1 Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA
Abstract
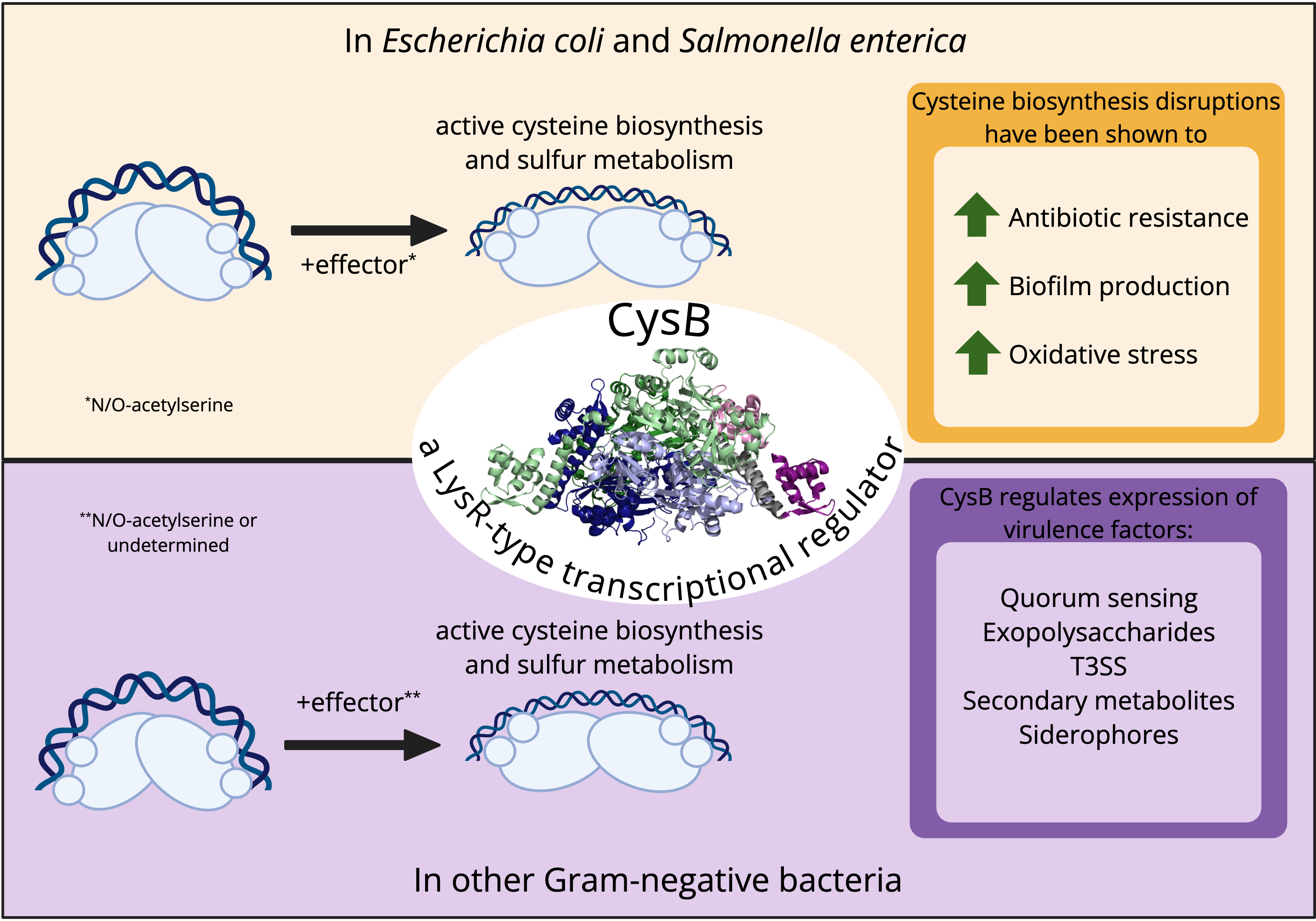
CysB is a member of the large bacterial LysR-type transcriptional regulator (LTTR) protein family. Like the majority of LTTRs, CysB functions as a homotetramer in which each subunit has an N-terminal winged-helix-turn-helix (wHTH) DNA-binding domain connected to an effector-binding domain by a helical hinge region. CysB is best known for its role in regulating the expression of genes associated with sulfur uptake and biosynthesis of cysteine in Gram-negative species such as Escherichia coli and Salmonella enterica. Activation of CysB target genes generally requires the effector N-acetyl-L-serine, which derives from an intermediate in the cysteine biosynthetic pathway. Here, we outline the established roles of CysB in controlling the cysteine regulon, complemented with an interpretation of DNA binding modes inspired by the recently published structure of full-length CysB that is consistent with the ‘sliding dimer’ model proposed for many LTTRs. Notably, CysB orthologs have been described for which N-acetyl-L-serine does not appear to be required as an effector, and CysB regulons frequently include genes that are not directly related to sulfur assimilation and cysteine biosynthesis. Examples include hslJ, which encodes a predicted membrane protein involved in novobiocin resistance in E. coli, and pqsR, encoding a transcriptional regulator involved in Pseudomonas Quinolone Signal production and virulence in Pseudomonas aeruginosa. These data suggest that CysB orthologs have diverged to ensure optimal function and incorporation in distinct gene regulatory networks.
Graphical Abstract
Keywords
- cysteine regulon
- gene regulation
- LTTR
- N-acetylserine
- sulfur assimilation
Sulfur is an essential and abundant element. On Earth, sulfur typically exists as sulfide or sulfate minerals. In living organisms, it is a key component of numerous essential factors, including the proteinogenic amino acids cysteine and methionine, the vitamins biotin and thiamine, and other cofactors such as coenzyme A [1]. In addition, many proteins contain iron-sulfur clusters, which are essential for their function, and metalloproteins in which a transition metal is part of the active site or zinc-containing proteins in which the metal ion serves a structural role often coordinate the metal ion by the sulfur atoms of cysteine [2]. Cysteine is redox active, and formation of disulfide bonds in proteins contributes to structural stability and function [3]. Cysteine is also one of the three amino acids in glutathione, which is an important antioxidant, as the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) in which two glutathione molecules are linked by a disulfide bond determine the redox status of a cell [4].
Bacteria can obtain sulfur from various sources, including inorganic sulfate, organic sulfur compounds, and sulfur-containing amino acids, and specific transporters typically mediate their uptake. The ability to utilize different sulfur sources varies among bacterial species, depending on their metabolic capabilities and environmental conditions. The preferred source is inorganic sulfate, and the reductive sulfur assimilation pathway, which converts inorganic sulfate or thiosulfate to the sulfide that is required for sulfur incorporation into biomolecules, occurs primarily in microorganisms and plants [5]. Assimilation of organosulfur compounds may be required if sulfate is inaccessible, and it is mediated by the sulfate starvation-induced (SSI) system [6]. Cysteine biosynthesis is central to the assimilation of both inorganic and organic sulfur, with cysteine in turn serving as a source of reduced sulfur for incorporation into other biomolecules. Given the key role of cysteine in furnishing sulfur for biosynthetic purposes, targeting cysteine biosynthesis has also been explored as a strategy for the generation of antimicrobial adjuvants; by reducing bacterial fitness, infectivity may also decrease, or the bacteria may become more susceptible to conventional antibiotics [7, 8].
Bacterial sulfur assimilation is carefully controlled. In Gram-negative bacteria, the conserved and ubiquitous LysR-type transcriptional regulator (LTTR) CysB plays a key role in this regulation by coordinately controlling expression of genes encoding both transporters of sulfur-containing compounds as well as enzymes involved in incorporating the sulfur into cysteine. These genes are organized in several operons. One circumstance in which CysB activity becomes apparent is during the transition from exponential growth to stationary phase, which is characterized by sulfur-limitation [9]. As outlined below, CysB responds to intracellular coactivators that signal sulfur limitation, while other factors prevent induction of the CysB regulon when sulfur-containing compounds are abundant to avert excessive sulfur uptake or cysteine biosynthesis.
The role of CysB in controlling cysteine biosynthesis has been described in some detail, particularly in species such as Escherichia coli and Salmonella enterica. Here, we summarize the known functions of CysB in regulation of cysteine biosynthesis, noting distinctions between bacterial species and possible DNA-binding modes consistent with the recently published structure of full-length CysB. More importantly, CysB—and sulfur assimilation in general—has been implicated as a determining factor in other events, including oxidative stress responses, antibiotic resistance, and regulation of the Type III Secretion System (T3SS), which is required for bacterial virulence. Available data suggest that the functions of CysB orthologs go well beyond regulation of sulfur assimilation, and that they have evolved to fit the needs of individual bacterial species.
LTTRs constitute one of the largest families of transcriptional regulators in bacteria [10, 11, 12]. The family is named for LysR, which regulates expression of an enzyme that functions in the biosynthesis of lysine [13, 14]. LTTRs often regulate basic metabolic pathways, but some members are involved in responses to environmental change. Originally denoted as transcriptional activators, they may act either as activators or repressors, and they may be encoded in a divergent orientation to genes under their control. They are frequently expressed at a low level and negatively autoregulated, allowing for a sensitive response to minor changes in the concentration of the effector; transcriptional regulation is typically achieved by binding of a small molecule effector, which may be a coactivator or corepressor, depending on the individual LTTR function. The structural and functional homology between orthologous LTTRs suggest a common evolutionary descent.
While LTTRs are quite diverse, some general features may be discerned. LTTRs
comprise two functional domains, and they most commonly exist as homotetramers.
Each subunit, which is ~300 amino acids in length, comprises an
N-terminal winged helix-turn-helix (wHTH) DNA-binding domain, a helical linker,
and an effector-binding domain. Helix
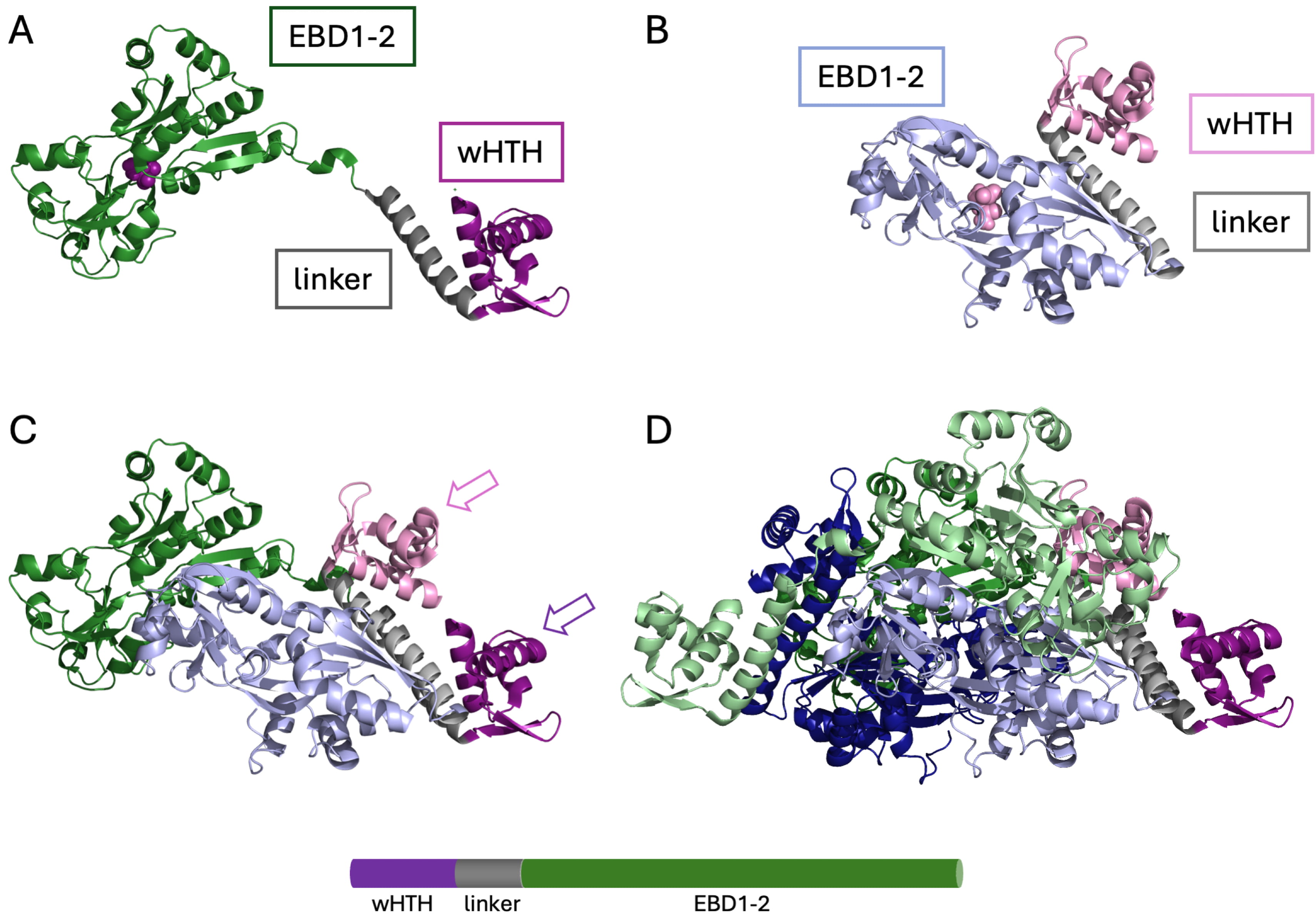
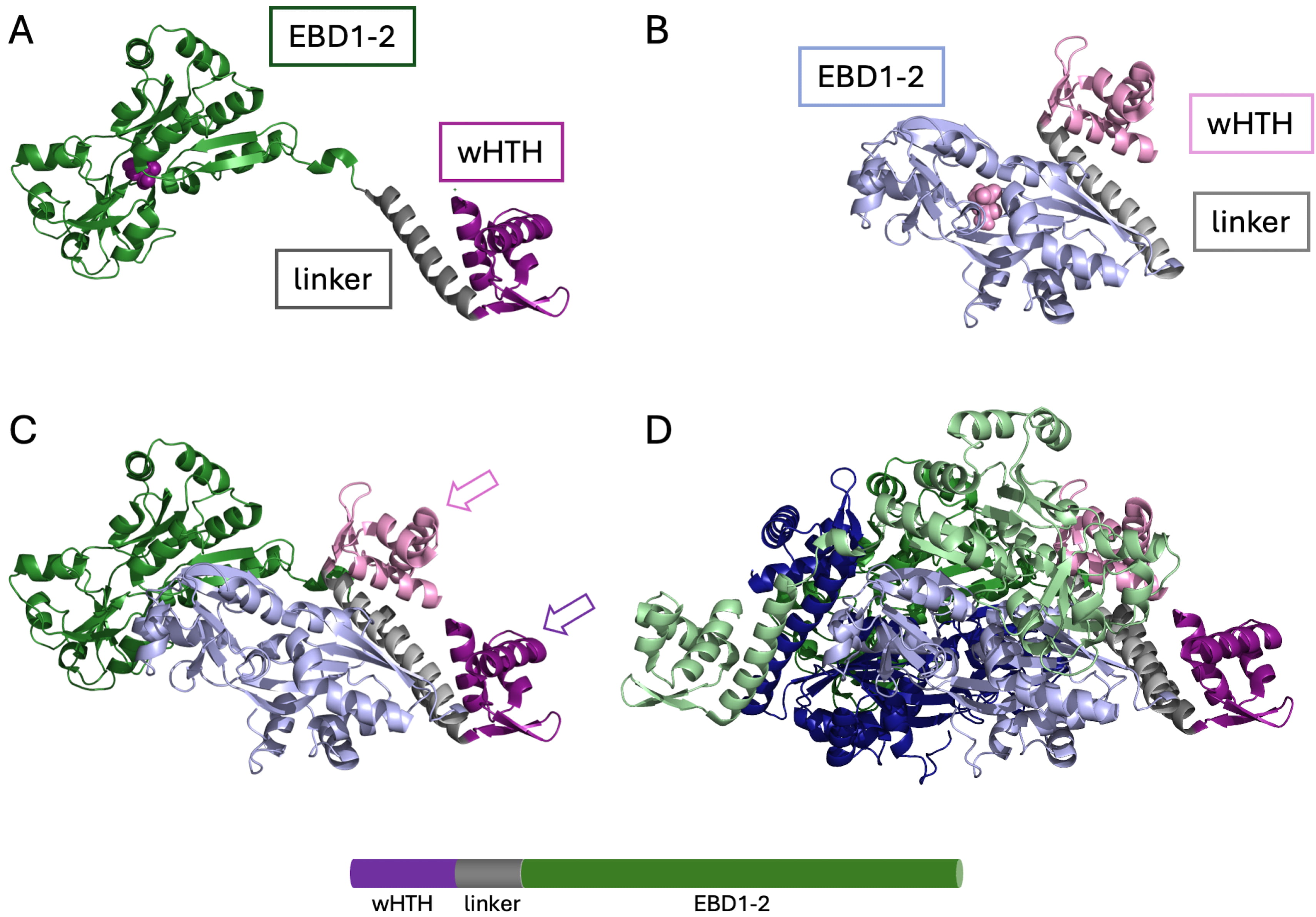 Fig. 1.
Fig. 1.
Structure of an LTTR. The structure of full-length CysB from Klebsiella aerogenes (PDB ID 9FDD). (A) A subunit in the extended conformation. Purple, N-terminal wHTH domain; grey, linker helix; green, effector-binding domains EBD1-2 (sometimes referred to as RD1 and RD2) with N-acetyl serine bound in the cleft between effector-binding domains (purple space-filling representation). (B) A subunit in the compact conformation. Pink, wHTH domain; grey, linker helix; light blue, effector-binding domains with N-acetyl serine bound (pink). (C) Association of an extended and a compact subunit with the two wHTH domains (arrows) in a position to bind consecutive DNA major grooves. Color coding as in (A) and (B). (D) Tetrameric assembly. Color coding for one dimer as in (A) and (B), with additional extended and compact subunits in light green and dark blue, respectively. LTTR, LysR-type transcriptional regulator; wHTH, winged-helix-turn-helix. Image was created with PyMol 3.1.6.1 (Schrödinger, LLC, New York, NY, USA).
The consensus sequence for LTTRs, the so-called LTTR-box, is T-N11-A [10, 11, 19]. This sequence is embedded within a short, usually ~15 bp palindromic region, typically centered at position –66 to –63 relative to the transcriptional start [20]. One pair of wHTH domains interacts with this high affinity Recognition Binding Site (RBS). Additionally, one or two more divergent low-affinity activation binding sites (ABS) may be located between the high affinity site and the transcriptional start site. Association with two sites requires introduction of a significant DNA bend, and binding to the ABS is involved in transcriptional control. Binding of an effector elicits conformational changes that lead to the LTTR binding a different combination of binding sites.
The C-terminal domain binds an effector, and it is joined to the DNA-binding
domain by a helical hinge region. The effector binds in a crevice between two
CysB is an LTTR first discovered, and most extensively studied, in S. enterica serovar Typhimurium (hereafter referred to as S. Typhimurium) and E. coli where it plays a vital role in cysteine biosynthesis [22]. In these species, cysB is not encoded divergently from a gene under its control. The cysteine biosynthesis pathway in E. coli and S. Typhimurium is a complex, multi-step process that converts inorganic sulfur sources into cysteine. The pathway involves three main branches: sulfate reduction, thiosulfate utilization, and serine activation. The serine activation branch focuses on preparing the carbon backbone for cysteine, while the sulfate reduction and thiosulfate utilization branches prepare the thiol group. For an authoritative review of sulfur metabolism and regulation, please see [23].
In the serine activation branch, L-serine is acetylated by serine transacetylase
(CysE), forming O-acetyl-L-serine (Fig. 2; center, orange frames). This step is
tightly regulated, as cysteine acts as a feedback inhibitor of CysE to prevent
overproduction of O-acetyl-L-serine [22, 23]. When sulfate is taken up from the
environment, it is captured by sulfate-binding protein (Sbp) in the periplasmic
space and carried to sulfate permease (encoded by genes cysTWA), for
transport across the cell membrane (Fig. 2; left, green frames). Sulfate is then
activated through two enzymatic reactions: the transfer of the sulfate group to
adenosine triphosphate (ATP) by ATP sulfurylase (encoded by cysDN) to
form adenosine 5′-phosphosulfate (APS) and the phosphorylation of APS by APS
kinase (CysC) to form 3′-phosphoadenosine 5′-phosphate (PAPS). PAPS is
subsequently reduced to sulfite by PAPS reductase (CysH), which is further
reduced to sulfide by NADPH-sulfite reductase (SiR, encoded by cysIJ)
[22, 23, 24]. SiR requires a siroheme cofactor for this step; siroheme is a modified
porphyrin molecule synthesized by siroheme synthase (CysG) [23, 24]. Sulfide
undergoes a
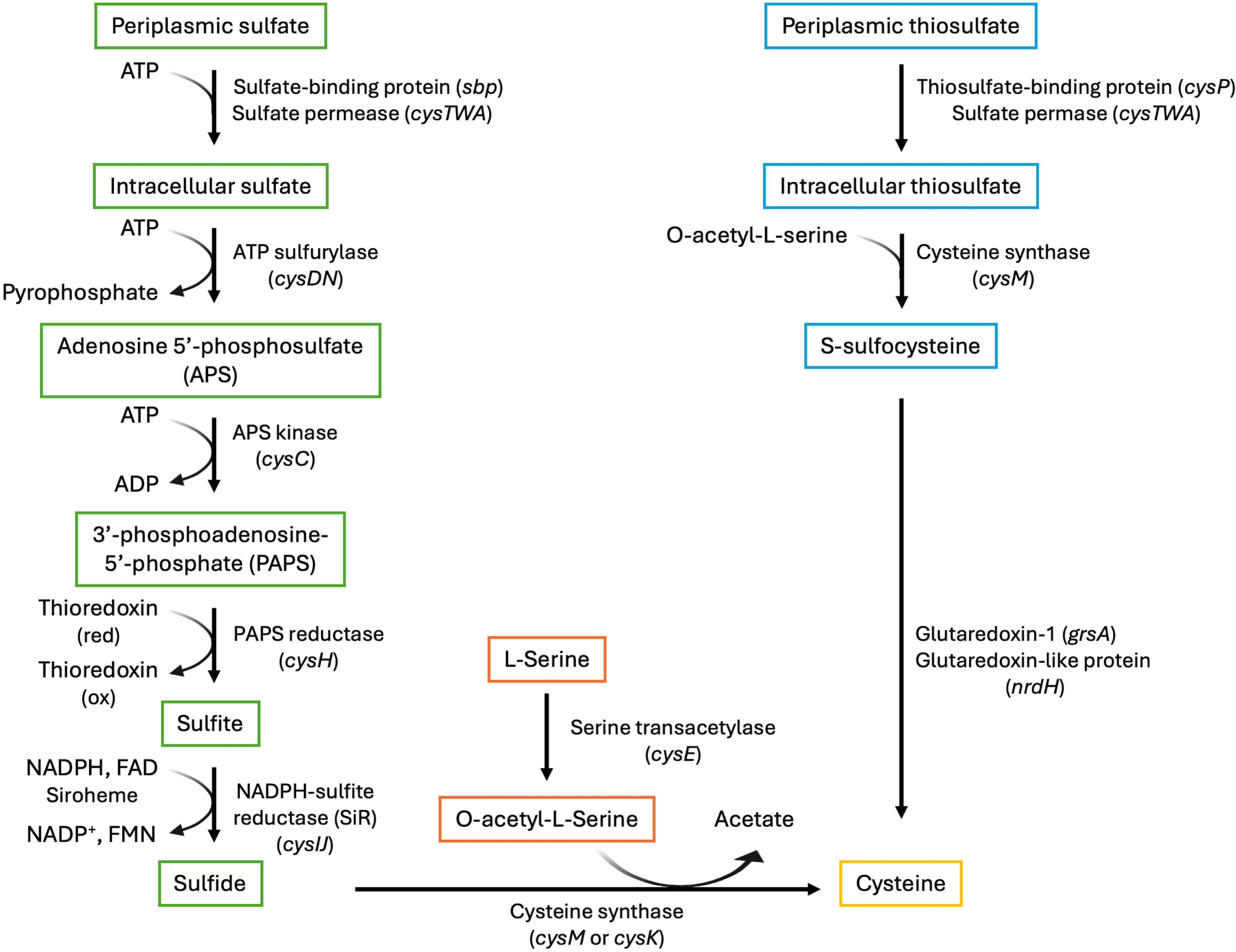
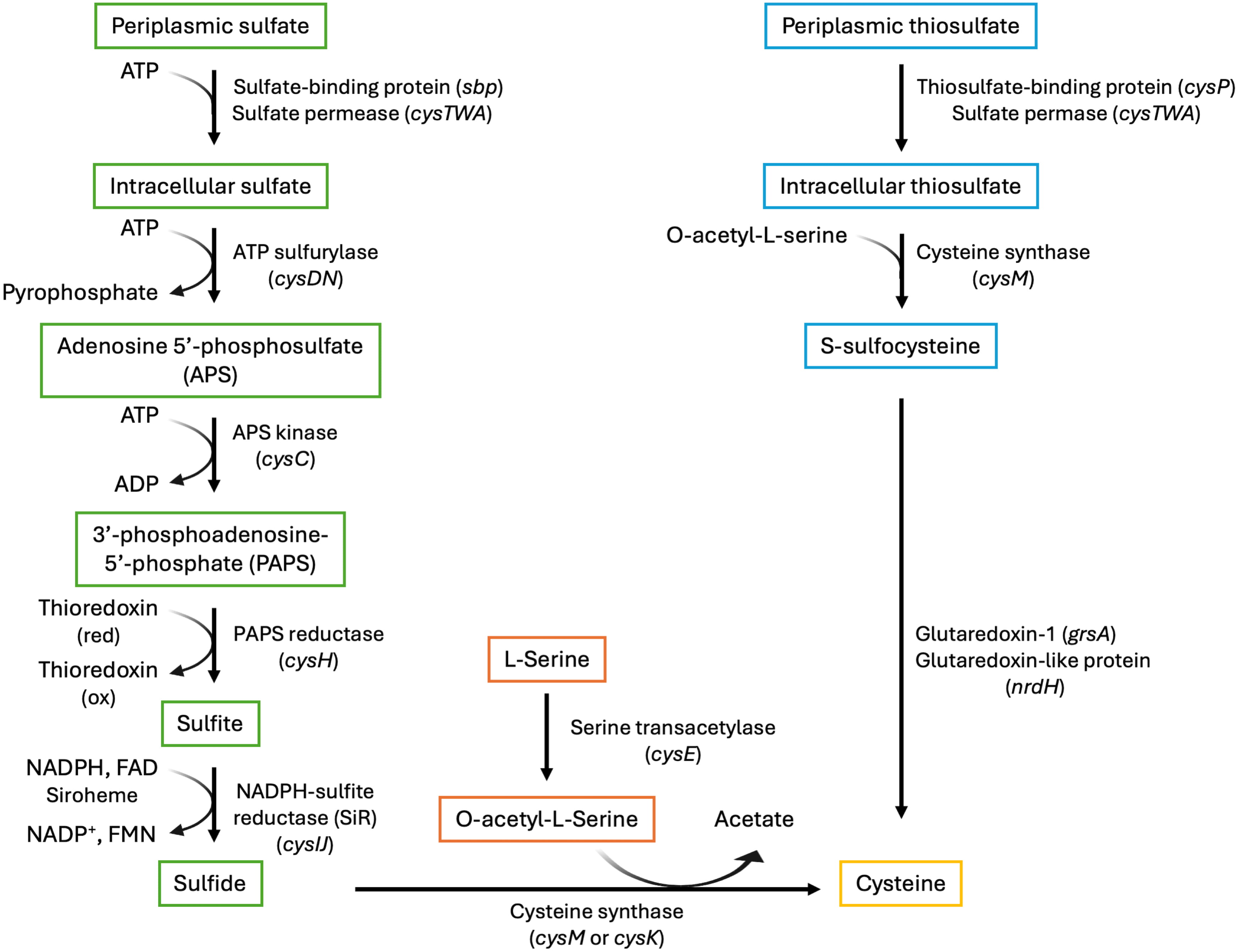 Fig. 2.
Fig. 2.
The cysteine biosynthetic pathway with enzymes, gene identifiers, and cofactors. The serine activation branch (center; orange frames) produces O-acetyl-L-serine. The sulfate reduction branch (left; green frames) reduces sulfate to sulfide, which then reacts with O-acetyl-L-serine to produce cysteine. Thiosulfate is an alternate sulfur source, which is converted to S-sulfocysteine (right; cyan frames) and ultimately to cysteine. Gene names are provided in parentheses. ATP, adenosine triphosphate; ADP, adenosine diphosphate; NADPH, nicotinamide adenine dinucleotide phosphate hydrogen; FAD, flavin adenine dinucleotide; NADP+, nicotinamide adenine dinucleotide phosphate; FMN, flavin mononucleotide.
Cysteine synthase exists as two different isoenzymes, designated as A and B,
which are encoded by cysK and cysM, respectively [23]. While
both can synthesize cysteine from O-acetyl-L-serine and sulfide, CysM has the
additional ability to use thiosulfate as an alternative sulfur source [21]. In
this thiosulfate utilization pathway, thiosulfate is captured in the periplasmic
space by thiosulfate-binding protein (CysP) and shuttled across the cell membrane
by sulfate permease (Fig. 2; right, cyan frames). There, thiosulfate can undergo
the same
E. coli can also utilize alkanesulfonates as a sulfur source due to the function of CysB-like protein (Cbl) [26, 27]. Alkanesulfonates are taken up by E. coli through two distinct ABC-type transport systems: a taurine-specific transporter (encoded by tauABC) and a general alkanesulfonate transporter (encoded by ssuABC) [23]. Once inside the cell, alkanesulfonates undergo desulfonation to release sulfite, which can then be incorporated into the cysteine biosynthetic pathway. Taurine is oxygenolytically cleaved by taurine dioxygenase (encoded by tauD) [23, 26]. Other alkanesulfonates are converted to sulfite by a two-component monooxygenase (encoded by ssuDE) [23].
CysB is widely conserved among Gram negative bacteria, specifically among
Proteobacteria, as evidenced by a BlastP search of the UniProt database using the
E. coli CysB sequence as a query. Orthologs from Proteobacteria account
for 993 of the top 1000 hits, of which 766 represent
CysB is classified as a Class I transcriptional activator, which means that it
binds upstream of the core promoter and interacts directly with the C-terminal
domain of the RNA polymerase (RNAP)
CysB functions as a homotetramer of 36-kDa subunits, each containing an
N-terminal wHTH DNA-binding domain and an effector-binding domain, the general
domain organization of LTTR proteins (Fig. 1) [18, 23, 34]. Within the N-terminal
DNA-binding domain, an activating region is located between the
CysB mediates changes in gene expression when it binds the effector N-acetyl-L-serine, and this binding results in activation of target genes while simultaneously decreasing CysB’s affinity for its own promoter region, thereby relieving autorepression [23, 36]. The interaction between N-acetyl-L-serine and CysB is finely tuned and influenced by environmental factors such as sulfur availability and the presence of the anti-inducers sulfide and thiosulfate, which bind CysB and are thought to prevent or alter the conformational changes normally associated with binding of N-acetyl-L-serine [23, 26]. O-acetyl-L-serine, the precursor to N-acetyl-L-serine, can also induce CysB binding, but N-acetyl-L-serine is believed to be the true inducer in vivo [23, 26]. This is due to the spontaneous conversion of O-acetyl-L-serine to N-acetyl-L-serine through O- to N-acetyl migration that occurs at a rate of 1% per minute at pH 7.6 and 10-fold faster at pH 8.6 [24]. Thus, accumulation of O-acetyl-L-serine would lead to production of N-acetyl-L-serine, triggering the activation of the cysteine biosynthetic pathway by CysB to clear the accumulating intermediate.
Additional evidence in support of N-acetyl-L-serine as the true effector for CysB include tryptophan fluorescence emission spectroscopy of K. aerogenes CysB [37]. In these experiments, the fluorescence emission increased markedly on addition of N-acetyl-L-serine to CysB, indicating a direct interaction with CysB that results in an altered environment of tryptophan residue(s). By contrast, changes in tryptophan fluorescence on addition of O-acetyl-L-serine increased with time, suggesting that the recorded signal depends on the spontaneous conversion of O-acetyl-L-serine to N-acetyl-L-serine. The structure of K. aerogenes CysB in complex with N-acetyl-L-serine reveals close proximity of Trp166 to the effector, explaining the fluorescence emission changes on addition of N-acetyl-L-serine to CysB [18]. This structure also indicated very close packing around the serine hydroxyl group of N-acetyl-L-serine, suggesting that O-acetyl-L-serine would not be readily accommodated in this binding pocket. By comparison, the structure of the S. Typhimurium CysB effector-binding domain in the apo form or in complex with either N-acetyl-L-serine or O-acetyl-L-serine identified a binding pocket capable of accommodating both effectors, with binding of O-acetyl-L-serine to this site allosterically modulating a separate site capable of binding N-acetyl-L-serine, but with an orientation different from that observed in K. aerogenes CysB [38]. It was argued that absence of the linker helix and the DNA-binding domain from the S. Typhimurium CysB structure resulted in the complex remaining in an uninduced state [18]. A structure of full-length S. Typhimurium CysB in complex with effector molecules should prove instructive to clarify the apparent discrepancies.
In E. coli, upregulation of CysB target genes is abolished in absence of a functional CysE, the enzyme that produces O-acetyl-L-serine—an observation that speaks to the critical importance of this intermediate for induction of CysB. E. coli cells in which cysE is disrupted also produce excess biofilm, suggesting that absence of CysE imposes stress [39]. By contrast, a CysB ortholog from Burkholderia cenocepacia was reported to activate target gene promoters in absence of cysE, indicating that B. cenocepacia CysB may not require O-acetyl-L-serine (or N-acetyl-L-serine) as a coinducer. That B. cenocepacia is a true ortholog of E. coli CysB is evidenced by the inability of a B. cenocepacia cysB mutant to utilize sulfate for cysteine biosynthesis [27]. Similarly, binding of CysB from Pseudomonas putida to a target promoter was not affected by addition of O-acetyl-L-serine, and regulation of cysH by P. aeruginosa was not induced by addition of O-acetyl-L-serine [40, 41]. In Vibrio fischeri, a marine bioluminescent bacterium that colonizes the light organ of the Hawaiian bobtail squid, CysB performs its conventional role in sulfur assimilation in the marine environment. However, the expression of CysB target genes varies markedly for V. fischeri populations that colonize different crypt spaces within the light organ, a heterogeneity that may correlate with the availability of host-derived cystine as a sulfur source [42]. These observations suggest that CysB proteins may have diverged to some extent depending on the needs of the individual bacterial species, a common feature of orthologous transcription factors [43, 44]. Such observations also indicate that care should be taken not to assume an equivalent mechanism for CysB orthologs despite their conserved role in regulating sulfate assimilation and cysteine biosynthesis.
CysB regulates a wide array of genes within the cysteine regulon, including those directly involved in cysteine biosynthesis (cysPUWAM, cysDNC, and cysJIH operons, as well as cysK) and those responsible for transport of cysteine and cystine (tcyJ, an L-cystine binding protein formerly known as fliY, ydjN, and genes for the L-cystine transport system CTS-1 in S. Typhimurium) [23, 35, 45]. In addition to these, CysB regulates many genes that are indirectly related to cysteine synthesis and sulfur metabolism, including sbp, ybiK (also known as spt, a protein involved in glutathione uptake), dcyD, cbl, and the tauABCD and ssuEADCB operons [23]. CysB’s regulatory role on these genes is enacted through direct binding to promoter regions or by other, yet uncharacterized mechanisms. The key role of CysB in activating these genes is evidenced by the deficiency of cysB mutants in utilizing sulfate, whereas they grow on sulfide.
In addition to regulating cysteine biosynthesis genes, CysB directly influences the expression of cbl, encoding a protein with high homology to CysB, particularly in its DNA-binding motif [23]. Under sulfur limitation, CysB activates the expression of cbl, enabling E. coli to utilize alkanesulfonates for cysteine biosynthesis. Since cbl expression is repressed by cysteine, it is considered part of the cysteine regulon. The regulatory relationship between CysB and Cbl extends to both the tauABCD and ssuEADCB operons, which require both proteins for activation. Expression studies have demonstrated that Cbl is the primary activator for these operons, while CysB plays a modulatory role [23, 26, 46].
S. Typhimurium CysB has been shown to bind directly to its own promoter as well as the promoters of cysJIH, cysK, and cysPUWAM. For these four promoter regions, distinct CysB binding sites have been identified, most of them generating DNase I footprints at least 40 bp in length, as summarized in Fig. 3A (Ref. [42, 43, 44, 45]) [23]. The cysB promoter features a single repressor site (CBS-B) centered around the transcription start site, with DNase I protection on the transcribed strand seen from position –10 to +36 relative to the transcriptional start [47]. This location would be expected to interfere with RNAP binding. CysB binding to this site is optimal in absence of effector, a common feature of LTTRs that activate transcription when bound to an effector [11]. The DNase I footprints did not show evidence of enhanced cleavage, arguing against CysB imposing marked DNA distortions upon binding to this site.
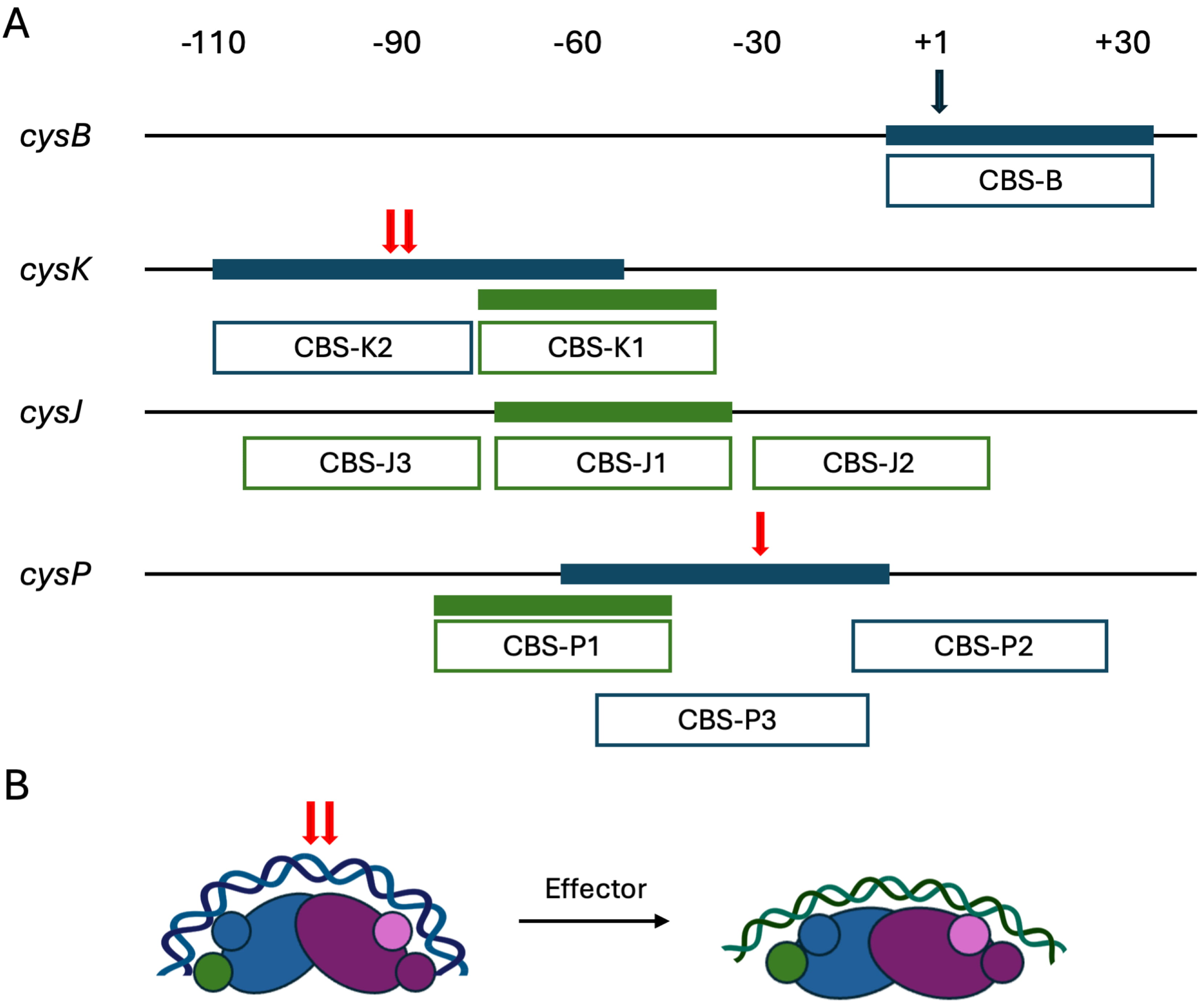
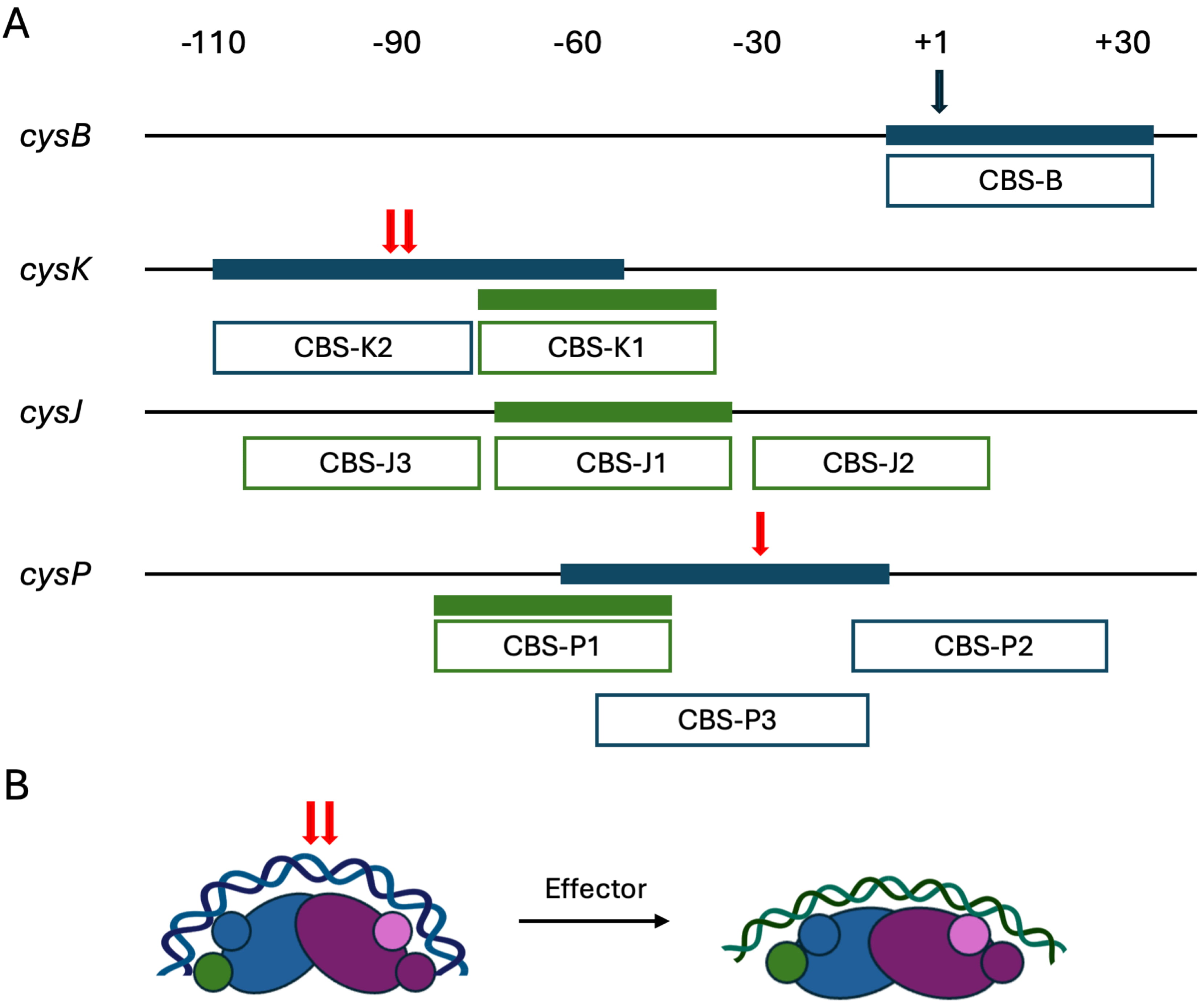 Fig. 3.
Fig. 3.
S. Typhimurium CysB binding patterns. (A) Binding to the cysB, cysK, cysJ, and cysP promoters determined by DNase I footprinting is illustrated, with filled blue bars representing binding by apo-CysB and filled green bars showing binding by CysB in the presence of O-acetyl-L-serine. Enhanced DNase I cleavage is marked with red arrows. Open boxes reflect the previously annotated binding sites, each proposed to contain two 19 bp half sites. On the transcribed strand, apo-CysB protects from –10 to +36 on the cysB promoter, with reduced binding in presence of effector (O-acetyl-L-serine) [42]. On the cysK promoter, apo-CysB protects from –110 to –56, and protection by effector-bound CysB extends from –78 to –44. On cysJ, effector-bound CysB protected from –76 to –35, while no protection was observed for apo-CysB [43]. Designation of sites CBS-J2 and CBS-J3 (for which the upstream edge appears uncertain) was inferred from hydroxyl radical footprinting [44]. Protection by apo-CysB on the cysP promoter extends from –63 to –14, and it spans from –85 to –43 when effector is present. Very weak protection was observed at site CBS-P2 at higher protein concentrations [45]. (B) Possible mode of CysB binding to different target sites, using the cysK promoter as an example. DNA-binding domains are shown as smaller circles. For apo-CysB, blue DNA represents the DNA shown to be protected from DNase I cleavage (panel A). Red arrows indicate sites of hypersensitive DNase I cleavage. Addition of effector is predicted to induce protein conformational changes associated with imposition of a shallower DNA bend and binding to a distinct DNA site (CBS-K1; green). S. Typhimurium, Salmonella enterica serovar Typhimurium.
S. Typhimurium CysB binding to cysJ, cysK, and cysP was mapped by multiple approaches. CysB binds as a tetramer to the cysJ, cysK, and cysP promoters in vitro, although binding of a second tetramer may be seen at higher protein concentration [34]. Binding is stimulated by N-acetyl-L-serine, with a variable increase in affinity for the different promoters, and a combined analysis of CysB:DNA stoichiometry with electrophoretic mobility shift assays suggests that the effector reduces the DNA bend angle at cysK and cysP promoters from ~100° to ~50° [48]. The latter is in accord with the mechanism of other LTTRs for which a lessening of the DNA bend angle on effector binding is associated with transcriptional activation [10, 11].
Based on DNase I footprinting, the promoters of cysJ, cysK, and cysP were described as containing multiple AT-rich binding sites, including activation sites (CBS-J1, CBS-K1, and CBS-P1) located just upstream of –35 (Fig. 3; green). These sites share little similarity with each other, and no obvious consensus binding sequence was identified; binding of CysB to these sites occurs in presence of O-acetyl-L-serine. These three sites were considered as consisting of two convergently oriented 19-bp half-sites separated by one or two base pairs [49]. However, as previously argued based on the structure of K. aerogenes CysB, the reported 19 bp half-sites are much longer than conventional LTTR half-sites, and each would span ~65 Å of B-form DNA [11, 18]. Since this distance is longer than the distance between paired wHTH domains in the tetrameric CysB, it appears more consistent with the CysB structure for the 19 bp sequences to harbor a pseudo-palindromic sequence to which a set of wHTH domains would bind. According to this model, a 60 bp duplex would be sufficient to engage both paired wHTH domains within a CysB tetramer, generating a sharp bend at the center (Fig. 3B).
So-called accessory sites (CBS-J2, CBS-J3, CBS-K2, CBS-P2, and CBS-P3) were identified downstream or upstream of the activation sites [23, 49]. The identification of these sites as ‘accessory’ appears inconsistent with what is now known about transcriptional regulation by most LTTRs. On the cysK and cysP promoters, apo-CysB protects a specific DNA segment from DNase I, and it imposes a DNA distortion in the middle of the protected region (Fig. 3A; blue bars with red arrow) [48, 50]. The reported accessory sites CBS-K2 and CBS-P3 cover part of these regions. While hypersensitive DNA cleavage does not necessarily imply bending, these locations are in very good agreement with measurements of DNA bend angles and positions using circular permutation assays [34]. This DNase I cleavage pattern would be consistent with both paired wHTH domains of the tetrameric CysB associating with these sequences (Fig. 3B; left). That the area of protection shifts on addition of effector is consistent with the ‘sliding dimer’ model in which CysB would occupy distinct binding sites due to effector-mediated conformational changes. In addition, no hypersensitive DNase I cleavage was seen on addition of effector, consistent with a reduced DNA bend angle and a shorter distance between binding sites for paired wHTH domains, a binding mode in which intervening DNA would not become hypersensitive to DNase I cleavage. By comparison, the structure of full length CbnR, which controls chlorobenzoate degradation in Cupriavidus necator NH9, was reported in free and DNA-bound state. While the conformation of individual domains is retained, the relative positions of domains are altered markedly on association with DNA [51]. Similarly, it cannot be ruled out that the CysB conformation changes on association with cognate DNA sites. On the cysJ promoter, no protection from DNase I by apo-CysB was evident, and accessory sites were identified based on hydroxyl radical footprinting at higher protein concentration. Similarly, protection at accessory site CBS-P2 required elevated protein concentrations. The physiological roles of CysB binding to CBS-P2 remain unclear.
Two distinct CysB binding regions have been identified within the E. coli tauABCD promoter, which controls expression of proteins associated with the transport of taurine (2-aminoethanesulfonate) and release of sulfite from this compound. This operon is also positively controlled by the Cbl. On this promoter, CysB binds CysB-BS1, located between positions –221 and –183, independently of N-acetyl-L-serine, though its binding is enhanced in the presence of the inducer [26]. However, removal of CysB-BS1 from the tauABCD promoter has no effect on expression of tauA, suggesting that CysB binding to this site does not directly activate transcription. In contrast, CysB-BS2, located between positions –120 and –11, exhibits weaker binding that is strongly stimulated by N-acetyl-L-serine. This larger site is hypothesized to allow for binding of multiple CysB tetramers. Cbl binds a single site located between positions –112 and –68, which overlaps CysB-BS2, and this binding is not affected by N-acetyl-L-serine. Though N-acetyl-L-serine seems to perform its usual inducer function on CysB at this promoter, sulfate and thiosulfate—typical anti-inducers of CysB—have unusual effects on this promoter, with sulfate having no effect and thiosulfate stimulating CysB binding [26].
CysB’s regulation of the E. coli ssuEADCB operon is more complicated. This operon encodes proteins required for utilization of sulfur from aliphatic sulfonates. Cbl is essential for expression of this operon, and it binds to a site located between positions –75 and –31 [46]. ssuEADCB is subject to negative regulation by APS, the first intermediate in the sulfate assimilation pathway (Fig. 2), which acts as a potent inhibitor of Cbl-dependent transcription initiation but does not interfere with Cbl binding [52]. The mechanism of action of this inhibition is unknown but could be due to APS-mediated conformational changes in Cbl that disfavor recruitment of RNAP. By contrast, CysB binds to the ssuEADCB promoter at a site overlapping the –10 and –35 positions and may serve a role as repressor. Paradoxically, it is also required to activate expression of cbl and therefore indirectly activates the ssu operon [53].
While CysB is best known for its roles in controlling expression of genes
related to sulfate assimilation, there is ample evidence for its participation in
regulating genes outside of this pathway. Disruption of cysB has been
associated with resistance to novobiocin, an inhibitor of DNA gyrase, and
mecillinam, a
In E. coli, CysB also regulates the stress-response gene hslJ,
which encodes a predicted outer membrane protein that is required for novobiocin
resistance, and adi, which encodes an arginine decarboxylase induced
under acidic conditions [33, 36, 59]. CysB acts as a repressor of hslJ
transcription by binding to the hslJ promoter, and this binding is
stimulated by N-acetyl-L-serine, a somewhat surprising observation considering
that CysB binds the hslJ promoter at a repressor site that overlaps –10
and –35 [59]. cysB mutants, and cells overexpressing hslJ,
exhibit increased resistance to novobiocin, underscoring the role of HslJ in
novobiocin resistance, though the exact mechanism remains unclear [36, 59]. The
precise mechanism of CysB’s involvement in adi regulation is not fully
elucidated, but it is proposed that CysB acts as a positive regulator of
adi through its interaction with RNAP or through a downstream gene
product [33]. rpoA341 mutations, which affect the
CysB has predicted binding sites on the promoters of several other E. coli genes with diverse functions, identified through sequence comparisons with promoters that have known CysB binding sites. These include yciW, a putative peroxidase; ybdN, a putative PAPS reductase/DUF3440 domain-containing protein; ariR, a putative two-component system connector protein; dgcZ, a diguanylate cyclase; and yoaC, a DUF1889 domain-containing protein. Overexpression of yciW confers tolerance to high concentrations of cysteine, while disruption of yciW increases cysteine concentration [60]. These findings suggest that YciW limits cysteine accumulation, possibly by converting it into other metabolites. For the other candidate CysB target genes, actual involvement of CysB has not been explored. Given the discovered and proposed involvement of CysB in regulation of multiple stress-response genes, it is clear that this regulator plays a larger role in E. coli beyond regulation of cysteine biosynthesis. It is also evident that the role of CysB in maintaining cysteine homeostasis is critical to maintaining redox balance.
In P. aeruginosa and P. putida, CysB retains its well-characterized function of regulating genes involved in cysteine biosynthesis and the sulfate starvation response [40, 41, 61]. The sulfate starvation response in P. putida involves a second transcription factor, SfnR, which is under regulatory control by CysB and induced by accumulating sulfate [40]. Additionally, CysB regulates the expression of several virulence factors and signaling genes in P. aeruginosa: pqsR, algD, and retS. CysB acts as a transcriptional repressor of pqsR, which encodes the Pseudomonas Quinolone Signal (PQS) receptor. The PQS, or 2-heptyl-3-hydroxy-4-quinolone, is a key player in P. aeruginosa’s quorum sensing network and influences the expression of multiple virulence factors. CysB regulates the expression of pqsR by direct interaction with its promoter and interference with the binding of LasR, an activator of pqsR transcription [41]. Repression of pqsR by CysB leads to reduced PQS production, as the PqsR is required for PQS biosynthesis, and reduced efficiency of quorum sensing [41]. This regulation by CysB is not affected by cysteine availability. Additionally, CysB serves as a transcriptional activator of algD, a gene encoding GDP-mannose dehydrogenase, the key enzyme in the synthesis of alginate. Alginate is a polysaccharide that contributes to the mucoid phenotype and antibiotic tolerance of P. aeruginosa strains often found in patients with cystic fibrosis [62]. The algD gene is regulated by numerous transcription factors with variable importance in controlling expression; through its interaction with the algD promoter, CysB promotes an increased production of alginate, and deletion of cysB not only reduces gene expression but also reduces the response to the messenger cyclic-di-GMP by a mechanism that remains unexplored [62, 63].
CysB regulates virulence genes associated with the T3SS in both P. aeruginosa and Ralstonia solanacearum. In P. aeruginosa, CysB is a transcriptional activator of the retS gene, which encodes a sensor kinase. RetS represses the GacS-GacA system, which activates the transcription of small RNAs that regulate the expression of virulence genes involved in the transition from acute to chronic infections, including those which encode the T3SS. CysB regulates the retS promoter through direct binding, as demonstrated by electrophoretic mobility shift assays [64]. This interaction activates retS, leading to the repression of the T3SS. cysB mutants exhibit reduced expression of retS and reduced virulence in a mouse acute pneumonia model [64]. CysB also positively regulates T3SS gene expression in R. solanacearum through activation of prhG, which encodes a transcriptional regulator that integrates plant-derived signals to the activation of T3SS genes. Other infection behaviors of R. solanacearum are also regulated by CysB, including exopolysaccharide production and swimming motility [65].
CysB regulates the production of several secondary metabolites in B. cenocepacia and Burkholderia pyrrocinia. B. cenocepacia cysW mutants exhibit defective sulfate transport and, as a result, are unable to make pyochelin, a siderophore crucial for iron acquisition. An adequate intracellular cysteine pool is required for the production of this secondary metabolite, as evidenced by the restoration of pyochelin production by supplementation with cysteine. Wild-type B. cenocepacia grown in sulfate-limiting conditions also exhibits reduced pyochelin production, although growth is not affected, indicating that CysB-mediated regulation ensures the prioritization of essential metabolites over secondary metabolites [66]. Siderophore production is also under CysB control in P. aeruginosa, where it directly activates expression of pvdS, which encodes an extracytoplasmic function (ECF) sigma factor important for the response to iron starvation [61]. PvdS in turn is required for expression of genes associated with production of the siderophore pyoverdine, and disruption of cysB results in attenuated expression of PvdS-dependent virulence phenotypes.
An adequate intracellular cysteine pool is also required for the antifungal activity of B. pyrrocinia JK-SH007, a strain known for its ability to control poplar canker [67]. Deletion of cysB in this strain leads to reduced antifungal activity and production of ornibactin, another siderophore in Burkholderia species, and cysteine supplementation restores both abilities [67, 68]. Transcriptome analysis of the cysB mutant of B. pyrrocinia JK-SH007 revealed significant changes in amino acid metabolic pathways and upregulation of small regulatory RNAs (sRNAs) involved in the iron-sulfur metabolic pathway [67]. Hfq is a conserved RNA-binding protein that, among other functions, facilitates the ability of such sRNAs to modulate mRNA translation [69]. In Pseudomonas syringae, hfq is directly activated by CysB under nutrient-limiting conditions [70].
Finally, the obligate human pathogen Neisseria gonorrhoeae presents an atypical case where cysB expression is downregulated during infection [71, 72]. CysB is one of the few transcriptional regulators in N. gonorrhoeae, and cysB is an essential gene in this bacterium [71, 73]. N. gonorrhoeae possesses a non-functional sulfate reduction pathway due to a 3500 base pair deletion in its genome, which results in the complete loss of cysH and cysD, truncated cysG and cysN, and premature stop codons in cysJ and cysI, and, as such, relies on thiosulfate for synthesis of cysteine [72]. CysB binds to the promoter regions of genes involved in this modified cysteine regulon and appears to still regulate them, however, this regulation is independent of the effector N-acetyl-L-serine, as noted for other species discussed above [72]. Since N. gonorrhoeae relies on glutathione (and therefore cysteine) to combat oxidative stress in its environment, and since cysB is essential, disruption of CysB function presents as a particularly promising avenue for antibacterial intervention in this species.
CysB orthologs are found in Gram-negative bacteria, and their roles in
regulating genes associated with sulfate assimilation have been examined
exceedingly in E. coli and S. Typhimurium [23]. In these
species, CysB binds the cysB promoter to repress transcription, a
repression that is relieved in presence of the effector N-acetyl-L-serine. On
activated genes, CysB in complex with effector binds a site just upstream of
–35, where it interacts directly with the RNAP
For both E. coli and S. Typhimurium CysB, N-acetyl-L-serine is the effector, which is required for transcriptional regulation. However, for other orthologs, transcriptional regulation appears to be independent of this effector. These data indicate that CysB orthologs may have evolved to optimize their regulatory function in sulfate assimilation in accord with the needs of the individual bacterial species. As further evidenced by the incorporation of target genes that are not directly related to sulfate assimilation into their regulons, these observations indicate that CysB function must be appreciated in the context of the individual bacterial species. Given that CysB regulates stress response- and virulence-associated genes in pathogens such as P. aeruginosa and B. cenocepacia, and that this transcriptional regulator is absent in humans and plants, CysB presents as a promising target for antibiotic development to combat infections affecting both human health and food crops. While potent inhibitors of CysE and CysK/CysM have been identified, their impact on pathogen growth has been minimal [7, 74]. As discussed in this review, cysteine biosynthesis operates through a complex network of pathways, and thus, the inhibition of a single enzyme is unlikely to disrupt cysteine biosynthesis in a biologically meaningful way. Targeting CysB, however, may offer a more effective approach by striking at the heart of this network. Since cysB is typically not an essential gene, such efforts may center on the generation of adjuvants that render the cells more susceptible to conventional antibiotics by reducing overall fitness [8].
ABS, activation binding site; APS, adenosine 5′-phosphosulfate; PAPS, 3′-phosphoadenosine 5′-phosphate; Cbl, CysB-like protein;
ENL and AG contributed to the acquisition of literature and creation of figures for the work. ENL and AG wrote the paper and approved the final manuscript. Both authors have participated extensively in the work and have agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
Research in the authors’ laboratory was supported by the National Science Foundation (MCB-2153410 to AG).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.