- Academic Editor
†These authors contributed equally.
Myocardial fibrosis is a key pathological driver of Hypertrophic Cardiomyopathy (HCM), contributing to adverse remodeling and poor prognosis. The transforming growth factor-β1/Smad3 (TGF-β1/Smad3) signaling cascade plays a central role in fibrogenesis; however, effective antifibrotic therapies remain limited. Astragalus polysaccharide (APS), a bioactive constituent of Astragalus membranaceus, has demonstrated cardioprotective potential. Nevertheless, the mechanisms underlying its effects in HCM-associated fibrosis remain unknown.
Pressure overload induced HCM was established in C57BL/6J mice using transverse aortic constriction (TAC), and animals were randomized to control, TAC, low-dose APS (50 mg/kg/day), or high-dose APS (100 mg/kg/day) groups. Cardiac function was evaluated by echocardiography, while myocardial hypertrophy and fibrosis were assessed by morphometry, Masson’s staining, and collagen I (Col-I) expression analysis. Parallel in vitro studies employed angiotensin II stimulated (Ang II-stimulated) H9C2 cardiomyocytes, with or without the TGF-β1/Smad3 agonist SRI-011381, to explore mechanistic pathways.
TAC induced marked cardiac dysfunction, ventricular dilation, and extensive fibrosis, accompanied by upregulation of TGF-β1, phosphorylated Smad3, and Col-I expression (all p < 0.05). APS treatment dose-dependently preserved systolic function, attenuated collagen deposition, and suppressed activation of the TGF-β1/Smad3 axis, with the strongest effects observed in the high-dose group. In vitro, APS significantly inhibited Ang II induced hypertrophy and fibrotic protein expression; these effects were abrogated by SRI-011381, confirming pathway specificity.
APS exerts cardioprotective and antifibrotic effects in HCM by inhibiting the TGF-β1/Smad3 signaling pathway. These findings highlight APS as a promising therapeutic candidate for targeting myocardial fibrosis and improving outcomes in HCM.
Hypertrophic Cardiomyopathy (HCM) is a common hereditary cardiovascular disease
characterized by the thickening of the ventricular walls, particularly in the
interventricular septum [1, 2]. Clinically, HCM manifests in a variety of ways,
including palpitations, chest pain, shortness of breath, and syncope, with severe
cases leading to heart failure or sudden cardiac death [3, 4]. Thus, there is an
urgent need to develop effective antifibrotic strategies clinical. Transforming
growth factor-
In this study, we employed a transverse aortic constriction (TAC) model to
induce HCM in mice and an angiotensin Ⅱ-induced (Ang Ⅱ-induced) hypertrophy model
in cardiomyoceytes to investigate the regulatory effects of APS on the classical
TGF-
Male C57BL/6J mice were provided by Beijing Huafukang Bioscience Co., Ltd.
(Beijing, China; license number: SCXK [Jing] 2019-0008). Forty mice after 1 week
of acclimatization, under anesthesia with 1% isoflurane, a 3-mm incision was
made at the proximal sternum to fully expose the aortic arch. A blunt 27-gauge
needle was positioned between the two carotid arteries, directly over the aortic
arch. Using 7-0 silk suture, the aortic arch was ligated against the needle at a
site immediately distal to the brachiocephalic artery. The needle was then
promptly removed, creating a reproducible TAC. Following the procedure, the
sternum and skin were closed with 6-0 polypropylene sutures, and the mice were
placed on a pre-warmed heating pad until full recovery from anesthesia.
Sham-operated animals underwent the identical surgical exposure without aortic
ligation. Beginning on postoperative day 2, the drug concentration is based on
previous literature [11, 12], mice assigned to the high-dose group (APS-H) and
low-dose group (APS-L) received intragastric administration of APS at 50
mg/kg/day and 100 mg/kg/day, respectively (APS: Solarbio, China, cat. no.AGV
7970, purity
At 4 weeks post-surgery, cardiac function was assessed using a high-resolution small-animal ultrasound system (The manufacturers and production addresses of the equipment used are listed in Table 1). Measured parameters included left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular end-diastolic internal diameter (LVIDd), left ventricular end-systolic internal diameter (LVIDs), left atrial diameter (LAD), and left atrial diastolic area (LA diastolic area).
| Reagent/Instrument name | Technology (cat. no) |
| Bovine serum albumin | Lanjieko Technology, Beijing, China |
| PMSF (100 Mm) | Beyotime, Shanghai, China |
| 5×sodium dodecyl sulfate-polyacrylamide gel electrophoresis | Beyotime, Shanghai, China |
| Enhanced chemiluminescence | NCM Biotech, Suzhou, China |
| Astragalus polysaccharides | AGV 7970, Solarbio, Beijing, China |
| Non-fat milk | Yili Global, Inner Mongolia, China |
| TEMED | Shanghai, China |
| Masson’s trichrome staining | abs9347, Absin, Shanghai, China |
| Commercial immunohistochemical kit | Kaiji, Shanghai, China (KGC3201-300) |
| Dulbecco’s modified Eagle’s medium | Sevicebio, Wuhan, China |
| 1% penicillin–streptomycin | Thermo Fisher, Shanghai, China |
| Fetal bovine serum | Thermo Fisher, Shanghai, China |
| 4’,6-diamidino-2-phenylindole | Beyotime, Shanghai, China |
| Cell Counting Kit-8 | C0038, Beyotime, Shanghai, China |
| SRI-011381 | 1629138-41-5, MedChemExpress, Princeton, NJ, USA |
| Collagen I Antibody (mouse) | 1:1000, sc-59772, Santa, Shanghai, China |
| TGF- |
1:1000, sc-130348, Santa, Shanghai, China |
| Smad3 (rabbit) | 1:1000, 9523, Cell Signaling Technology, Danvers, MA, USA |
| p-Smad3 (rabbit) | 1:1000, 9520, Cell Signaling Technology, Danvers, MA, USA |
| 1:1000, 2144, Cell Signaling Technology, Danvers, MA, USA | |
| Upright microscope | Chongqing UPO Optoelectronic Technology Co., Ltd., Chongqing, China |
| Fluorescence microscopy | Nikon, Beijing, China |
| High-resolution small-animal ultrasound system | VINNO Corporation, Suzhou, China |
| Precision gas anesthesia system | MIDMARK, Versailles, OH, USA |
Cardiac tissues were paraffin-embedded, sectioned, and subjected to Masson’s
trichrome staining according to the instructions provided by the manufacturer’s
instructions (The manufacturers, production addresses, and batch numbers of the
kits used are shown in Table 1). Images were acquired using an upright microscope
under identical settings (magnification:
Paraffin-embedded sections were subjected to deparaffinization, hydration, and antigen retrieval before immunohistochemical staining, which was carried out using a commercially available kit (The manufacturers, production addresses, and batch numbers of the kits used are shown in Table 1). Expression of collagen I (Col-I) was detected under a light microscope, and quantitative evaluation was subsequently performed.
Cardiac sections were first fixed and permeabilized, then blocked with 5%
bovine serum albumin. After overnight exposure to anti-Col-I antibodies, samples
were incubated with fluorophore-conjugated secondary antibodies and
counterstained with DAPI. Fluorescence images were acquired under identical
conditions at
H9C2 rat cardiomyoblasts (ATCC® CRL-1446™) were
obtained from ATCC (Manassas, VA, USA). Cell lines were authenticated by short
tandem repeat profiling, regularly screened for mycoplasma contamination with the
MycoAlert™ kit (Lonza, Switzerland), and cross-referenced with
ICLAC and Cellosaurus databases. Cells were maintained in high-glucose DMEM
supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. After
reaching ~50–60% confluence, cultures were serum-deprived for
24 h. Hypertrophy was induced by Ang II (1
Cells were seeded into 96-well plates and incubated for 24 hours
(1
Proteins were extracted from ventricular tissues or cultured cells, quantified, and separated by SDS-PAGE. After transfer onto PVDF membranes, blocking was performed with 5% non-fat milk. Membranes were incubated with primary antibodies overnight, followed by HRP-conjugated secondary antibodies (The manufacturers, batch numbers, and preparation ratios of the antibodies used are shown in Table 1). Protein bands were visualized using enhanced chemiluminescence and analyzed with ImageJ software.
Data analysis was conducted with GraphPad Prism 8.4.0 (GraphPad Software Inc.,
San Diego, CA, USA). One-way ANOVA followed by Tukey’s multiple comparison test
was applied to determine intergroup differences. A two-tailed p-value
Four weeks after TAC surgery, compared with controls, mice displayed significant cardiac impairment. TAC mice showed marked declines in LVEF and LVFS, accompanied by enlarged LVIDd, LVIDs, LAD, and LA diastolic area, consistent with ventricular remodeling, and the heart weight/body weight (HW/BW) ratio was significantly elevated. These changes are consistent with the typical features of HCM. For example, studies have shown that the TAC model leads to a significant reduction in EF, with FS decreasing from 53.2% to 32.3%, indicating impaired cardiac contractile function [13]. In addition, TAC-induced pressure overload can trigger cardiac remodeling, manifested as ventricular wall thickening and chamber dilation. These alterations correspond well with the imaging characteristics observed in clinical HCM [14]. Administration of APS ameliorated these abnormalities in a dose-dependent manner. The APS-H exhibited notable improvements in systolic performance and reduced ventricular dilation, whereas the APS-L showed moderate but significant benefits. These findings highlight the cardioprotective action of APS against pressure overload induced dysfunction (Fig. 1A–I).
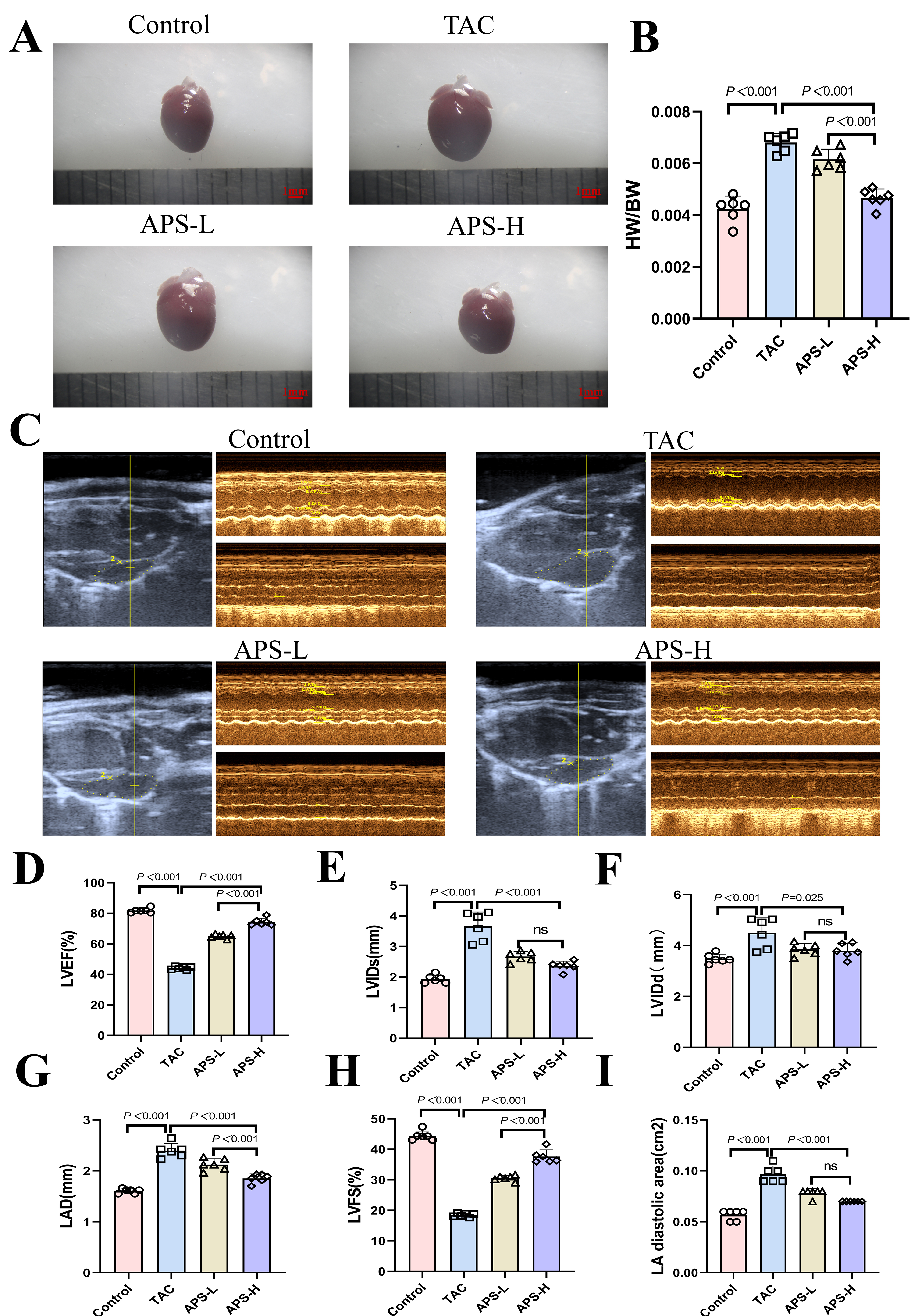 Fig. 1.
Fig. 1.
Astragalus polysaccharide (APS) improves cardiac function in transverse aortic constriction (TAC) mice. (A) Heart photo (scale bar = 1 mm). (B) Heart weight/body weight (HW/BW). (C) Transthoracic echocardiography. (D) Left ventricular ejection fraction (LVEF). (E) End-systolic left ventricular diameter (LVIDs). (F) Eend-diastolic left ventricular diameter (LVIDd). (G) Mean left atrial straight diameter (LAD). (H) Left ventricular short axis shortening (LVFS). (I) Left atrial diastolic area (LA diastolic area). ns, no statistical difference. APS-L, low-dose APS, 50 mg/kg/day; APS-H, high-dose APS, 100 mg/kg/day. n = 6.
Masson’s staining demonstrated a robust increase in collagen accumulation in the TAC group, confirming extensive fibrotic remodeling. Quantitative analysis revealed a substantial rise in collagen volume fraction relative to controls. Treatment with APS effectively reduced collagen deposition, with the most profound suppression observed in APS-H mice. Consistently, Col-I expression, as assessed by immunohistochemistry and immunofluorescence, was significantly diminished following APS treatment, indicating its potent antifibrotic effect (Fig. 2A–F).
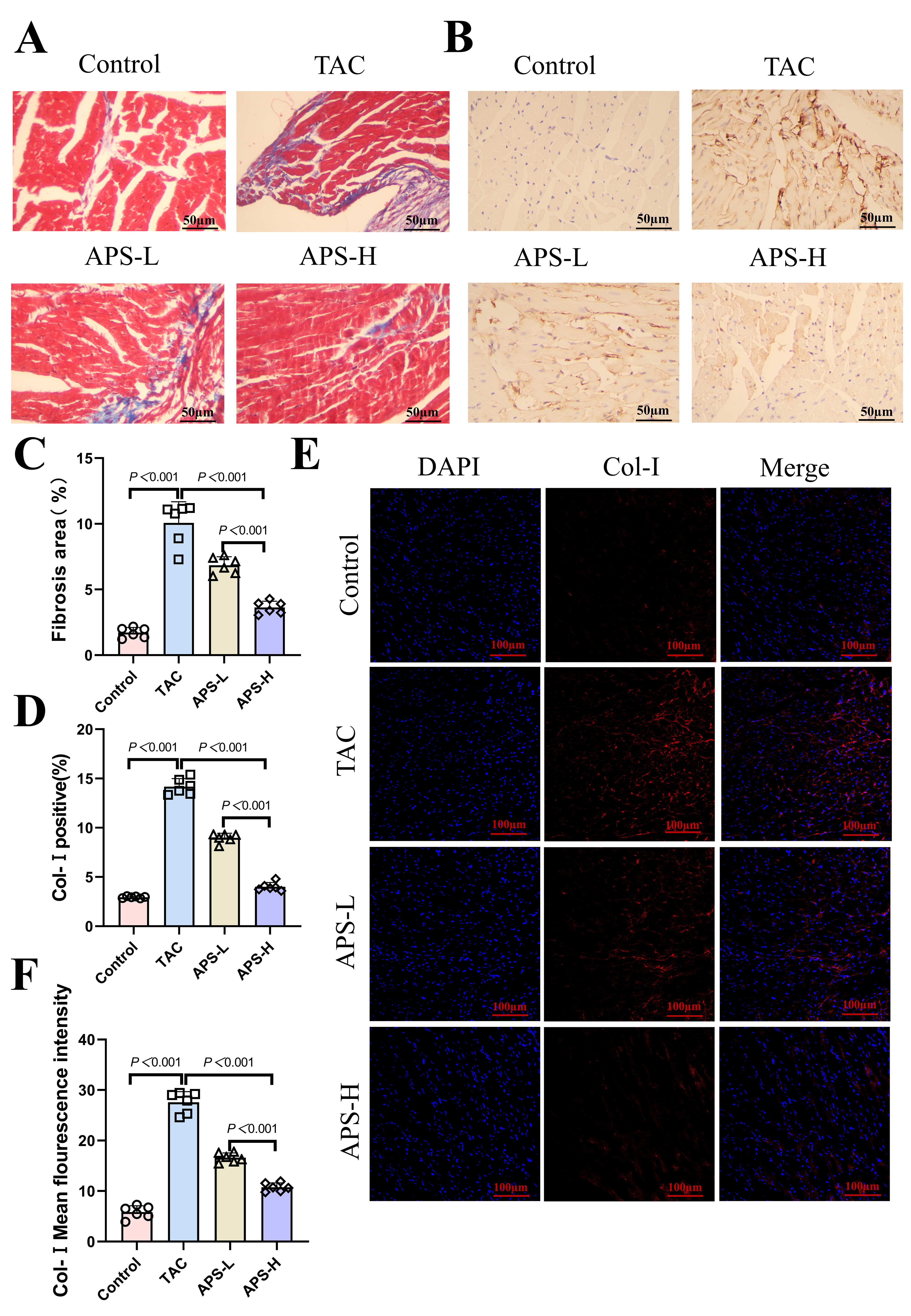 Fig. 2.
Fig. 2.
APS attenuates TAC-induced myocardial fibrosis. (A) Ventricular Masson staining. (B) Collagen I (Col-I) is a representative immunohistochemical staining of the ventricle. (C) Statistical analysis of the atrial fibrosis area (scale bar = 50 µm). (D) Col-I average histochemical analysis. (E) Col-I (red) and DAPI (blue) representative ventricular immunofluorescence staining (scale bar = 100 µm). (F) Col-I average fluorescence intensity analysis. n = 6.
Western blot analysis revealed that TAC-induced remodeling was associated with
increased myocardial expression of TGF-
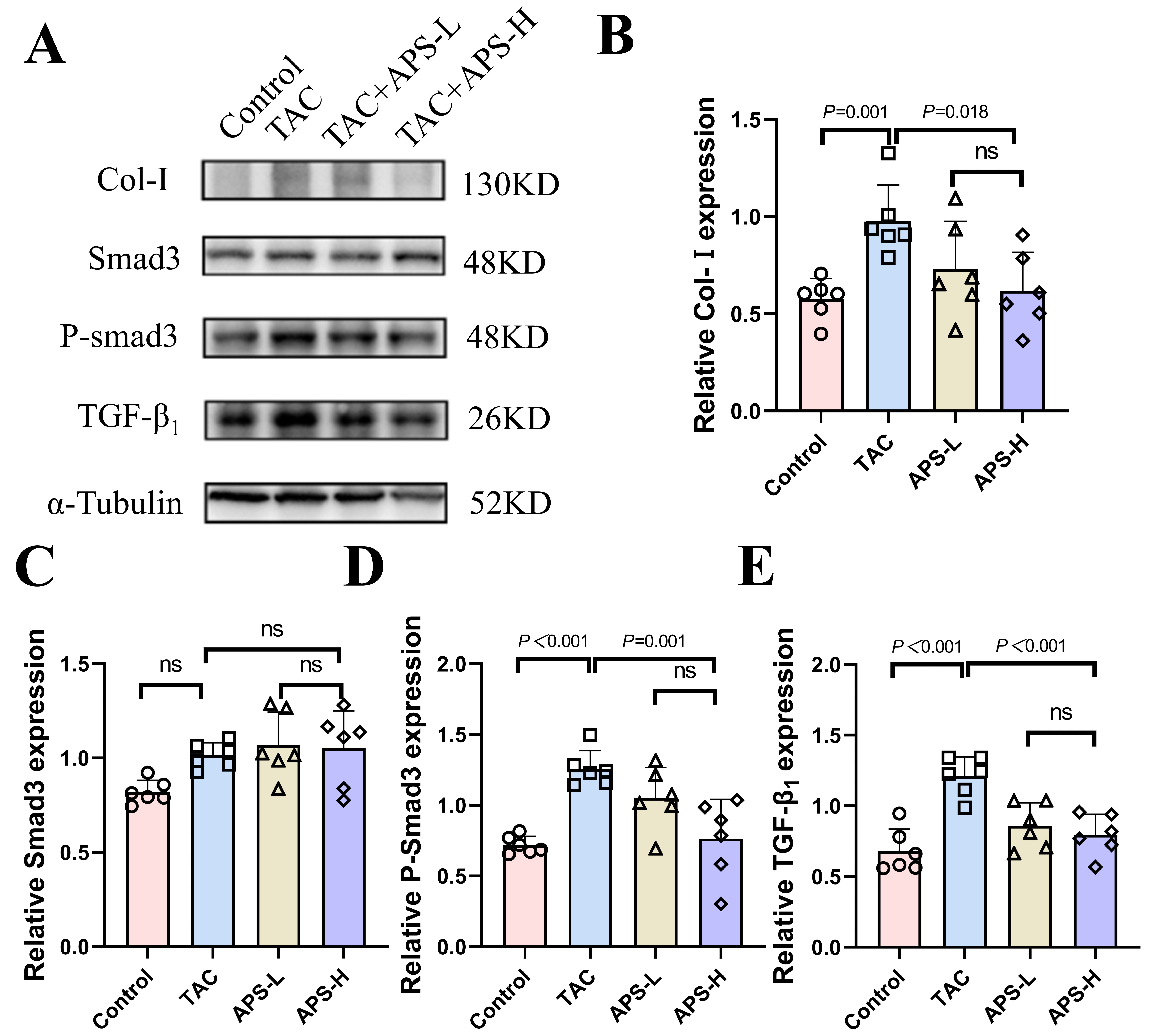 Fig. 3.
Fig. 3.
APS modulates the expression of growth factor-
With increasing concentrations of Ang II, cell viability decreased
significantly. At 10-5 mmol/L, cell viability was approximately 70% of the
control, which is commonly used as the experimental concentration; at
10-4 mmol/L, notable cell damage occurred, indicating potential toxicity.
Therefore, 10-5 mmol/L was selected for subsequent experiments (Fig. 4A).
Exposure of H9C2 cardiomyocytes to Ang II markedly upregulated the expression of
TGF-
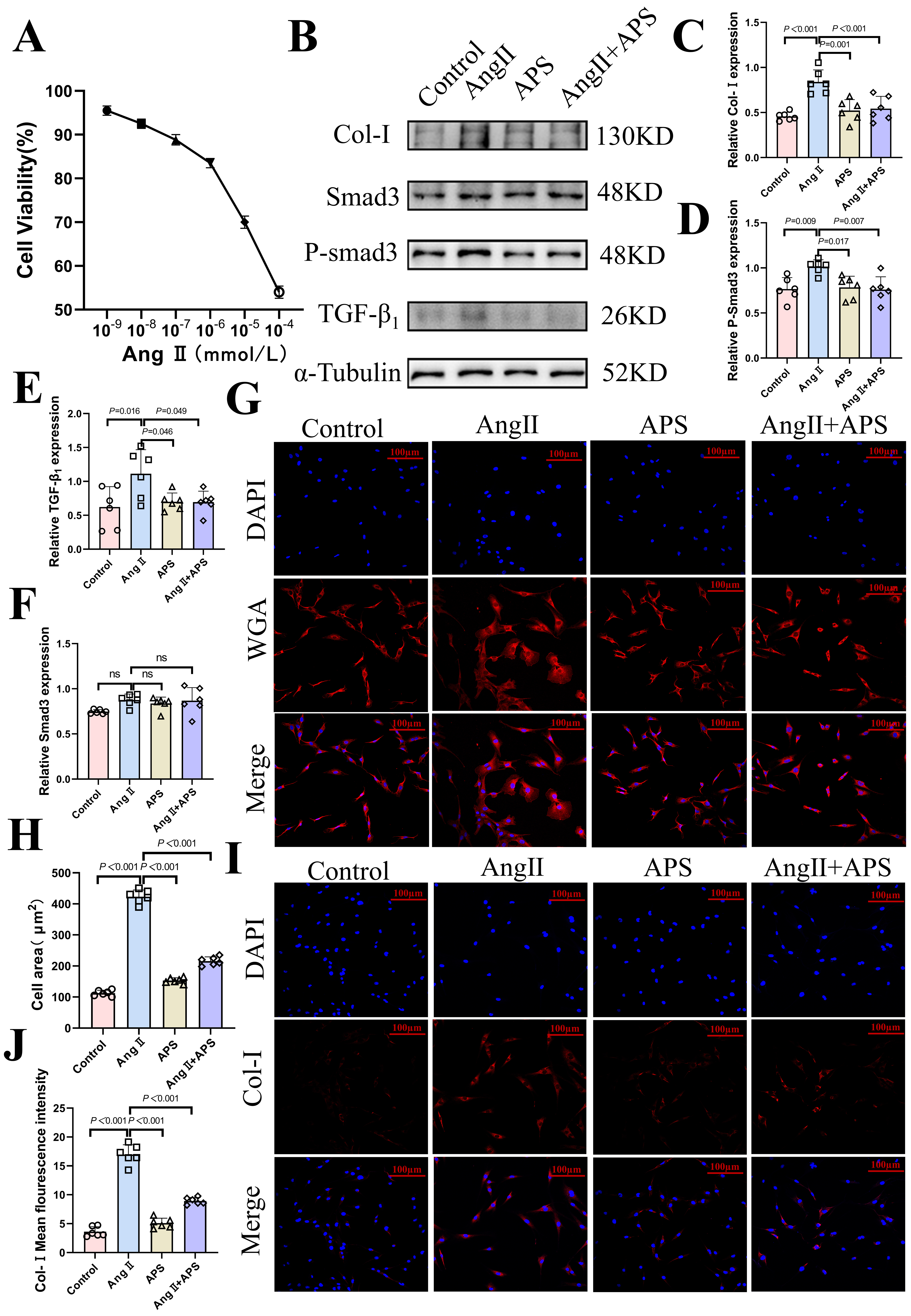 Fig. 4.
Fig. 4.
APS suppresses angiotensin IIstimulated (Ang II)-induced
hypertrophy in H9C2 cells. (A) Cell counting kit-8 (CCK8) assay of cell
viability at different concentrations of Ang II. (B) Western blotting detecting
the expression of TGF-
To further confirm pathway specificity, Ang II-stimulated cells were
co-incubated with the TGF-
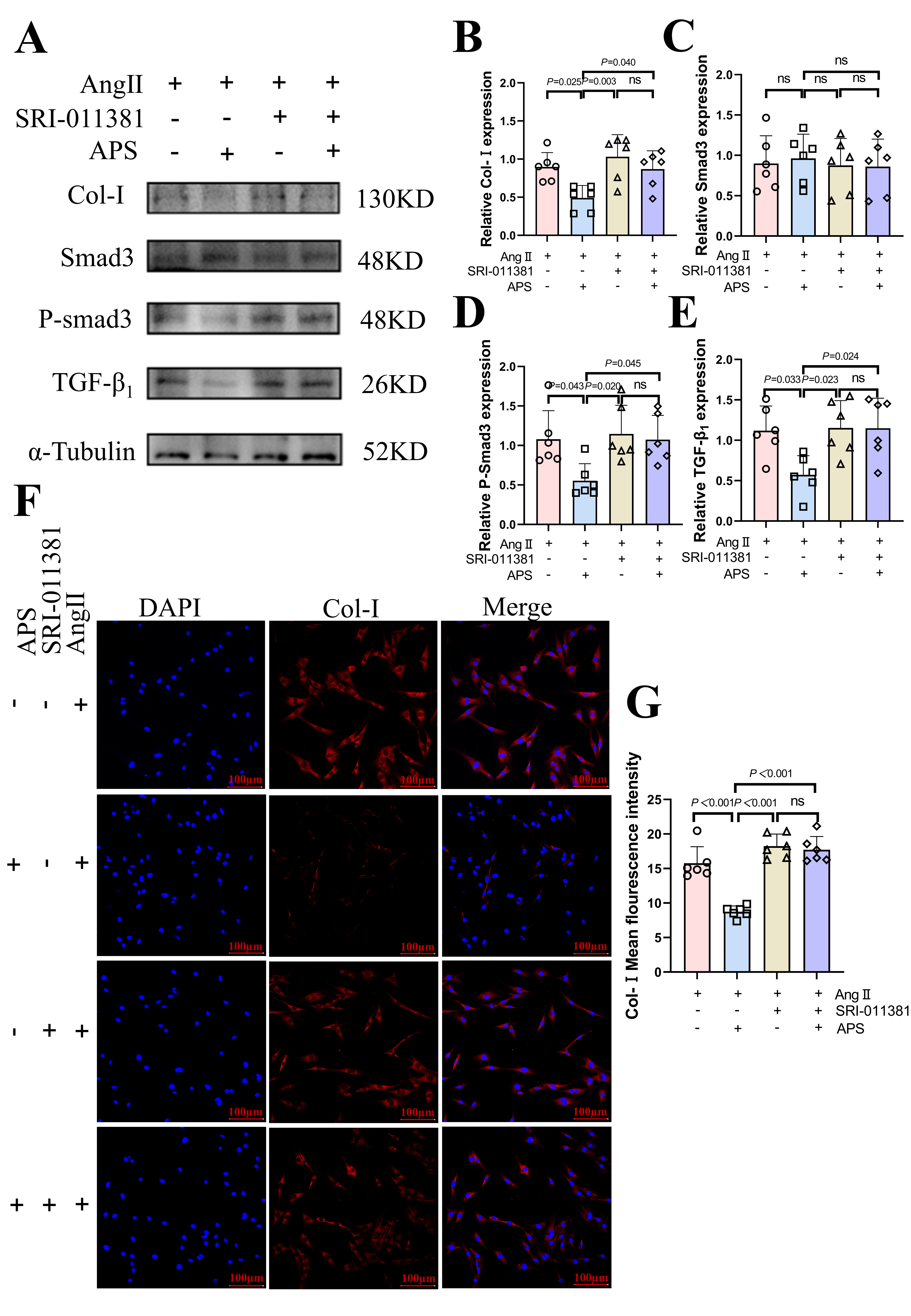 Fig. 5.
Fig. 5.
APS exerts antifibrotic-Fibrotic effects via the
TGF-
HCM is the most common genetic heart disorder and continues to pose a major challenge to cardiovascular health worldwide [15]. A key feature of HCM pathophysiology is maladaptive cardiac remodeling, characterized by myocyte hypertrophy, interstitial fibrosis, and microvascular dysfunction [3]. Myocardial fibrosis plays a crucial role in disrupting normal cardiac structure and function, contributing to impaired diastolic and systolic performance, and increasing the risk of arrhythmias [16]. Furthermore, myocardial fibrosis is strongly associated with adverse clinical outcomes, including sudden cardiac death. Therefore, targeting myocardial fibrosis has become a promising therapeutic strategy in the management of HCM [17].
Among diverse antifibrotic strategies, traditional Chinese medicine has
demonstrated unique advantages due to its multi-target and holistic regulatory
properties. Astragalus membranaceus, a widely used Qi-tonifying herb in
clinical practice, contains major active components such as astragaloside IV and
APS [18, 19]. As a natural polysaccharide, APS exerts diverse pharmacological
effects, including antioxidant, anti-apoptotic, anti-inflammatory, and
immunomodulatory activities [20]. Recent studies have shown that APS plays
important roles in attenuating fibrosis across multiple organs. For instance, APS
significantly suppresses isoproterenol-induced myocardial hypertrophy and
improves cardiomyocyte morphology [21]. In renal fibrosis models, APS reduces ECM
deposition by inhibiting the TGF-
In the present study, we employed a TAC mouse model to mimic pressure overload-induced HCM. Echocardiographic analysis revealed that HCM mice exhibited significantly increased LAD, LVIDd, and LVIDs, along with reduced LVEF and LVFS, reflecting ventricular dilation and impaired contractile function—typical features of HCM. Pathological examination further demonstrated elevated Col-I expression and extensive collagen fiber deposition, confirming pronounced myocardial fibrosis. These findings underscore the central role of fibrosis in TAC-induced HCM.
Mechanistically, the TGF-
The significance of this study lies in providing both systemic and molecular
evidence that APS exerts protective effects against HCM by alleviating myocardial
fibrosis via TGF-
Nevertheless, several limitations of this study should be acknowledged. First,
TAC mice and H9C2 cardiomyoblasts may not fully replicate the complex
pathophysiology of human HCM. Validation studies using primary human cardiac
fibroblasts and large animal HCM models are essential to confirm translational
relevance. Second, the long-term safety, pharmacokinetics, and potential
off-target effects of APS require systematic evaluation. Third, although this
study confirmed that the antifibrotic effects of APS are dependent on the
TGF-
Myocardial fibrosis is a major pathological manifestation of HCM. APS may
effectively ameliorate HCM by suppressing myocardial fibrosis through modulation
of the TGF-
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
NNQ, WJW and BYL were responsible for study conception and design. BYL performed the data analysis and interpretation. WJW and NNQ drafted the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the ARRIVE Guidelines The research protocol was approved by the Ethics Committee of Animal Ethics Committee of the General Hospital of the Northern Theater Command (Ethic Approval Number: 2024-09).
We would like to express our gratitude to all those who helped me during the writing of this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
