- Academic Editor
-
-
-
Type 2 cardiorenal syndrome (CRS) is a complex disease characterized by the interplay between the heart and kidneys. The pathophysiology of type 2 CRS involves multiple molecular signaling pathways. Transient receptor potential melastatin 2 (TRPM2) is a reactive oxygen species (ROS)-sensitive and non-selective calcium-permeable cation channel, which plays a regulatory role in intracellular Ca2+ homeostasis. Thus, this study aimed to explore the biological functions and mechanisms of the ROS–TRPM2 signaling axis in type 2 CRS.
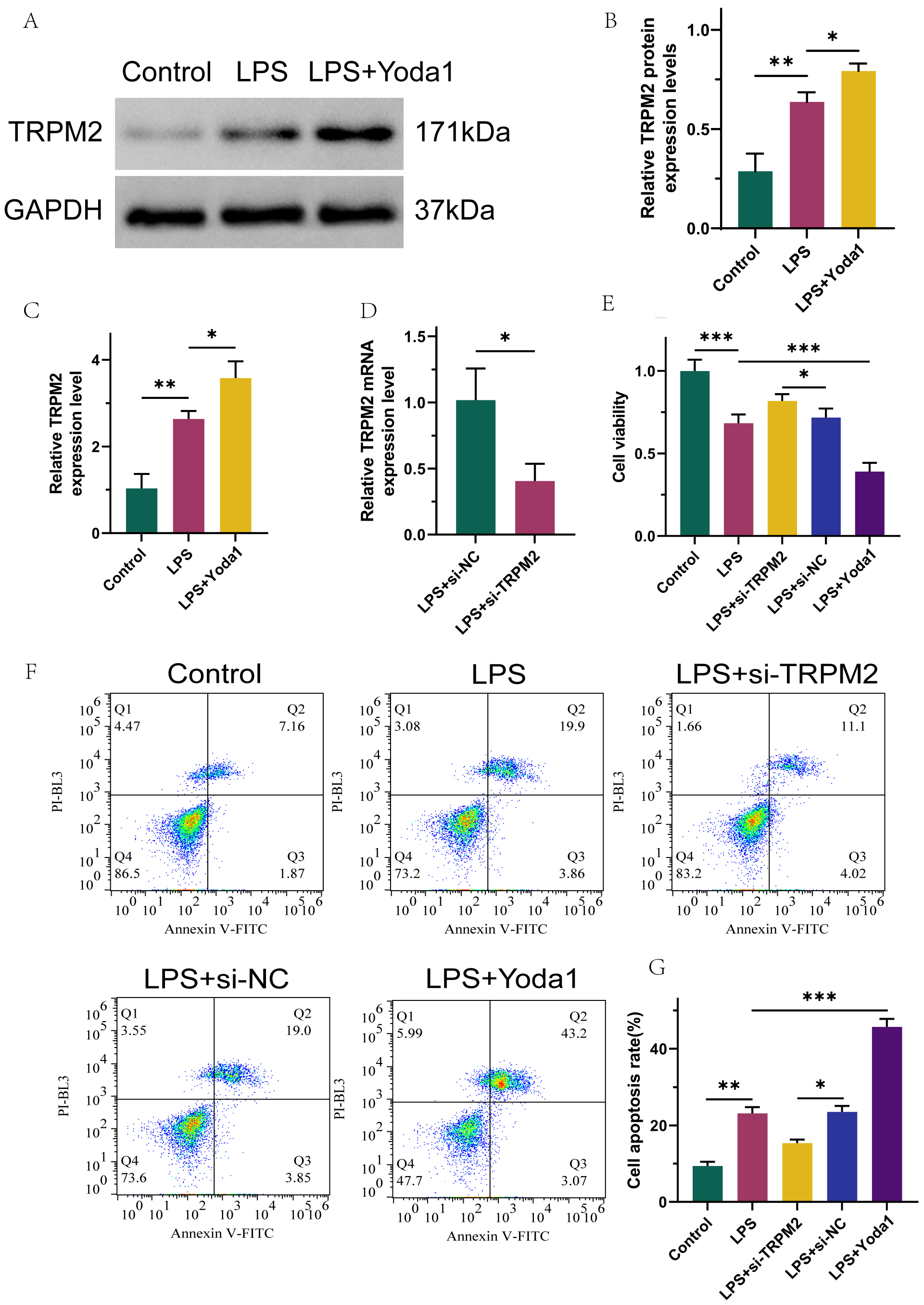
Type 2 CRS model rats (a rat model of type 2 CRS induced through left anterior descending coronary artery ligation combined with 5/6 total nephrectomy) and lipopolysaccharide (LPS)-induced CRS cell lines, human kidney-2 (HK-2), were transfected with small interfering RNA (siRNA) to knock down TRPM2 or a calcium ion channel activator Yoda1 to evaluate the involvement of the ROS–TRPM2 signaling axis on type 2 CRS. Changes in kidney tissue morphology were observed using H&E staining; cell viability and apoptosis were monitored using CCK-8, Annexin V-FITC/PI, and TUNEL kits, alongside quantitative real-time polymerase chain reaction (qRT-PCR), Western blot, ELISA, and immunofluorescence assays to confirm the interaction between ROS, TRPM2, and Ca2+.
TRPM2 is highly expressed in HK-2 cells after LPS stimulation and renal tissues of type 2 CRS rats. Intervention via TRPM2 improves injured cell viability, mitigates apoptosis, inhibits the inflammatory cytokines interleukin 10 (IL-10) and tumor necrosis factor-α (TNF-α), as well as indices of oxidative stress—malondialdehyde (MDA) and ROS—promotes total antioxidant capacity (T-AOC) expression, and alleviates pathological changes in CRS; Yoda1 promoted a contrasting effect to the biological effect induced by TRPM2 deletion.
TRPM2 is abnormally highly expressed in damaged kidneys during the pathogenesis of type 2 CRS. Silencing TRPM2 can inhibit inflammatory and oxidative stress responses, reduce cell apoptosis, promote survival, and alleviate pathological loss; this may be related to the inhibition of Ca2+ influx. This suggests that the ROS–TRPM2 signaling pathway is significant for CRS development, and TRPM2 may be an effective therapeutic target for type 2 CRS.