- Academic Editor
Ageing is a progressive functional decline in health conditions and a risk factor for many chronic diseases. To address the elevated burden of age-related pathologies, the ageing process has been extensively studied over the past decades, and yet the underlying mechanisms remain to be fully understood. One of the prominent features of ageing is cellular senescence, a special form of durable cell-cycle arrest. While senescent cells release the senescence-associated secretory phenotype (SASP) molecules that recruit immune cells to facilitate the clearance of senescent cells, senescence is also indispensable for many essential physiological functions. However, a ‘chronic’ nature of senescence arises due to immune deficiencies and persists during ageing. Immunosenescence, the ageing of immune cells, is the underlying key driving the pathological burdens of senescence, leading to systemic ageing as demonstrated by animal studies. Thymic regeneration has been shown by several studies to be a potential anti-ageing intervention, restoring immunity as well as reversing immunosenescence and ageing. The specific targeting of senescent cells by senolytic and/or senomorphic drugs is also promising but needs to be dealt with caution to protect the essential physiological roles of senescence. A deeper understanding of the biological origins of immunosenescence is crucial for unveiling the potential root cause of ageing.
The ageing of the world’s population is becoming an enormous undertaking spanning across medical, social, economic, political, and psychological chores. With an unmet need to prolong a healthy life span, population ageing has intensified interest in understanding the ageing process over the decades [1]. Ageing is a gradual functional decline across multiple organ systems, causing progressive deterioration in health and well-being. Consequently, age is a risk factor for numerous chronic pathologies such as neurodegenerative diseases, cardiovascular disorders, and various types of malignancies [2]. Despite these pathological burdens, the underlying biological inception of the ageing process remains to be fully unveiled. Studies in the past few decades have identified common cellular and molecular traits called ‘hallmarks of ageing’, including several abnormalities such as cellular senescence, genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, stem cell exhaustion, and so on [3]. These hallmarks helped conceptualise much ageing research dedicated to biomedical approaches to ameliorating aging in animal models, and some of these led to enthusing prospects of delaying or reversing multiple aspects of ageing.
Among the hallmarks of ageing, cellular senescence, a special form of durable cell-cycle arrest, has been directly implicated as a key driver of ageing and age-related diseases [4]. In fact, diverse forms of age-associated traits, including genomic instability, telomere attrition, and mitochondrial dysfunction, converge on a common cellular fate of stable growth arrest or senescence. This led to the hypothesis that tissue ageing is caused by cells progressively losing their ability to proliferate, which is supported by studies showing cumulative enrichment of senescent cells in many tissues, including skeletal muscle, lung, brain, adipose tissue, bone, and kidney with advancing age [5].
Deciphering the embedded role of senescence in ageing warrants a comprehensive perception of the biological origin and relevance of senescence. Cellular senescence was formally described in 1961 when Leonard Hayflick and Paul Moorhead showed that diploid fibroblast cell lines stop dividing after a limited number of passages, known as replicative senescence [6]. In addition to replicative senescence caused by telomeric erosion and induction of a DNA damage response, the induction of cellular senescence by other stressors, including but not limited to the hallmarks of ageing as well as oncogenic- and therapy-induced stress, and viral infections, has established this phenomenon as a stress-induced, durable cell-cycle arrest [7].
Senescent cells are unique in that while their growth is arrested, they remain metabolically active and continue to release chemicals that can trigger inflammation, which facilitates the removal of senescent cells by recruiting immune cells (Fig. 1). Hence, senescent cells play essential physiological roles including embryonic development, childbirth, wound healing, and tumour suppression [8, 9, 10]. Compared to the ‘acute’ nature of senescence devoted to the coordination of biological processes as well as to the clearance of senescent cells as a protective mechanism, ‘chronic’ senescence takes place due to the failure of immune cells to clear senescent cells and thereby leads to a persistent form of senescence-associated secretory phenotype (SASP), a distinguishing features of senescent cells that drive age-associated pathologies across multiple organs [11]. The SASP is remarkably heterogeneous and differs by cell type and various stimuli. Components of the SASP include a wide spectrum of bioactive factors, including cytokines, chemokines, growth factors, proteases, oxylipins, and other signalling molecules. Thus, SASP has important effects on the surrounding cells and can induce secondary senescence via both proximal and distal effects, thereby propagating and enhancing the overall senescence burden [12, 13]. This magnitude of SASP explains its impact on multiple pathophysiological conditions associated with chronic senescence. Much of the SASP is proinflammatory, and thus senescent cells are the major driver of age-associated inflammation, called “inflammaging” [14]. The persistent SASP along with an unresolved and continued encounter with immune cells, including natural killer (NK) cells, macrophages, and T cells, plays a critical role in exacerbating both local and systemic inflammation [15, 16]. Immune dysregulation, therefore, seems to be the underlying factor turning senescence from helpful to harmful (Fig. 1).
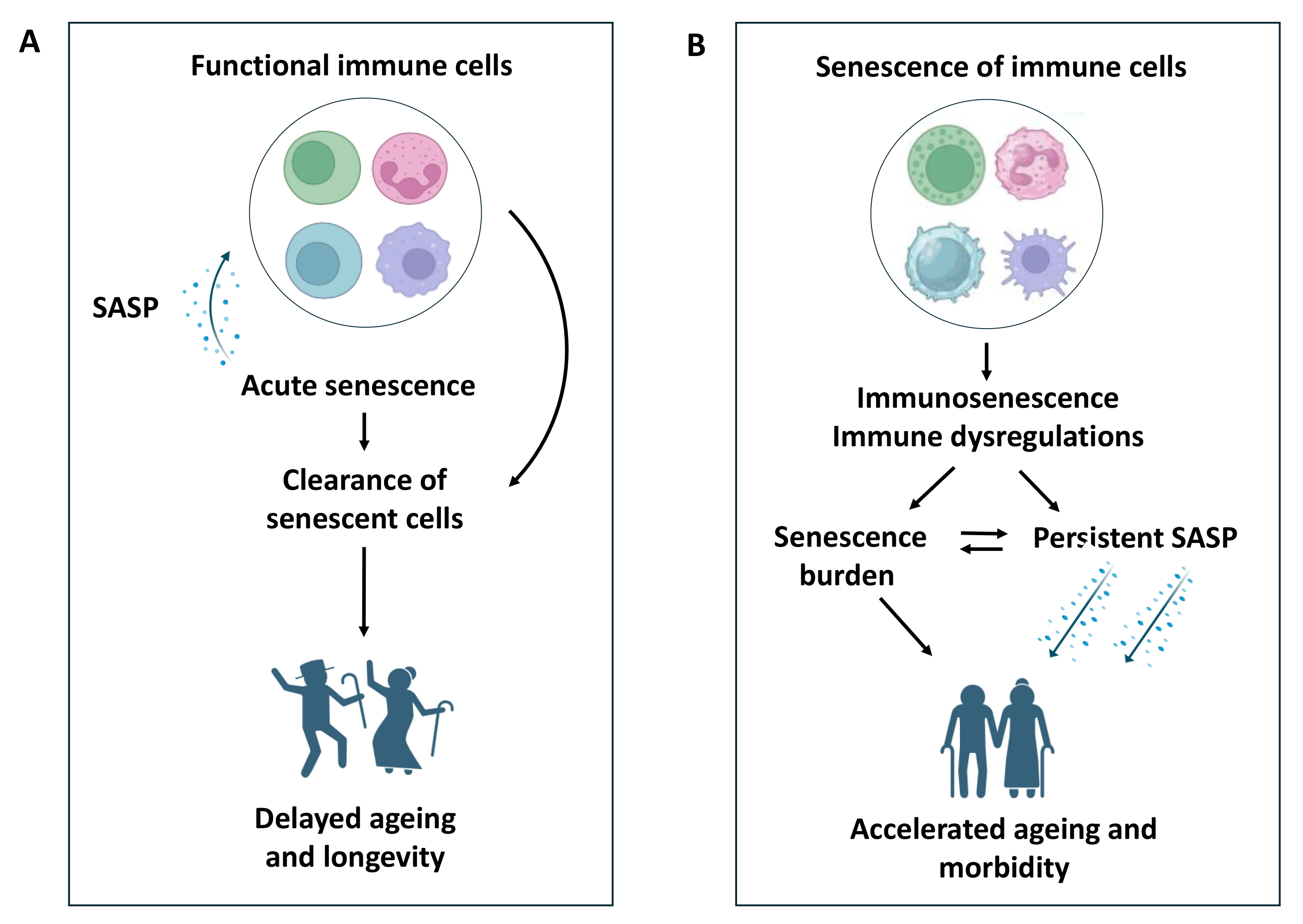 Fig. 1.
Fig. 1.
The impact of immunity on senescence and ageing. (A) Functional immune cells drive the clearance of senescent cells via senescence-associated secretory phenotype (SASP)-mediated recruitment and thereby prevent both senescence burden and persistent SASP, leading to acute senescence. Thus, functional immune cells favour delayed ageing and longevity by minimising any detrimental impact of sustained senescence on ageing. (B) Senescence of immune cells that leads to immunosenescence and immune dysregulations fails to clear senescent cells, and thereby leads to both senescence burden and persistent SASP. Hence, immunosenescence accelerates the ageing process and associated morbidity.
During ageing, the immune system exhibits a declining ability to constitute effective defences against pathogens and cancer cells. This age-related decline in immune function, termed immunosenescence, is a state of dysregulated immune cell function characterized by not only senescence-associated phenotypes (such as cell cycle arrest, telomere shortening, markers of cellular stress, epigenetic reprogramming, and secretion of proinflammatory mediators) but also by other senescence-independent changes in the naive:memory T cell ratio, CD4:CD8 ratio, lymphocyte-to-monocyte ratio (LMR), and thymic atrophy [15]. Activated by the intracellular metabolic sensor adenosine monophosphate (AMP)-activated protein kinase (AMPK), glucose deprivation, or genotoxic stress, senescent T lymphocytes exhibit elevated endogenous p38 phosphorylation and a reduced T-cell receptor (TCR) diversity, limiting their ability to recognise foreign antigens [16]. While AMPK is a well-known regulator of cellular metabolism and energy balance, it plays a key role in the ageing process [17] and appears to have both positive and negative effects on T cell functions [18, 19]. Moreover, the immune function is intricately linked to metabolic regulation [20, 21], which is further emphasised by the coexistence of weakened immunity and an imminent risk of metabolic diseases during the ageing process. In fact, the state of T cell senescence primarily manifests not only during the aging process but also in the context of metabolic disorders such as obesity, diabetes, and cardiovascular diseases. Interestingly, metformin, a well-known diabetes medication, was also shown to have an anti-aging role by reducing the number of senescent T-cells [22]. Consequently, senescent T-cells are emerging as a noteworthy therapeutic target. Remarkably, a critical role of immunosenescence in driving systemic ageing has been established by an elegant study in a mouse model [23]. Using a genetic deletion of a crucial DNA repair protein in mouse haematopoietic cells, this study has shown not only a premature onset of immunosenescence with impaired immune function but also increased senescence and damage in non-lymphoid organs, leading to systemic ageing phenotypes. Consequently, immunosenescence has been attributed as one of the key drivers of the ageing process [24], associated with human morbidity and mortality (Fig. 1).
As a potential anti-ageing therapeutic intervention to restore immune function, thymic regeneration has been attempted by several studies. Thymic function and the supply of T cell progenitors from the bone marrow are interconnected and interdependent. While thymic function depends on the supply of T cell progenitors, the migration of T cell precursors from bone marrow also appears to depend on thymic function [25, 26]. The growing output of myeloid haematopoietic stem cells in comparison with the T cell progenitors during ageing contributes to an age-related decline in thymic function [27]. Several earlier studies showed thymus regeneration and restoration along with the reversal of age-related immune deficits in animal models via the administration of growth hormone [28, 29]. Using a recombinant human growth hormone, thymic regeneration was also significantly reinstated in ageing men, accompanied by changes in age-related immunological parameters, including increased LMR and decreased exhausted programmed cell death protein 1 (PD-1+) CD8 T cells, leading to improvements in a variety of disease risk factors [30].
While the emergence of pharmacological interventions targeting senescent cells, called senotherapeutics, has been shown to be promising in delaying the development of many age-related disorders by ameliorating senescence-associated symptoms [31, 32], challenges remain in assessing the clinical efficacies of these drugs in terms of any associated toxicities [33]. At the same time, preserving the essential ‘acute’ senescence would be of paramount importance. Senotherapeutics can be broadly categorised into ‘senolytics’ which selectively eliminate senescent cells, and ‘senomorphics’ which transform the characteristics of senescent cells by intervening with senescence-associated signalling pathways. Examples of senolytic drugs include dasatinib and quercetin, targeting the apoptotic resistance of senescent cells. In contrast, senomorphic drugs were initially discovered by serendipity, including rapamycin, metformin, and aspirin. The implications of these drugs on the tissue structural integrity and blood-tissue barrier in the elderly, due to a high percentage of senescent cells in some tissues, need to be dealt with caution [34]. For the elderly with a prevalence of senescent cells, senomorphics would therefore seem to be a more effective and safer alternative to senolytics. However, the mechanistic action of these drugs remains to be fully identified, especially in vivo, and their relevance to senescent cell types has further cast some doubts over the long-term use of these drugs [32]. Moreover, both short-term and long-term side effects of these drugs are largely unknown due to their associated off-target effects [33]. Compared to drugs, the specific targeting of senescence and age-related diseases using monoclonal antibodies could be an attractive and reliable alternative, even though the immune response-associated side effects are common [35]. Another physiologically safe and effective strategy for targeting senescent cells would be to enhance the immune function since immune cells such as NK cells, macrophages, and CD8+ T cells are inherently capable of recognising and eliminating senescent cells. Interestingly, a number of peripheral immune markers associated with the oldest centenarians have indicated such therapeutic targets. For example, the number of cytotoxic CD4+ T cells is elevated in the peripheral blood of the oldest centenarians, which correlates positively with the number of senescent fibroblasts in the skin and is attracted by C-X-C motif chemokine ligand 9 (CXCL9) produced by senescent fibroblasts [36]. The effector memory subset of CD8+ T cells that clears senescent cells is significantly increased in the oldest centenarians [37, 38]. An age-related increase in CD56+ CD16+ NK cells that play a crucial role in the immune surveillance of senescent cells is also predominantly associated with the oldest centenarians [39]. Novel therapeutic strategies aimed at enhancing such immune subsets could lay the foundation for safer and effective anti-ageing interventions.
Considering the restoration of immunity as a therapeutic strategy to treat age-related senescence burden and diseases, precise targeting of immunosenescence, facilitating both systemic and chronic senescence, would be vital to overcome the existing clinical needs. In the context of T cell dysfunction, a careful distinction between exhausted and senescent T cells would be critical not only to identify the underlying mechanism but also to define prognostic information and therapeutic strategies [16]. The main differences between T cell senescence and exhaustion lie in their underlying causal mechanisms as well as the potential for functional recovery. T cell exhaustion usually arises from persistent exposure to antigens and is characterized by the up-regulation of immune checkpoints such as PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), and such exhaustion could be reversed by checkpoint inhibitors. T cell exhaustion is also characterised by impaired metabolic activities, including reduced glycolysis and oxidative phosphorylation (OXPHOS) along with a decline in the number of mitochondria [40, 41]. On the other hand, senescent T cells are characterised by AMPK activation and p38 phosphorylation, leading to the inhibition of T cell proliferation and telomerase activity [42]. In addition, senescent T-cells tend to adopt aerobic glycolysis to produce energy instead of using OXPHOS, leading to the accumulation of reactive oxygen species (ROS) and mitochondrial dysfunction [43]. Despite the extensive research dedicated to the role of immunosenescence in ageing over the decades, a complete picture of its distinctive traits needs to be revealed for the future development of efficacious therapeutic interventions. Toward that goal, deciphering a full comprehension of the molecular cues that render functional immune cells into senescent cells holds the key.
AUA single-handedly conducted all literature searches, conceived the idea of this manuscript, drafted the manuscript, edited the manuscript, and prepared Fig. 1. AUA also contributed significantly to all editorial changes as a part of the revision process. AUA read and approved the final manuscript. AUA participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The author is an employee of Aeterna Health Services Pty Ltd. The judgments in data interpretation and writing were not influenced by this relationship.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
