- Academic Editor
Acute macular neuroretinopathy (AMN) is a rare retinal condition that predominantly affects young females. The incidence of AMN increased significantly during the COVID-19 pandemic, thereby providing a unique opportunity to elucidate the etiology of this disease. In the present study, 24 articles reporting 59 patients were reviewed. The average age of the patients was 33.51 ± 14.02 years, ranging from 16 to 75 years, with females comprising 71.19% of the cases. The average duration of ocular symptoms post-infection was 8.22 ± 10.69 days, ranging from 4 to 150 days. This study investigated the potential pathogenesis of AMN, including the impact of COVID-19 on retinal neurovascular structure and function, immune-mediated inflammatory factor production, blood-retinal barrier disruption, and retinal microvascular damage, as well as potential clinical therapeutic interventions. This research provides a theoretical framework that can inform further investigations of AMN.
Acute macular neuroretinopathy (AMN) is a rare retinal disease first described by Bos and Deurman [1]. It is most commonly observed in young and healthy females, and is characterized by the sudden onset of monocular or binocular symptoms that can progressively worsen over days or weeks. AMN lesions often exhibit varying shades of reddish-brown, brown, or purple. Imaging with frequency-domain optical coherence tomography (OCT) reveals various abnormal findings, including rupture of the ellipsoid zone, increased reflectivity of the outer nuclear layer, thinning of the outer nuclear layer, decreased reflectivity of the photoreceptor/retinal pigment epithelium (RPE) complex, and extensive loss of the outer retina [2, 3, 4]. Clinical associations with AMN include the use of oral contraceptives, and exposure to vasoconstrictive substances such as epinephrine or ephedrine, trauma, shock, intravenous contrast, preeclampsia, postpartum hypotension, and caffeine intake [3, 5, 6, 7, 8, 9].
An association between the development of AMN and respiratory or influenza-like illnesses was reported previously [10]. COVID-19 has been the most prevalent influenza-like respiratory disease in recent years, and an increased incidence of AMN has been observed in COVID-19 patients and in individuals who received COVID-19 vaccinations [4]. Although this suggests that COVID-19 infection contributes to an increased prevalence of AMN, the potential mechanisms linking these two conditions remain unclear, thus presenting a challenge for the clinical prevention and treatment of AMN. Investigation and elucidation of the underlying mechanism for COVID-19-induced AMN is therefore of considerable importance for the prevention and treatment of AMN, as well as other ocular complications caused by the COVID-19 pandemic.
Currently, most research suggests the primary mechanism of AMN involves vascular damage to the deep retinal capillary plexus, leading to occlusion of the ocular microvessels. This results in ischemic retinopathy, which triggers retinal hemorrhage, damage to photoreceptors and macula, and ultimately induces AMN [11, 12]. However, some studies have reported that patients with AMN often suffer decreased visual acuity, possibly indicating damage to the retinal ganglia and disruption of the blood-brain barrier, although the precise mechanism of AMN is still unclear [7, 8, 10]. A study has also shown that COVID-19 induces the development of microvascular thrombosis and endothelial cell dysfunction, suggesting a potential link between COVID-19 and AMN [13]. Indeed, there is increasing evidence of an association between COVID-19 and ocular diseases [14]. Transmission of COVID-19 via the ocular surface was postulated during the early stages of the pandemic, with recent findings supporting this hypothesis [15]. Nevertheless, the clinical features, pathogenesis, and treatment of COVID-19-related AMN have yet to be fully characterized.
The aim of this review was therefore to compile and analyze the clinical manifestations and diagnostic features of AMN associated with COVID-19, and to compare our findings with those of previous reports. Based on existing studies, we investigated the effects of COVID-19 on the structure and function of the retinal neurovascular unit. These effects may underline potential pathogenic mechanisms involving the secretion of immune storm factors, disruption of the blood-retinal barrier (BRB), and damage to the retinal microvasculature. Our objectives were to provide a theoretical framework and foundation for further investigation of AMN, and to identify areas for possible therapeutic intervention.
The inclusion criteria for this literature search were: (1) studies on AMN caused by COVID-19 or SARS-CoV-2 infection; (2) publication between April 2021 and April 2024; (3) original research or case reports. The exclusion criteria were: (1) studies on AMN that were not associated with COVID-19 or SARS-CoV-2 infection; (2) studies related to AMN following COVID-19 vaccination.
The pathogenesis of a disease is closely associated with its clinical manifestations. To elucidate the molecular mechanisms of AMN, it is essential to investigate and identify the epidemic-like characteristics of AMN within the context of the COVID-19 pandemic. The clinical features of AMN induced by COVID-19 were studied by conducting a comprehensive review of relevant articles published in the PubMed database between April 2021 and April 2024. The keywords used in the search were “SARS-CoV-2”, “COVID-19”, and “acute macular neuroretinopathy”. All articles containing these keywords were systematically screened and evaluated, together with their references (Fig. 1). In all, 24 articles [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39] containing 49 AMN patients were identified. The data collected included the number of cases, patient age and gender, clinical characteristics, time of disease onset after COVID-19 diagnosis, and disease duration (Table 1, Ref. [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]). This data was subsequently analyzed using IBM SPSS Statistics software V27.2.1 (IBM Corp., Armonk, NY, USA).
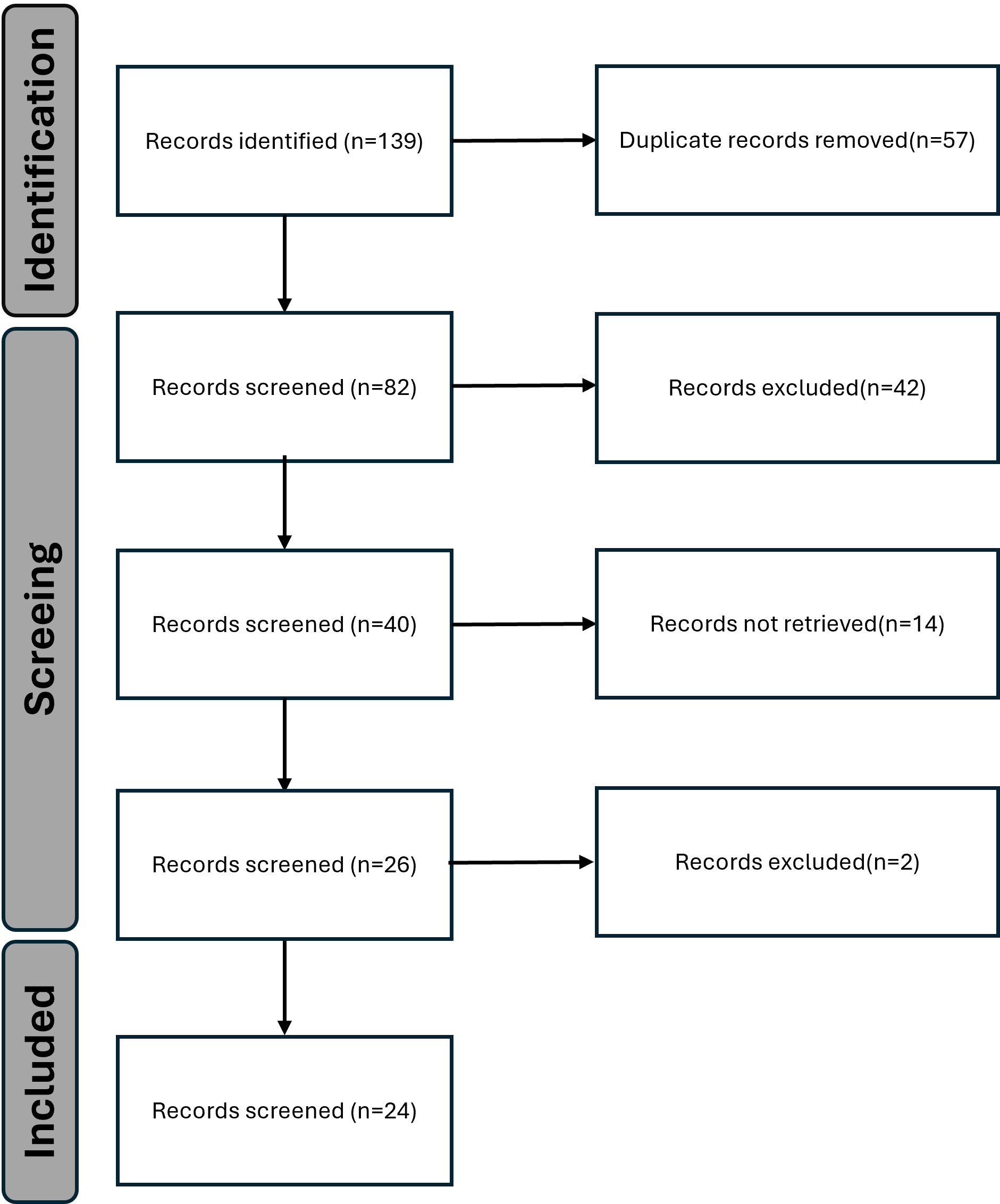 Fig. 1.
Fig. 1.
PRISMA flow diagram showing the process of data collection and selection.
| Article | Sex | Age (years) | Symptoms | 1 Time of onset (days) | Severity of COVID-19 | Unilateral or bilateral | Imaging | Follow-up (days) |
| Ajaz S et al. [16] | Female | 28 | Paracentral scotoma | NR | NR | OU | OCT, OCTA | 35 |
| Female | 22 | Paracentral scotoma | NR | NR | OD | OCT, OCTA | 60 | |
| Female | 27 | Paracentral scotoma | NR | NR | OU | OCT, OCTA | NR | |
| Jalink and Bronkhorst [17] | Female | 29 | Scotomas | 14 | Moderate | OS | OCT, VF | NR |
| Female | 40 | Scotomas | 150 | Mild | OD | OCT, VF | NR | |
| Female | 21 | Scotomas, photopsias | 42 | Mild | OD | OCT, VF | 90 | |
| Female | 42 | Scotomas | 45 | Mild | OD | OCT, VF | NR | |
| Preti et al. [18] | Male | 70 | Scotomas, diaphoresis | 4 | Severe | OS | OCT | 30 |
| Aidar et al. [19] | Female | 71 | Blurry vision | 14 | Moderate | OS | OCT | 60 |
| David and Fivga [20] | Female | 22 | Scotomas | 14 | Moderate | OU | OCT, OCTA, ICGA | 280 |
| Zamani et al. [21] | Female | 20 | Scotomas | 9 | Mild | OD | OCT | 6 |
| Gascon et al. [22] | Male | 53 | Scotoma | NR | NR | OS | OCT, ICGA | 11 |
| Virgo and Mohamed [23] | Male | 32 | Paracentral scotoma | 16 | Moderate | OD | OCT, VF | NR |
| Female | 37 | Paracentral scotoma | 35 | Mild | OD | OCT, VF | NR | |
| Giacuzzo et al. [24] | Female | 23 | Paracentral scotoma | 14 | Moderate | OU | OCT, VF | 30 |
| Masjedi et al. [25] | Female | 29 | Paracentral scotoma | 14 | Moderate | OS | OCT | 90 |
| Bellur S et al. [26] | Female | 64 | Persistent central vision loss | 3 | Severe | OU | OCT, OCTA | 14 |
| Capuano V et al. [27] | Female | 37 | Paracentral scotoma | NR | NR | OU | OCT | 30 |
| Male | 27 | NR | NR | NR | OU | OCT | 30 | |
| Goyal M et al. [28] | Male | 32 | Paracentral scotoma | 120 | Mild | OU | OCT | NR |
| El Matri et al. [29] | Female | 75 | Paracentral scotoma | 30 | Moderate | OD | OCTA | NR |
| Macé and Pipelart [30] | Female | 39 | Paracentral scotoma, photopsia | 2 | Mild | OU | OCTA | 30 |
| Diafas A et al. [31] | Male | 59 | Paracentral scotoma | NR | NR | OU | OCTA | 42 |
| Strzalkowski et al. [32] | Female | 18 | Central scotomas | NR | NR | OU | OCTA | 30 |
| Liu YC et al. [33] | Male | 19 | NR | 5 | Moderate | OU | OCTA | NR |
| Male | 35 | Paracentral scotoma | 5 | Moderate | OU | OCTA, OCT | NR | |
| Female | 17 | NR | 4 | Moderate | OU | OCT | NR | |
| Male | 28 | Scotomas | 4 | Moderate | OU | OCTA, OCT | NR | |
| Male | 37 | Decreased vision | 2 | Moderate | OU | OCT | NR | |
| Female | 49 | Blurred vision | 5 | Moderate | OU | OCTA, FFA | NR | |
| Female | 23 | Scotoma, decreased vision | 1 | Moderate | OU | OCTA, FFA | 20 | |
| Female | 32 | Decreased vision | 4 | Moderate | OU | OCTA | NR | |
| Male | 16 | NR | 2 | Moderate | OU | OCTA | NR | |
| Kovalchuk et al. [34] | Female | 16 | Paracentral scotomas | 1 | Severe | OU | OCTA, OCT | 30 |
| Hawley and Han [35] | Female | 21 | bilateral Paracentral scotomas | 2 | Severe | OU | OCT | NR |
| Reddy S et al. [36] | Female | 24 | scotomas | 5 | Moderate | OU | OCT, OCTA | NR |
| Sonmez et al. [37] | Female | 41 | Paracentral scotoma | 30 | Moderate | OD | OCTA | 30 |
| Feng et al. [38] | Male | 34 | Paracentral scotoma | 2 | NR | NR | OCT, OCTA | 56 |
| Male | 42 | Blurred vision | 3 | NR | OU | OCT, OCTA | 84 | |
| Male | 37 | Central Scotomas | 10 | NR | NR | OCT, ICGA | 84 | |
| Female | 39 | Blurred vision, Paracentral scotoma | 1 | NR | OU | OCT, OCTA | 112 | |
| Female | 36 | Central scotoma | 2 | NR | OU | OCT, OCTA | 105 | |
| Female | 41 | Decreased vision | 3 | NR | OU | OCT, ICGA | 56 | |
| Tang J et al. [39] | Male | 27 | Scotoma, photopsia | 2 | Mild | OU | OCT | NR |
| Female | 25 | Scotoma, photopsia | 2 | Mild | OS | OCT | NR | |
| Female | 27 | Scotoma, Decreased vision | 2 | Severe | OU | OCT | NR | |
| Female | 29 | Scotoma, Decreased vision | 10 | Mild | OU | OCT | NR | |
| Female | 16 | Scotoma | 2 | Severe | OS | OCT | NR | |
| Female | 54 | Scotoma | NR | NR | OS | OCT | NR | |
| Average | 33.5 | 8.22 | Mild: 28.57% | OU 67.79% | 59.51 | |||
| (SD 14.02) | (SD 10.69) | Moderate: 54.28% | OS 32.21% | (SD 40.71) | ||||
| Severe: 17.14% |
1 Time of onset: number of days after COVID-19 diagnosis until AMN was identified.
Abbreviations: OCT, Optical Coherence Tomography; OCTA, Optical Coherence Tomography Angiography; FFA, Fluorescein Fundus Angiography; VF, Ventricular Fibrillation; ICGA, Indocyanine Green Angiography; NR, Not Reported; AMN, Acute macular neuroretinopathy; OU, oculus unati; OS, oculus sinister; OD, oculus dextrus.
The demographic and clinical characteristics of the patients are shown in Table 1. Among the 49 participants, females predominated and comprised 71.19% of the study cohort. The mean age of patients was 33.51 years, with a range of 16 to 75 years. Data on the time of onset of ocular symptoms post-infection was available for 42 patients. Of these, 39 developed ocular symptoms after a mean of 8.22 days, with a range of 4 to 150 days. In the 3 remaining patients, ocular symptoms manifested concurrently with COVID-19. All patients underwent OCT or OCTA imaging, which revealed abnormalities in all cases. Moreover, we have observed that the severity of COVID-19 is related to the incidence of AMN. Among the 49 patients, 35 patients had reported COVID-19 severity, including 10 mild cases (10/35, 28.57%), 19 moderate cases (19/35, 54.28%), and 6 severe cases (6/35, 17.14%), further indicating the correlation between the incidence of AMN and COVID-19.
The most prevalent ocular manifestation was acute onset of a scotoma (32/49,
65.30%), occasionally associated with blurred vision (6/49, 12.24%), decreased
vision (6/49, 12.24%), and photopsia (5/49, 10.20%). Additional reported
symptoms included persistent and central vision loss [26]. Among the patients
with follow-up data (average follow-up period: 59.51
 Fig. 2.
Fig. 2.
Infrared and optical coherence tomography images of patient. The infrared image (A) of the eye showed the macular hypo-reflectance focus (arrow), and the corresponding right macular optical coherence tomography (B) showed the high reflection of the outer plexiform layer, thinning of the outer nuclear layer (arrow) and interruption of the continuity of the ellipsoid zone (arrowhead). The scale bar is 200 μm.
The incidence of AMN increased substantially during the COVID-19 pandemic, accompanied by a concomitant rise in related conditions. Because the neurological and vascular clinical manifestations of AMN are more obvious in patients with COVID-19, we hypothesized these may be related to alterations in the retinal neurovascular unit (RNVU) and in the BRB. The specific pathogenesis of AMN is explored in detail in subsequent sections.
The neurovascular unit (NUV) concept was formulated during the initial Stroke Progress Review Group meeting organized by the National Institute of Neurological Disorders and Stroke, a division of the National Institutes of Health (https://www.ninds.nih.gov).This concept was developed to highlight the intricate interplay between cerebral cellular components and the brain’s vascular network, whose structural and functional impairments underlie many neurodegenerative and neuroinflammatory disorders [40, 41]. The NVU denotes the functional coupling and interdependence among neurons, glial cells, and the vascular system [42]. As an extension of the brain, the eye has a similar structure to the NVU, referred to as the RNVU. This consists mainly of retinal neurons, neuroglia and the retinal microvascular system distributed within the corresponding retinal structures [43, 44].
COVID-19 enters host cells mainly via the spike (S) protein, which exists as a
homotrimer on the viral surface [45, 46]. The S protein interacts with
angiotensin-converting enzyme 2 (ACE2), which serves as the host receptor for
COVID-19. After this interaction, the virus enters the cell through a mechanism
facilitated by transmembrane serine protease 2 (TMPRSS2) [47]. Expression of ACE2
has been detected on the surface (cornea and conjunctiva) and on the inside of
the eye (iris, trabecular meshwork, and retina) [48, 49, 50, 51]. The virus can invade all
structures in the retinal tissue, and COVID-19 virus particles have been found in
autopsy samples of retinopathy [52]. The S protein interacts with ACE2 and is
detected within the deeper ocular structures, specifically in the retinal
ganglion cell layer, inner plexiform layer, inner nuclear layer, and outer
segment of the photoreceptor [53]. The ACE2 receptor has also been detected
at the mRNA and protein levels in rat and porcine retinas [54, 55], and its
expression in the retina hasbeen demonstrated by in situ hybridization.
These observations suggest that COVID-19 is located in the RNUV following
infection, and that the interaction between COVID-19 and ACE2 may result in
retinal inflammation and significant upregulation of inflammatory cytokines,
including TNF-
Five types of neurons exist in the RNVU: photoreceptors, horizontal cells,
bipolar cells, amacrine cells, and ganglion cells. Photoreceptor cells detect
light stimuli and transmit signals to bipolar and ganglion cells, which
subsequently convey these signals to the central nervous system (CNS) via the
optic nerve [58]. Retinal ganglion cells (RGCs) are neural retinal elements that
connect visual receptors to the brain to form the neurovisual system. The
functional and morphological involvement of RGCs is essential for retinal
function, making them critical targets for protective strategies in retinal
diseases [59]. The ACE2 receptor is readily detected in eye and brain tissues,
and is expressed ubiquitously throughout the human visual system and CNS [60, 61, 62, 63].
COVID-19 exhibits a preferential infection and replication pattern in the RGCs of
human retina-like organs. A comparative analysis of differentially expressed
genes in retinal organoids revealed the enrichment of TGF-
The optic nerve and the visual circuit of the brain contain significant amounts of ACE2 receptor mRNA and protein [66]. The abundance of ACE2 receptors correlates with the propensity to attract and bind COVID-19, thereby facilitating entry of the virus into human host cells. Elevated expression of ACE2 receptors on the outermost surface of the eye and extending through the RGCs into the brain could potentially impact vision and the CNS, thus contributing to the development of AMN.
In retinopathy, the vascular system is considered to be the primary site of pathology and is therefore the principal focus of research for elucidating visual dysfunction. However, the potential contribution of photoreceptor cells, which comprise the majority of retinal structures and drive metabolic activity, remains largely overlooked in the context of vascular disease. Even under conditions that induce the degeneration of photoreceptor cells, little attention has been directed towards damage to retinal capillaries resulting from the decline of photoreceptor cells [48, 49, 50, 51, 67, 68, 69].
The photoreceptor is the predominant cell type in the retina and the most metabolically active neuron in the RNUV, containing at least 75% of the retinal mitochondria. Photoreceptors serve a unique function in the body by absorbing light and transducing it into electrical energy, thereby facilitating vision. Research has found that alterations or impairment of photoreceptors in the retina are related to the pathogenesis of AMN [70, 71, 72].
Studies have revealed the presence of defects in the junction between the inner and outer segments of the photoreceptor, attenuation of the outer nuclear layer (ONL) [73, 74, 75, 76], thinning of the photoreceptor outer segments, and localized hyper-reflectivity in the outer retina, which subsequently progress to degenerative changes [76, 77, 78]. Hansen et al. [79] employed adaptive optical scanning ophthalmoscopy (AOSLO) and SD-OCT to examine photoreceptor chimeras in patients with AMN and to evaluate the structures of rods and cones. SD-OCT images showed that the two bands in the outer retina, which are usually associated with the photoreceptor structure, exhibited diminished intensity. Moreover, AOSLO imaging revealed the cone cells exhibited significant structural deterioration in regions with diminished retinal sensitivity, as determined by microperimetry. In retinal organoids infected with COVID-19, the S protein of the virus was predominantly localized in photoreceptors, leading to their structural and functional impairment and consequent retinal dysfunction [63].
The COVID-19 targeting and degeneration of retinal photoreceptors subsequently damages the retinal capillaries, thus potentially affecting the normal functioning of the retina and initiating AMN.
The glial component of the RNVU is comprised of macroglia (astrocytes and Müller cells), microglia, and oligodendrocytes. These encompass neurons, establish retinal defense structures, and contribute to the maintenance of retinal homeostasis. Glial cells serve as the interface between neurons and the vascular system, and are critical regulators of functional communication [80]. COVID-19 affects not only cells within the retina, but also other retinal cells including Müller cells, microglia, and astrocytes. In patients with COVID-19, binding of ACE2 protein to COVID-19 S protein results in the activation of Müller cells [81, 82, 83, 84, 85]. A significant correlation has been observed between Müller cell activation and cone cell alterations [86, 87].
Microglial activation is also prominent in the retina following COVID-19 infection. Albertos et al. [88] reported the presence of microglial nodules surrounding retinal blood vessels in the superficial vascular plexus, analogous to those observed in the brain tissue of COVID-19 patients. They also noted an increase in the perivascular periphery of all vascular plexuses, and a higher proportion of microglia. Increased levels of pro-inflammatory factors in the blood of COVID-19 patients may facilitate the activation and migration of microglia, potentially affecting the development and function of retinal photoreceptors, RPE, and ganglion cells. This may lead to impaired retinal outcomes and function, thereby inducing AMN [89, 90].
The potential mechanism for COVID-19-induced AMN may therefore involve neuronal and glial damage in the RNUV that alters its structure and function, which in turn triggers AMN.
Changes to the retinal microvasculature and disruption of the BRB have been implicated in the pathogenesis of AMN. Inflammatory responses following COVID-19 infection may trigger vascular or embolic events that lead to ischemia in the deep retinal capillary plexus, thereby disrupting the BRB and manifesting as relatively hypodense regions in the retina [91, 92, 93]. OCT and OCTA have revealed the presence of superficial or deep capillary plexus (DCP) and choroidal capillary ischemia in AMN [94, 95, 96, 97].
Approximately 30% of COVID-19 patients experience complications caused by thrombosis. Inactivation of ACE2 can disrupt the equilibrium of the renin-angiotensin system, resulting in vasoconstriction and inadequate retinal vascular perfusion [98]. This makes patients vulnerable to retinal ischemia, which can potentially lead to AMN. Azar et al. [99] proposed that virus-induced endothelial injury may be the primary mechanism underlying the pathogenesis of AMN caused by COVID-19, which could correspond to microvascular damage in the DCP. Using OCTA, Liu et al. [100] observed decreased vascular density in the superficial capillary plexus (SCP), DCP, choriocapillaris, and large vessel layer in all AMN cases, with DCP exhibiting the most decrease. This suggests that pathogenesis of the acute phase of AMN may be associated with decreased vascular density resulting from microvascular contractions in the deep retina. A prospective study utilizing OCT to analyze the retinal microcirculation found that changes in macular microvessel density in the plexiform layer served as a clinical marker for the severity of COVID-19, and also indicated possible immune thrombosis [101]. Based on these findings, it was hypothesized that AMN is associated with decreased microvascular circulation.
Damage and destruction of vascular endothelial cells (ECs) plays a crucial role
in the impairment of microvascular function. A recent study found that markers
associated with endothelial inflammation and injury pathways, including IL-6,
TNF-
Microvascular dysfunction is significantly affected by the senescence of
vascular ECs. COVID-19 infection specifically elicits this process, which
functions as a critical stress response. The aggregate effects of
COVID-19-induced senescence and angiogenesis may vary depending on the disease
phase. Treatment of human ECs with both TNF-
Consequently, one of the potential mechanisms for AMN is that COVID-19 infection induces the senescence or secondary senescence of ECs. This leads to vascular endothelial inflammation and the expression of associated factors, as well as disruption of the BRB, resulting in dysfunction of microvascular circulation and alterations in the structure and function of the retina.
RPE cells are essential for various aspects of eye function, including nutrient transport, light absorption, and the production of cytokines and chemokines. Impairment of RPE function can lead to visual disturbances and potentially trigger the onset of AMN. A recent study of COVID-19-associated AMN found distinctive OCT features, including increased reflectivity in the macular outer plexiform and outer nuclear layers, as well as disruptions in the ellipsoid and interphalangeal regions, as well as in the RPE layers. Of note, the enhanced reflectivity observed by OCT in the outer retinal layers may be an indirect indicator of damage to nearby photoreceptors and the RPE [112]. These clinical observations suggest that COVID-19 may have a detrimental effect on the RPE, potentially contributing to the development of AMN.
COVID-19 and its spike proteins can induce cellular senescence. Zhang et
al. [113] demonstrated the S protein of COVID-19 could induce senescence of
ARPE-19 cells in vitro by increasing the level of cytosolic reactive
oxygen species (ROS), endoplasmic reticulum (ER) stress, and activating the
NF-
When the immune system fails to recognize specific body components, it can produce autoantibodies that target cells, tissues, or organs. This phenomenon results in inflammatory reactions, and ultimately leads to the development of autoimmune diseases [119]. The specific pathogenic mechanism associated with COVID-19 infection has yet to be fully elucidated. However, a study has shown that COVID-19 can induce excessive production of inflammatory cytokines [120]. This immune response can facilitate the fight against the virus, or contribute to excessive production of inflammatory chemokines, potentially leading to a “cytokine storm” that exacerbates the patient’s severe condition [121].
COVID-19 infection also damages mitochondrial structure and function, thereby promoting the production of inflammatory cytokines and triggering AMN. Ajaz et al. [16] found that COVID-19 appropriates the host mitochondria, which is an essential organelle in innate immune signaling. COVID-19 then induces mitochondrial dysfunction, resulting in oxidative stress and the production of pro-inflammatory cytokines, and subsequently causing damage that affects retinal function.
Finally, the mechanism of COVID-19-induced AMN may not be limited to single cell damage. Rather, COVID-19 could potentially inflict varying degrees of harm on multiple retinal cell types, thus affecting the structure and function of the retina and consequently leading to disease (Fig. 3).
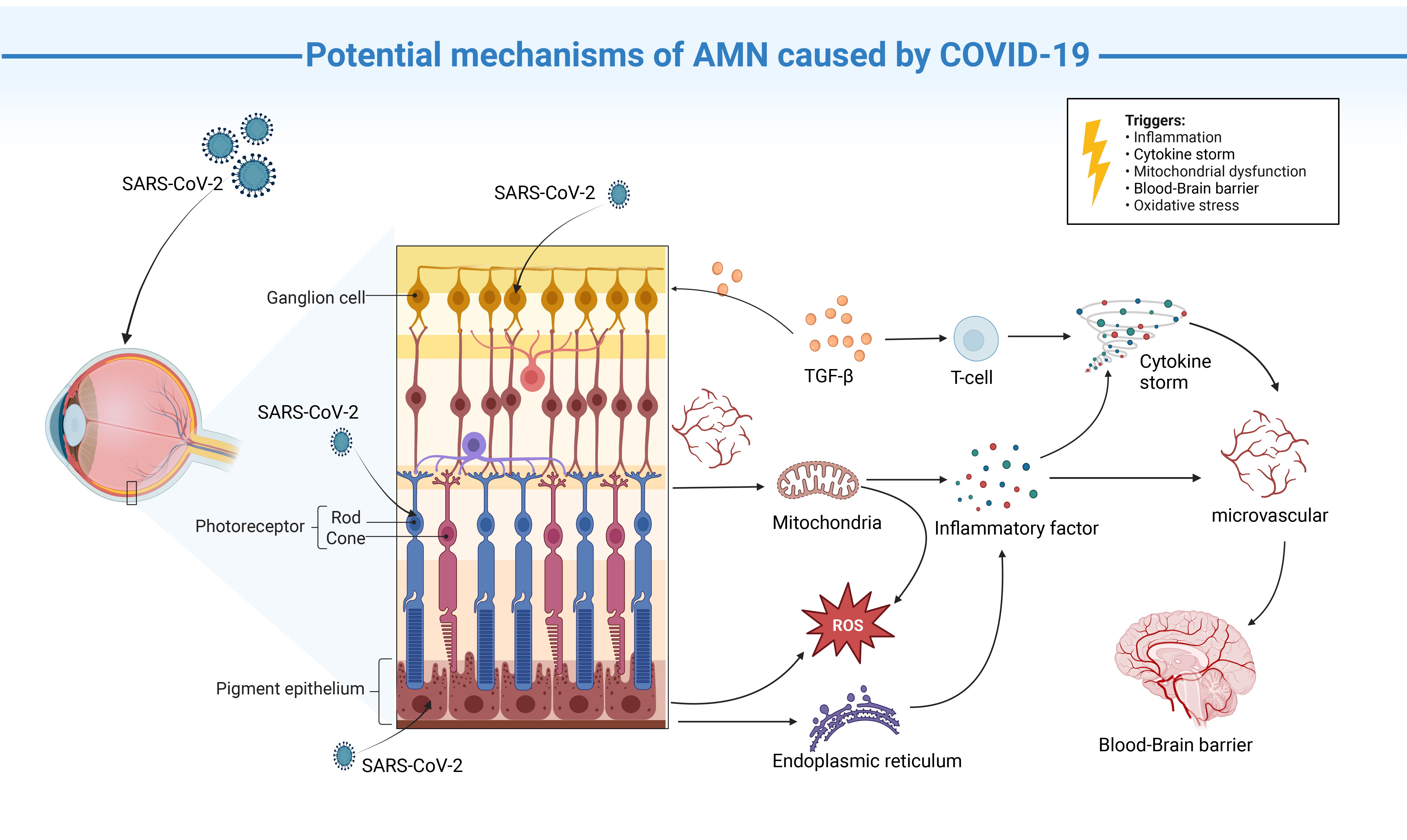 Fig. 3.
Fig. 3.
Proposed mechanisms for AMN caused by COVID-19 infection.
COVID-19 infects the inner retina (ganglion cells, photoreceptors, RPE) and
recruits TGF-
At present, no established treatment exists for COVID-19-related AMN. Consequently, the pharmacological interventions discussed below have proven effective against COVID-19, or represent potential therapeutic approaches.
Corticosteroids have been used extensively to treat cytokine storm syndrome, which is characterized by excessive inflammation resulting from the overproduction of immune cells and cytokines [122]. This syndrome can manifest during various infections or autoimmune conditions, and a significant increase in several cytokines has been observed in a subset of COVID-19 patients [123]. The primary mechanism of corticosteroid action involves the inhibition of viral entry and replication by targeting viral proteins, or by modulating the host immune response to reduce hyperinflammation caused by COVID-19 infection [124, 125, 126, 127]. This is achieved by decreasing the expression of two principal proteins utilized by COVID-19 for cellular uptake: TMPRSS2 and ACE2 [128].
Nevertheless, the high dosage and prolonged course of corticosteroids required to treat severe cases can result in adverse effects and undesirable reactions, including longer virus elimination and increased susceptibility to opportunistic infections. Consequently, attention has turned to the development of corticosteroid-reducing medications (immunomodulators) and novel corticosteroid administration methods aimed at reducing the required dosage and hence the associated drug-induced risks [129].
Interferons are a group of cytokine-signaling mediators that exhibit critical
antiviral activity and play significant roles in innate and adaptive cellular
immune responses [130]. Their immunomodulatory and antiviral activities are
thought to involve activation of the innate immune response after viral
infection, inhibition of viral replication, and stimulation of protein synthesis
through interaction with toll-like receptors [131]. Interferons are widely used
in ophthalmology for the treatment of ocular surface tumors, macular edema, and
other anterior and posterior chamber pathologies. Recombinant forms of interferon
have also been employed to treat various viral infections and neoplastic diseases
[132, 133]. The efficacy of interferon
Arbidol hydrochloride is an antiviral drug that inhibits interaction between the spike protein and ACE2 [136]. It has been used extensively in the prevention and treatment of influenza, and is currently being investigated for its potential efficacy against COVID-19 using an identical dosing regimen (200 mg every 8 h). Although preliminary results from a small, non-randomized study has shown promise, further research is needed to comprehensively evaluate its effectiveness [137]. As with other pharmaceutical agents, no ocular adverse effects associated with its use have been reported. Arbidol is a non-nucleoside antiviral agent that prevents viral entry into host cells by inhibiting the virus lipid envelope from fusing with the host cell membrane. Arbidol can also boost the host immune system by inducing the production of interferons and activating macrophages [138].
Carmustine mesylate exerts its effect on the host enzyme TMPRSS2, a member of the serine protease family. This mechanism inhibits viral entry and impedes virus-cell membrane fusion, thereby effectively suppressing viral replication [139]. Regulatory approval for this pharmaceutical agent has been received for the treatment of pancreatitis in Japan, and it also exhibits some efficacy against other coronaviruses. However, definitive evidence for its effectiveness against COVID-19 remains to be established [140].
Tocilizumab is an immunomodulatory drug recently approved by the U.S. Food and Drug Administration for Phase III clinical trials of critically ill COVID-19 patients. COVID-19 infection results in compromised vascular integrity, activated coagulation, and increased inflammation due to IL-6 trans-signaling. The therapeutic mechanism of tocilizumab involves the targeting of endothelial IL-6 signaling transduction to mitigate COVID-19-induced cytokine storm symptoms [141]. This medication is typically administered either as a monotherapy or in combination with other drugs to manage various autoimmune disorders, including rheumatoid arthritis [142, 143].
The presence of COVID-19 in the eye can result in various ocular diseases. AMN is a rare retinal disorder with few cases reported prior to the COVID-19 pandemic. However, the incidence of AMN is significantly higher in patients with COVID-19. This observation suggests the virus plays a crucial role in the increased prevalence of AMN, highlighting the need for further investigation into its underlying mechanisms in order to improve disease management. In this study, we examined the demographic and clinical characteristics of COVID-19-induced AMN utilizing the existing literature. We comprehensively analysed the potential mechanisms of COVID-19-induced AMN, focusing not only on microvascular damage but also the effects of COVID-19 on immunological factors and the BRB. We posit that COVID-19 induces damage to the retina and retinal cells, resulting in impairment of the microvascular circulation and subsequently triggering the development of AMN. This hypothesis provides a theoretical foundation for further investigation of the specific pathophysiological mechanisms underlying AMN.
The continuous mutation of COVID-19 enhances its immune escape and transmission capabilities, thus underscoring the importance of clinical prevention and treatment strategies. This review examined agents that can effectively reduce viral infection and associated toxicity in COVID-19. However, certain drugs exhibit varying adverse effects that necessitate careful consideration in the clinical setting. These therapeutic interventions may provide a foundation for future clinical treatments.
Conceptualization, GD and YX; Methodology, YX, GD, and TC; Investigation, YX, and ZZ; Resources, YX; Data curation, YX and ZC; Writing—original draft preparation, ZC and YX; Writing—review and editing, ZC, GD, and YX; Visualization, YX and ZZ; Supervision, GD and ZC; designed the research study, ZC. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Northwestern University Clinical Research.
We deeply appreciate Dr. Yannian Hui from Dept. Ophthalmology of Xijing Hospital for his critical thinking and constructive suggestions for this work.
This study was supported by grants from the National Natural Science Foundation of China (Nos. 82371071, 81970814, and 82000905); clinical AFFMU foundation support (2021JSTS28); Science and Technology Project of Xi’an (22YXYJ0053).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
