treat LX2 cells, respectively. And the exosomes derived
from LX2 culture medium were set as control. All the exosomes were added with
same concentration (10
The data was represented as the Mean
Cancer cells’ supernatant contains bioactive components that can influence the
activity and phenotypic of recipient cells [1]. Therefore, we hypothesized that
the substance released by tumor cells after flow subjection has greater
capability of activating fibroblasts through bioactive components. Initially, we
collected the conditioned medium from HepG2 cells in the absence or presence of
fluid shear stress (FSS) subjection (the medium for HepG2 cells without fluid
shear stress was referred to as static condition medium (CM); while the medium
for HepG2 cells with fluid shear stress subjection was referred to as fluid shear
stress-induced medium (FM)) to study their effect on the activation of hepatic
stellate cell LX2. Western blot analyses were conducted to measure the expression
of activated fibroblast markers. A slightly increased expression of FAP and
 Fig. 1.
Fig. 1.Fluid shear stress-induced cancer cell medium promoted the
activation and proliferation of CAFs. (A) Western-blot analysis of the
expression of FAP and
The condition medium contained various bioactive substances, such as exosomes, cytokines, and proteins [26, 27]. To determine whether exosomes in medium activate CAFs, exosomes derived from HepG2 static condition medium (CM) and fluid shear stress-induced medium (FM) were isolated and used to treat LX2 cells, respectively. And the exosomes derived from LX2 culture medium were set as control. First, the classical methods were used to isolate and identify exosomes in each group [25]. After differential centrifugation and ultracentrifugation, the exosomes from each group were resuspended in PBS, and then validated by transmission electron microscopy (TEM). As shown in Fig. 2A, the exosomes were characterized by the cup-like structure of the membrane, and exosomes with a size range of 50–160 nm were confirmed using nanoparticle tracking analysis (NTA). Furthermore, the vesicles tested positive for exosome markers CD63, Alix and Hsp70 (Fig. 2B).
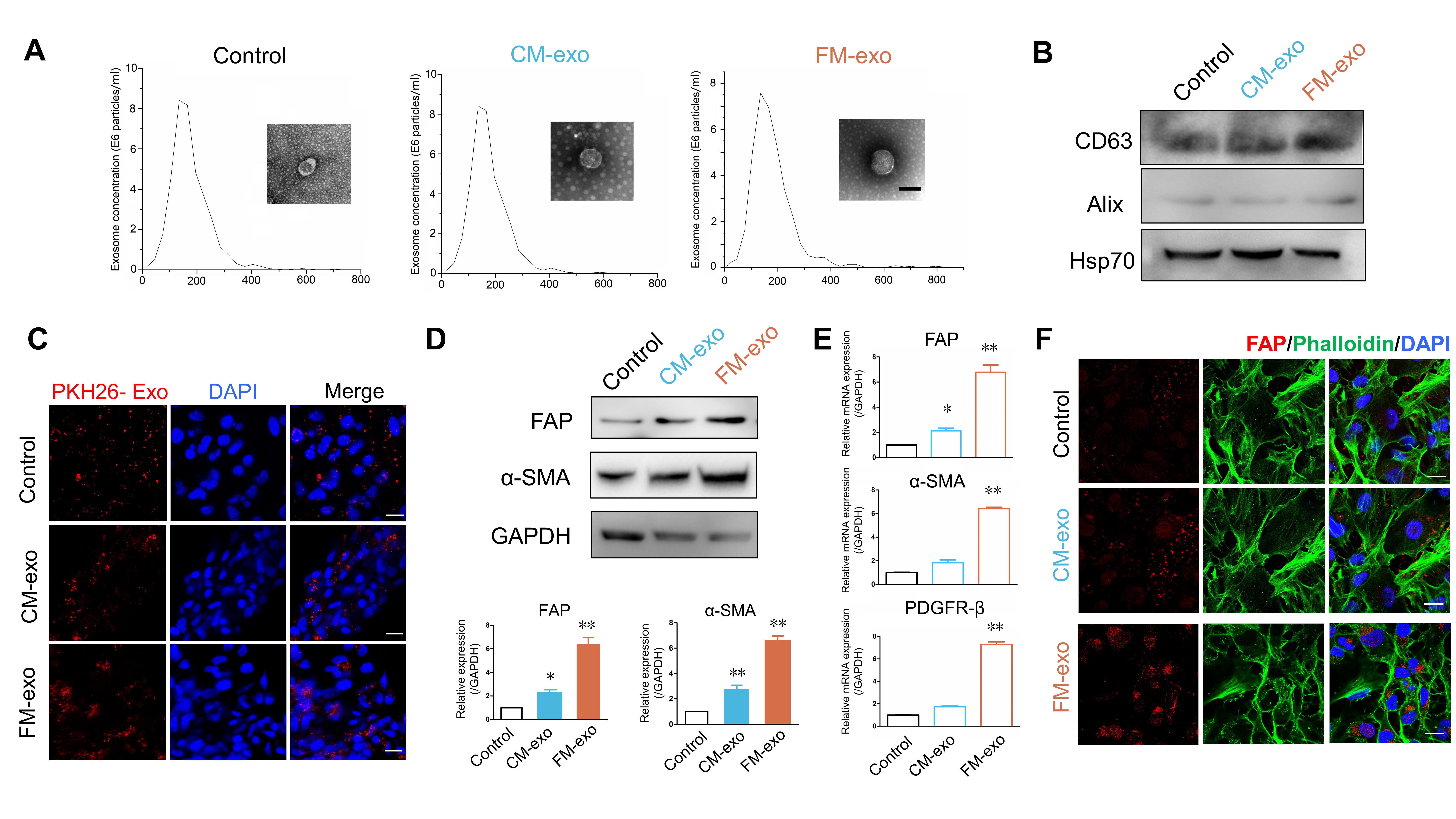 Fig. 2.
Fig. 2.Exosomes derived from sheared medium promote CAFs activation.
Exosomes were isolated from LX2 culture medium (Control), HepG2 static condition
medium (CM-exo) and HepG2 fluid shear stress-induced medium (FM-exo), by
differential centrifugation and ultracentrifugation, followed NTA and TEM (A) to
identify the size and structure of the exosome in each group. Scale bar = 100
Next, LX2 cells were incubated with PKH26-labeled exosomes (10
Although IGF2 was upregulated in many cancers as reported previously [23], it
generally promotes cancer development in a ligand-receptor way [24, 28], and few
studies focused on IGF2 delivered by exosomes. We found that the FSS upregulated
the expression of IGF2 (Fig. 3A,B) in two liver cells (HepG2 and QGY-7703).
Consistently, the significantly increased IGF2 was observed in liver cancer cells
exposed to FSS as shown by immunofluorescence (Fig. 3C). Furthermore, the high
expression of IGF2 was also detected in the exosome derived from fluid shear
stress-induced medium, suggesting that the exosomes from FM contained more IGF2
(Fig. 3D). In addition, recombinant human IGF2 could effectively activate LX2 by
upregulating FAP and
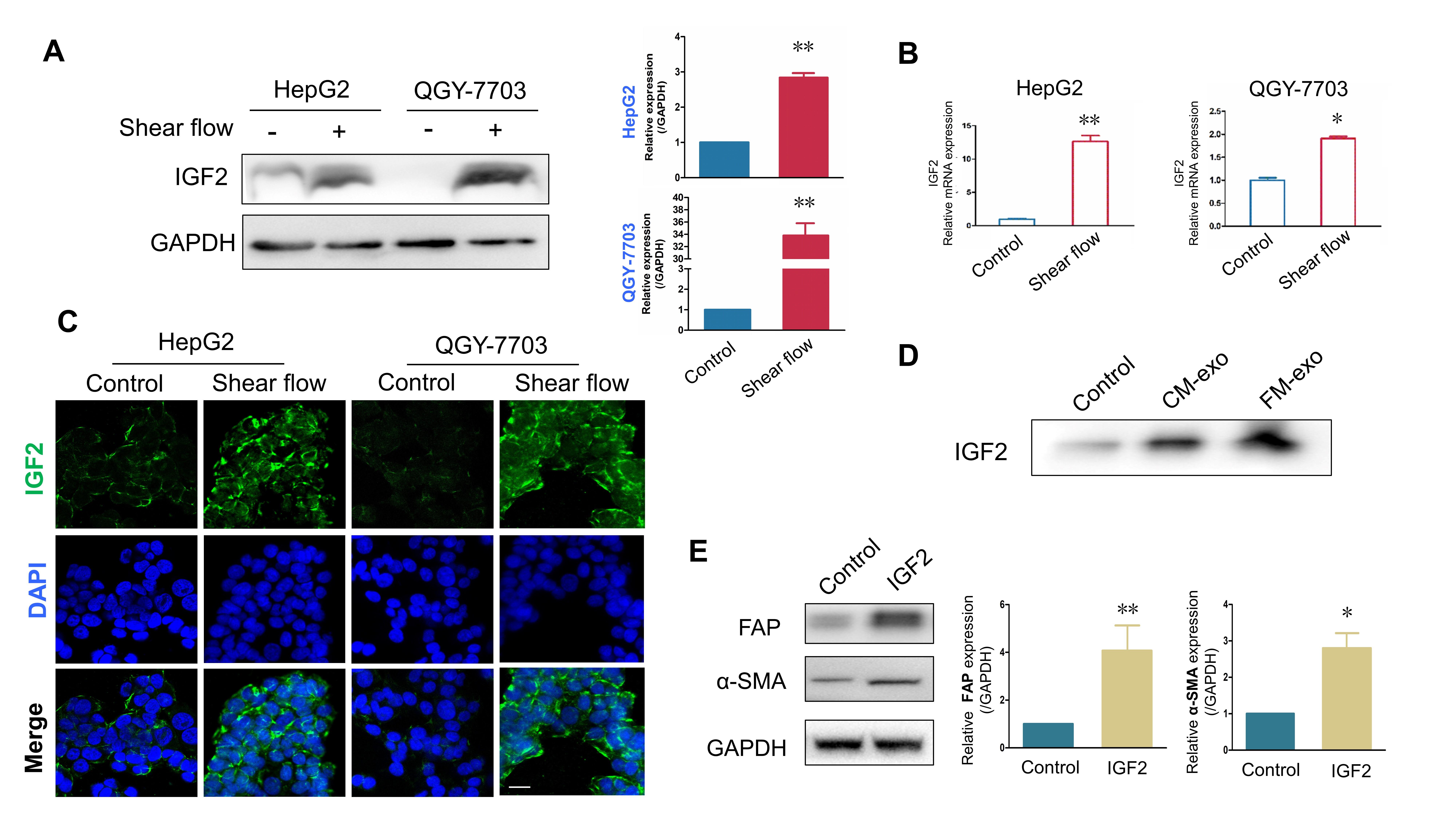 Fig. 3.
Fig. 3.The expression of IGF2 was upregulated by fluid shear stress in
liver cancer cells and exosomes. (A) Images showing the expression of IGF2 in
HepG2 and QGY-7703 cells with or without shear flow stimulation, measured by
Western blot (n = 3). (B) Graphs showing the expression of IGF2 in HepG2 and
QGY-7703 cells, measure by qPCR (n = 3). (C) Images showing the expression of
IGF2 in HepG2 and QGY-7703 cells with or without shear flow stimulation, measured
by immunofluorescence staining. Scale bar = 100
The PI3K/AKT signaling pathway is critical for cell proliferation, differentiation, apoptosis, and mobility [29, 30]. Increasing evidence has identified that PI3K/AKT pathway could promote the differentiation of various cells into CAFs. IGF2 was identified as a key factor that interacted with PI3K pathway to promote carcinogenesis [31]. We previously demonstrated the high level of IGF2 in exosomes derived from fluid shear stress-induced liver cancer cells (Fig. 3D). To investigate the molecular mechanism of flowed-exosome inducing LX2 activation, we examined the IGF2-PI3K-AKT signals in LX2 cells.
As shown in Fig. 4A, exosomes derived from fluid shear stress-induced medium
significantly upregulated the expression of IGF2, p-PI3K, and
p-AKT in LX2 cells, compared with the static condition medium group
(Fig. 4A). PI3K inhibitor was used to study the role of PI3K in CAFs activation;
as expected, the expression of FAP and
 Fig. 4.
Fig. 4.IGF2-PI3K axis participated in CAFs activation. (A) The
expression of key factors in IGF2-PI3K-Akt axis of LX2 treated with exosomes
derived from CM or FM (n = 3). (B) The expression of CAFs marker proteins in LX2
cells treated with PI3K inhibitor (n = 3). (C) The expression of CAFs markers in
LX2 cells treated with Xentuzumab (n = 3). *p
Primary liver cancer is a highly fatal disease with high mortality, which has become one of the most malignant tumors and threatens human health [32]. Recent studies strengthened the conception that cross-talk signaling between malignant cells and the liver cancer microenvironment contributed to tumor progression and the pathogenesis of liver cancer [2, 33]. As well known, cellular communication in the tumor microenvironment plays a pivotal part in carcinogenic processes; therefore, it necessitates the study of the cellular communication between liver cancer cells and CAFs, especially in the tumor microenvironment (TME).
In recent decades, the physical traits in TME gained more attention. The tumor
physical microenvironment, particularly the liquid microenvironment, is
particularly crucial in tumor progression because of the pervasive fluid flow in
our bodies [15]. The fluid shear stress affects the biology and function of TME
in several ways, including facilitating angiogenesis and lymphangiogenesis [34],
regulating matrix metalloproteinase activity as well as cell motility [35], and
enhancing cancer cell ability of migration and invasion [36]. Our previous
studies have confirmed that elevated fluid shear stress greatly promoted the
migration of liver cancer cells [17, 18]. In this study, we first analyzed the
CAFs activation markers expression both at gene and protein level when typical
liver fibroblast cell line LX2 co-culture with condition medium from static HepG2
cell and 1.4 dyn/cm
Exosomes widely exist in body fluids and are significantly associated with the
progression of tumors. Exosomes served as mediators in intercellular
communications between various cell types by transporting information cargos,
such as nucleic acids, proteins, and lipids, to the recipient cells and changing
the recipient cells’ behaviors. Therefore, we further investigated if the
bioactive substance rests in exosomes and is absorbed by LX2 cells. So, we
isolated the exosomes from HepG2 static condition medium and fluid shear
stress-induced medium, characterized the exosomes by transmission electron
microscopy, nanoparticle tracking analysis and Western blotting, and then they
were used to co-culture with LX2. The results showed that the expression of CAFs
markers FAP,
IGF2, a mitogenic peptide hormone regulating various physiological functions,
which is over-expressed in many cancers and is associated with poor prognosis
[23]. In the present study, high expression of IGF2 was detected in liver cancer
cell lines HepG2 and QGY-7703 cells when treated with 1.4 dyn/cm
In conclusion, tumor cells in the flowed condition released exosomes are more capable of activating normal fibroblasts to CAFs through transporting IGF2 to the recipient cells and activating the PI3K/Akt signaling. These findings illustrated the fundamental role of intercellular communication in the tumor physical microenvironment and revealed a new molecular mechanism elucidating the activation of CAFs in liver cancer.
XL and HY conceived the idea and designed the research study. TF and FF performed the research. TL and YS provided help and advice on data analysis. TF, CZ and JH analyzed the data. TF and FF wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This study was supported by grants from the National Natural Science Foundation of China (31971239, 11932014).
The authors declare no conflict of interest.
