- Academic Editor
-
-
-
†These authors contributed equally.
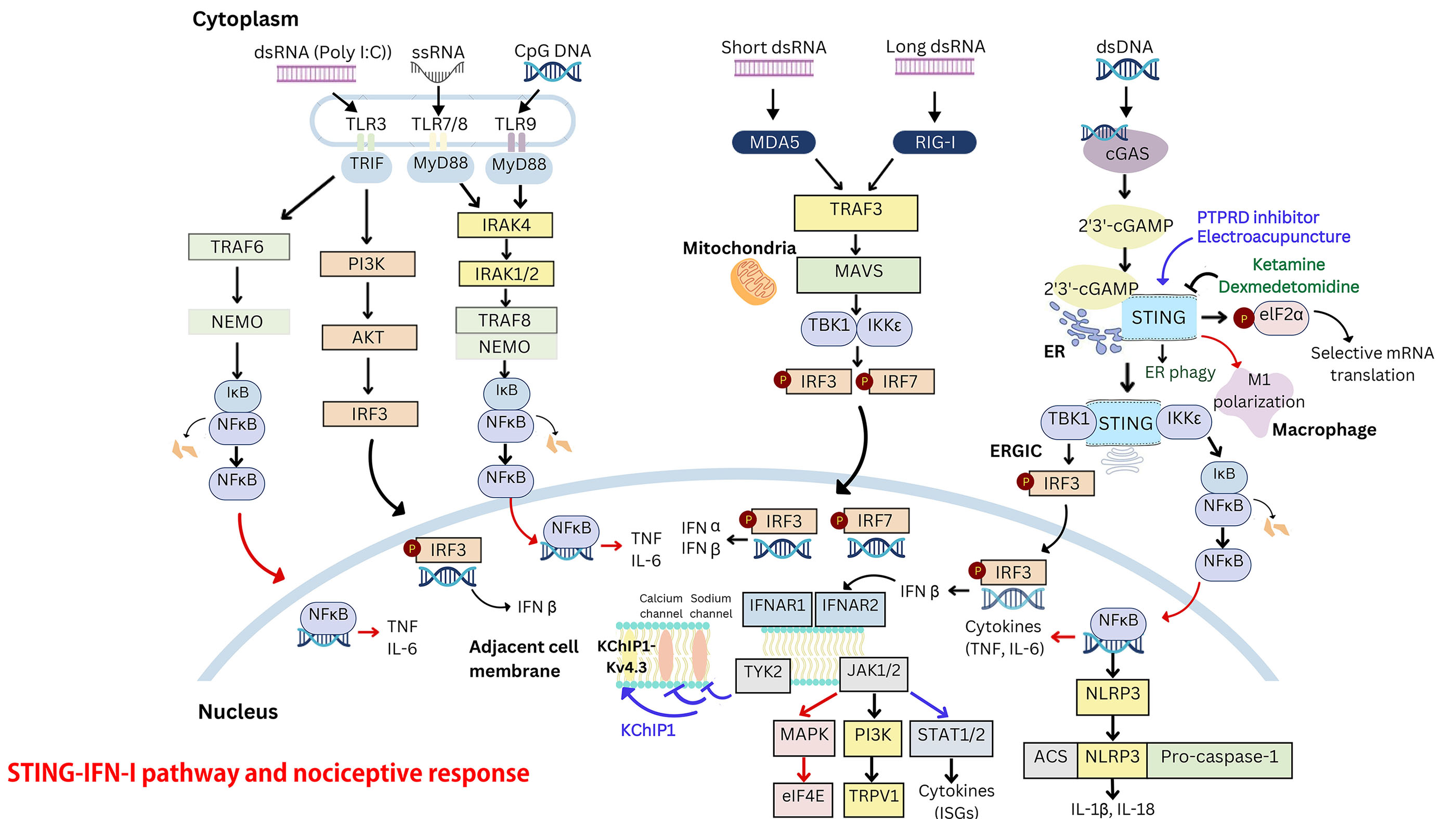
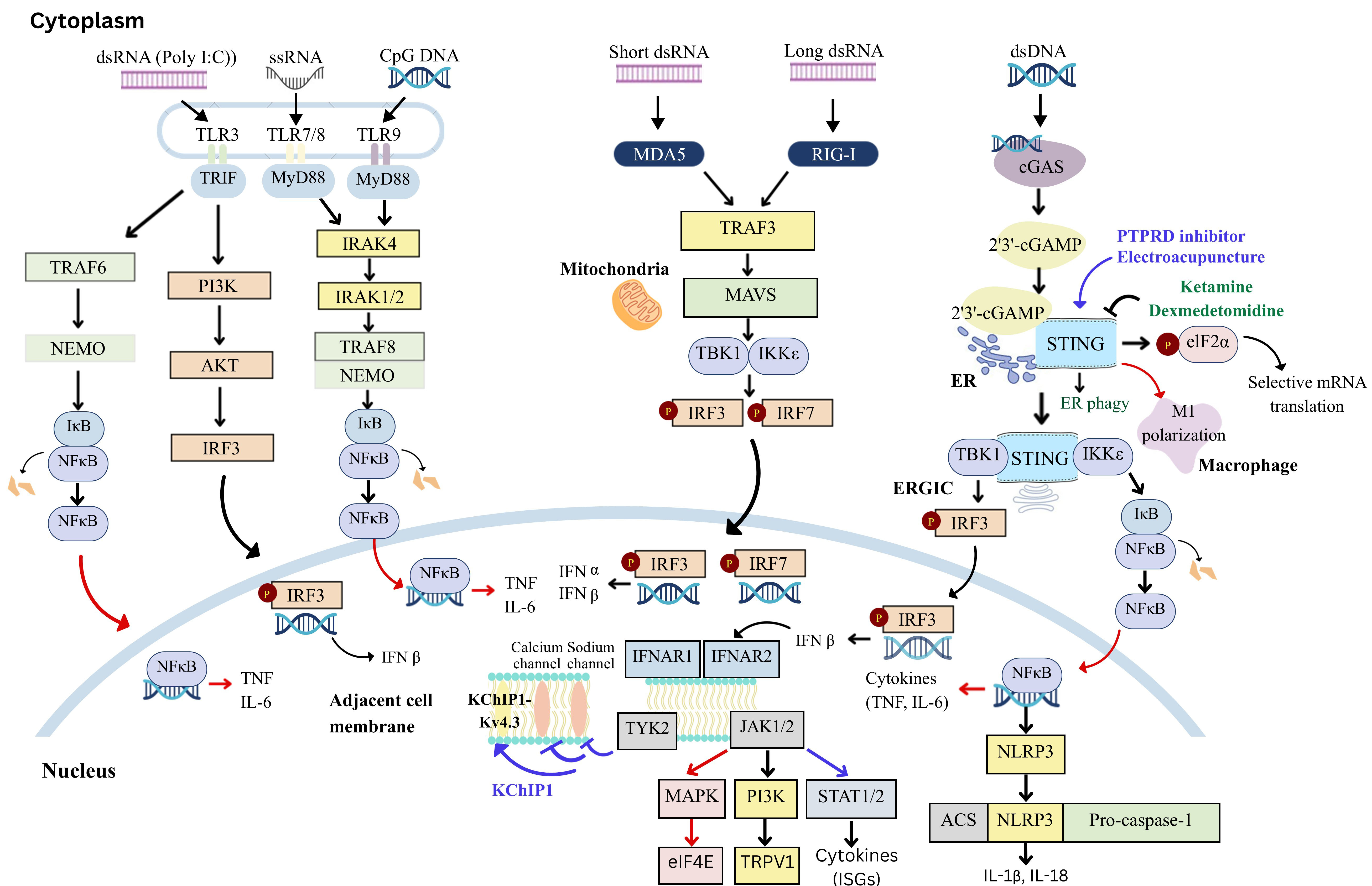
Interferons (IFNs) are cytokines with diverse functions, possessing antiviral, antiproliferative, and immunomodulatory effects. IFN-α and IFN-β, key members of the type I interferon (IFN-I) family, are widely used in the treatment of diseases such as hepatitis and multiple sclerosis. In the nervous system, microglia, astrocytes, and neurons express IFN-I receptors. Beyond their classical transcriptional roles, IFN-Is can suppress neuronal activity and synaptic transmission through nongenomic mechanisms, producing potent analgesic effects. However, IFN-Is are active in signaling pathways such as phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK), and the MAPK-interacting serine/threonine-protein kinase (MNK)-eukaryotic initiation factor 4E (eIF4E) pathway, which can sensitize peripheral nociceptors and contribute to nociceptive responses. This narrative review explores recent advances in understanding the roles of IFN-I and the cyclic-GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling cascade in acute and chronic nociceptive responses, which are increasingly recognized but remain a subject of debate. Recent studies suggest that the STING–IFN-I pathway has complex, stage-dependent effects on nociception. In the middle to late stages of the nociceptive response, this pathway can activate signal transducer and activator of transcription (STAT) signaling, as well as microglial mediated STING pathways and tumor necrosis factor (TNF) receptor-associated factor (TRAF) family member-associated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB activator) collectively referred to as TANK. These pathways increase pro- and anti-inflammatory cytokine production, promote microglial M1 polarization, and inhibit endoplasmic reticulum-phagy (ER-phagy) in the central nervous system (CNS). These mechanisms contribute to central sensitization while modulating the analgesic effects of IFN-Is. Thus, the STING-IFN-I pathway plays a dual role in nociception, with both pro-nociceptive and analgesic effects that are dependent on the stage of the nociceptive response. Understanding the differential roles of STING–IFN-I signaling in nociceptors under physiological and pathological conditions could pave the way for the development of targeted nociceptive response management therapies.