- Academic Editor
-
-
-
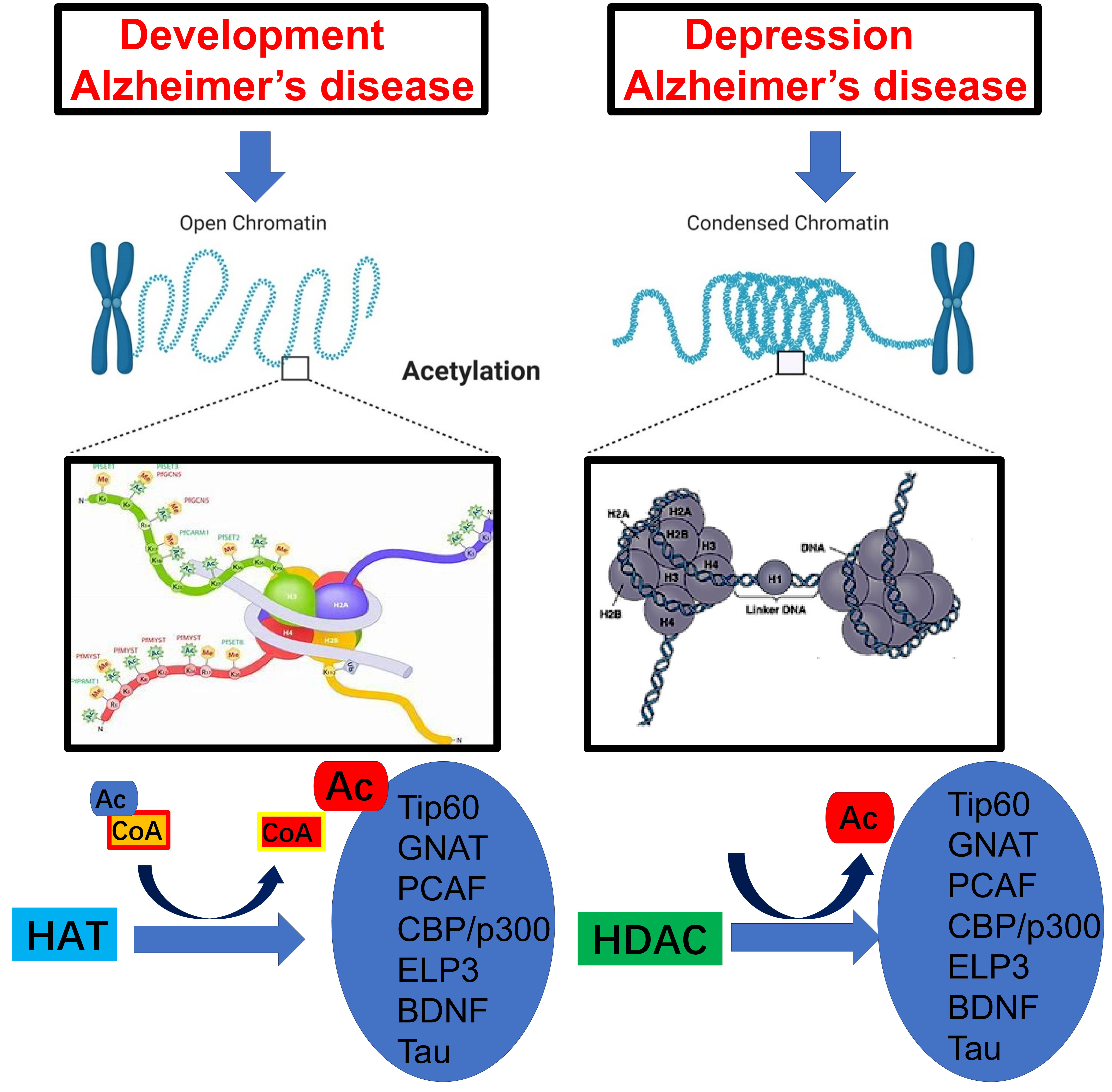
The human DNA double helix is wrapped around proteins known as histones, which play a critical role in regulating gene expression. The goal of this opinion piece is to provide an overview of how histone sensing drives Alzheimer’s disease (AD). Histones are proteins enriched in basic amino acids. Histone acetylation plays an important role in the progression of AD as its dysregulation can lead to neuroinflammation and neurodegenerative diseases. Specifically, abnormal histone acetylation, a post-translation modification, is a key factor in AD as it contributes to brain cell inflammatory pathology. Thus, higher levels of histone acetylation could potentially serve as important biomarkers for the progression of AD. Here, we report that increased levels of acetylation of histones H2B, H3, and H4 in the promoter regions of Tip60 lysine acetyltransferase protein, p300/CREB-binding protein (CBP), GCN5-related N-acetyltransferases, p300/CBP-associated factor, elongator protein 3, brain-derived neurotrophic factor, and Tau genes in the hippocampus and temporal lobe are associated with the development of AD-associated learning and memory impairment.