- Academic Editor
Despite extensive research, the systemic biological mechanisms underlying exercise-induced physiological adaptations remain incompletely understood. While animal models (e.g., rodents, non-human primates) have been instrumental in elucidating exercise-mediated benefits in aging and disease, interspecies differences in genomics, epigenetics, and metabolic regulation limit their translational relevance. The advent of induced pluripotent stem cell (iPSC)-derived 3D organoids revolutionizes exercise biology research by enabling human-specific modeling of tissue architecture and donor genomic/epigenetic profiles. This review highlights three transformative strategies: (1) Athlete-derived organoids preserving exercise-induced epigenetic memory to study muscle/neural adaptations; (2) Engineered systems integrating optogenetics and microfluidics to simulate mechanical forces (e.g., muscle contraction) and systemic signals (e.g., cytokines); (3) multi-omics mapping revealing exercise-responsive pathways like mitochondrial biogenesis. Collectively, these patient-specific models bridge pathophysiology with high-throughput screening, advancing precision medicine—from personalized training regimens to therapies counteracting sedentary-related diseases.
Biological aging describes a complex process involving the gradual decline of functional traits, leading to reduced physical and mental abilities and a higher risk of diseases. In recent decades, alternative therapies have gained attention as potential anti-aging treatments because of their ability to provide systemic benefits. Among these, physical exercise stands out as one of the most effective non-drug strategies for promoting healthy aging. It offers widespread protective effects across various body systems and increases the body’s resilience to environmental stressors—factors often linked to the speeding up of aging processes [1, 2]. It has been demonstrated that physical exercise could improve cardiovascular health, strengthen muscles and bones, support mental well-being, and is consistently linked to a longer health span and lifespan. However, responses to physical activity vary greatly among individuals and are influenced by genetic, epigenetic, and environmental factors. Gaining a deeper understanding of the molecular and cellular mechanisms behind these responses is crucial for designing personalized exercise plans that maximize health benefits for different populations.
Recent studies demonstrated a series of key biological pathways derived from physical activity, including those involved in gene expression regulation, metabolic homeostasis, oxidative stress responses, and immune regulation. Most of these biological mechanisms have been reported to serve as systemic regulators of aging-related processes. Animal models, particularly rodent models, are widely used for clarifying the exercise-mediated biological benefits, which provide a systematic cross-organ overview. Rodents offer an efficient platform for anti-aging research, given the relatively short lifespan, well-established experimental readouts, and well-characterized genetic backgrounds. Experimental paradigms of exercise, such as voluntary wheel running, forced treadmill running, and swimming protocols, have been successfully utilized to investigate the physiological and molecular effects of physical activity. These models provide a feasible framework for investigating the long-term impact of exercise on aging and the onset of chronic diseases within a realistic experimental timeframe. In contrast, non-human primates (NHPs) offer a translational model due to their higher-order cognitive capabilities, complex social behaviors, and closer genetic homology to humans. NHP studies facilitate the evaluation of exercise-induced changes in cognitive function, emotional regulation, and social interactions, which are particularly relevant to the study of aging-related neuropsychiatric conditions [3]. Despite logistical and ethical challenges, the use of NHPs provides valuable insights into the neurological and behavioral dimensions of exercise that are not easily captured in rodent models.
Developing in vitro models of exercise involves the challenge of replicating the complex physiological and biochemical networks associated with physical activity. While these models inherently lack the integrated systemic responses of whole-body exercise, they offer powerful platforms to dissect cell-type-specific and tissue-level molecular mechanisms. This reductionist strategy is especially useful for elucidating the direct cellular or organ-level targets of exercise while minimizing the influence of confounding systemic factors such as hormonal variations or neural signaling. Advances in induced pluripotent stem cell (iPSC) technology greatly promote the development of organoids, which recapitulate key aspects of the three-dimensional architecture, cell diversity, and functional complexity of specific human tissues [4]. The iPSC-derived organoids have been successfully used to model a wide range of diseases, including neurological, muscular, metabolic, and inflammatory disorders [5, 6]. Their ability to retain individual genetic backgrounds and reflect disease-specific phenotypes provides a promising tool for precision medicine and mechanistic research. Although the application of organoids in modeling the effects of physical exercise remains in its infancy, their potential is considerable. By applying mechanical stimulation, metabolic stressors, cytokine exposure, or electrical impulses to organoid cultures, researchers may begin to mimic key facets of the exercise microenvironment. For example, engineered muscle organoids exposed to cyclic strain or electrical pacing can simulate muscle contraction and mitochondrial adaptations, while brain organoids may be interrogated for neurotrophic responses to exercise-mimicking conditions. However, substantial challenges remain, including the standardization of stimulation protocols, the scalability of organoid cultures, and the translation of observed responses to in vivo physiology. Moreover, the absence of systemic cues such as vascular perfusion, endocrine signaling, and inter-organ crosstalk limits the full extrapolation of organoid-based findings to whole-body exercise responses.
This perspective review highlights recent advances and conceptual developments in adapting organoid systems as in vitro platforms for studying the biological responses elicited by exercise. Here, we discussed the application of advancing technologies such as bioengineering approaches and single-cell omics to enhance the role of organoids in exercise research. Furthermore, this review suggested the current limitations of organoid models and outlines future directions to bridge the gap between in vitro systems and in vivo exercise physiology.
Voluntary wheel running and forced endurance training remain cornerstone
paradigms for investigating exercise physiology in rodents, providing critical
insights into neuromuscular adaptation, mitochondrial biogenesis, and metabolic
regulation [7, 8]. Emerging modalities, including resistance exercise (ladder
climbing/weighted running) and high-intensity interval training (HIIT), have
enabled stimulation of mechanotransduction pathways and energy substrate
utilization [9, 10]. In Table 1 (Ref. [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]), contemporary murine studies
reveal exercise-induced biological effects operating across multiple temporal
scales - from acute post-translational modifications to chronic epigenetic
remodeling. These adaptations converge on tissue regeneration (enhancing
hippocampal neurogenesis via brain derived neurotrophic factor/tropomyosin
receptor kinase B (BDNF/TrkB) signaling and muscular repair through Pax7+
satellite cell activation [27]), antioxidant defense mediated by nuclear factor
erythroid 2-related factor 2 (Nrf2)- antioxidant response element (ARE) pathway
upregulation, mitochondrial biogenesis governed by Peroxisome
proliferator-activated receptor gamma coactivator 1
| Animal model | Procedure | Key component | Anti-aging mechanisms | Physiological adaption mechanisms |
| Voluntary wheel running | Free access to running wheels (5–14 days to lifelong). | BDNF, insulin-like growth factor 1 (IGF-1), Exosomal miR-206, Cathepsin B | Hippocampal neurogenesis, synaptic plasticity, age-related cognitive decline [11, 12]. | Increased heart ventricular weights and reduced visceral/epididymal fat weights, as well as blood triglyceride level compared [13]. |
| Forced treadmill running | Gradual intensity (10–20 m/min, 30–60 min/day, 4–12 weeks). | Irisin, FGF21, microRNA-21 (miR-21), Lactate | Mitochondrial biogenesis (peroxisome proliferator-activated receptor gamma coactivator-1 |
Maintain the expression level of Ostn, Ak4, Srrm4os, and Zfhx4 in skeletal muscle [17]. |
| Resistance training | Climbing ladders with weighted loads (3×/week for 6 weeks). | IGF-1, Follistatin, Myostatin, miR-133a | Muscle protein synthesis (Ak strain transforming (Akt)/mechanistic target of rapamycin (mTOR)), sarcopenia, preserves muscle mass in aging [18, 19]. | Stimulating the processes of fibre-type specificity and contraction mode-dependent key pathways, including mechanistic target of rapamycin complex 1 (mTORC1), AMP-activated protein kinase (AMPK), and the ubiquitin–proteasome system [20]. |
| High-intensity interval training | Short intense bursts (e.g., 1 min sprint/2 min rest, 8–10×/week for 2 weeks). | FGF21, |
AMPK/SIRT1 (Sirtuin 1) activation, metabolic flexibility, age-related insulin resistance [21, 22]. | Increased aerobic capacity (VO(2) max) adaptations in hypoxia [23]. |
| Swimming | Forced swimming (30–60 min/day, 5 days/week). | Interleukin-6 (IL-6), IL-15, heat shock protein 70 (HSP70) | Antioxidant defenses (SOD2, catalase), NF-κB-driven inflammation and cellular senescence [24, 25]. | Enhanced mitochondrial activities in adipocytes and liver tissues, upregulate fibronectin type III domain containing protein 5 (FNDC5), fibroblast growth factor 21 (FGF21), and brain-derived neurotrophic factor (BDNF) in serum [26]. |
Murine systems have proven indispensable for mapping cross-organ effects of
exercise. However, rodent models robustly demonstrate the limitations in explore
benefits of exercise, which could be summarized into three translational chasms
persist: (1) Genetic/sexual dimorphism: it was reported that human populations
exhibit extensive polymorphisms in exercise-responsive genes (e.g., ACTN3 R577X variant affecting muscle performance, PPARGC1A polymorphisms influencing
aerobic capacity reducing mitochondrial content via impaired coactivator
recruitment) [34, 35] or motor nerve injury activates the nuclear factor kappa B
(NF-
While animal models (e.g., rodents, non-human primates) have provided
foundational insights into exercise-mediated systemic benefits (e.g., aging
attenuation, metabolic disease prevention), their translational utility is
fundamentally restricted by interspecies divergence. Key discrepancies in
lifespan dynamics, genomic regulation (e.g., over-activated NLR family pyrin
domain containing 3 (NLRP3) promotes the release of IL-1
PSC-derived organoid systems uniquely address the limitations of traditional models by integrating human specificity, physiological complexity, and systemic interactivity. Leveraging donor-specific genetic and epigenetic profiles, these models capture individualized exercise responses, such as athlete-derived organoids retaining exercise-primed DNA methylation patterns that enhance muscle repair or neuronal plasticity. Their 3D architecture replicates native tissue microenvironments, enabling precise study of exercise-driven processes like mechanosensitive skeletal muscle hypertrophy or neurovascular coupling, which are irreproducible in 2D cultures. Till now, scientists have developed the methods to induce iPSCs into different organoids (Fig. 1), such as ectodermal progenitor-derived brain organoids [44], mesodermal progenitor-derived muscular organoids [45], neuromesodermal progenitor-derived neuromuscular organoids [46], as well as vascular organoids, as well as endodermal progenitor-derived liver organoids [47]. Beyond single-tissue modeling, bioengineered assembloids and organ-on-chip platforms reconstitute cross-organ dynamics, such as liver-muscle metabolic coordination during simulated exercise or cortical-spinal-muscle circuits modeling motor learning. By mirroring human pathophysiology at multiscale resolutions, organoids provide an unprecedented bridge between molecular mechanisms and whole-organism exercise physiology, paving the way for personalized therapeutic discovery.
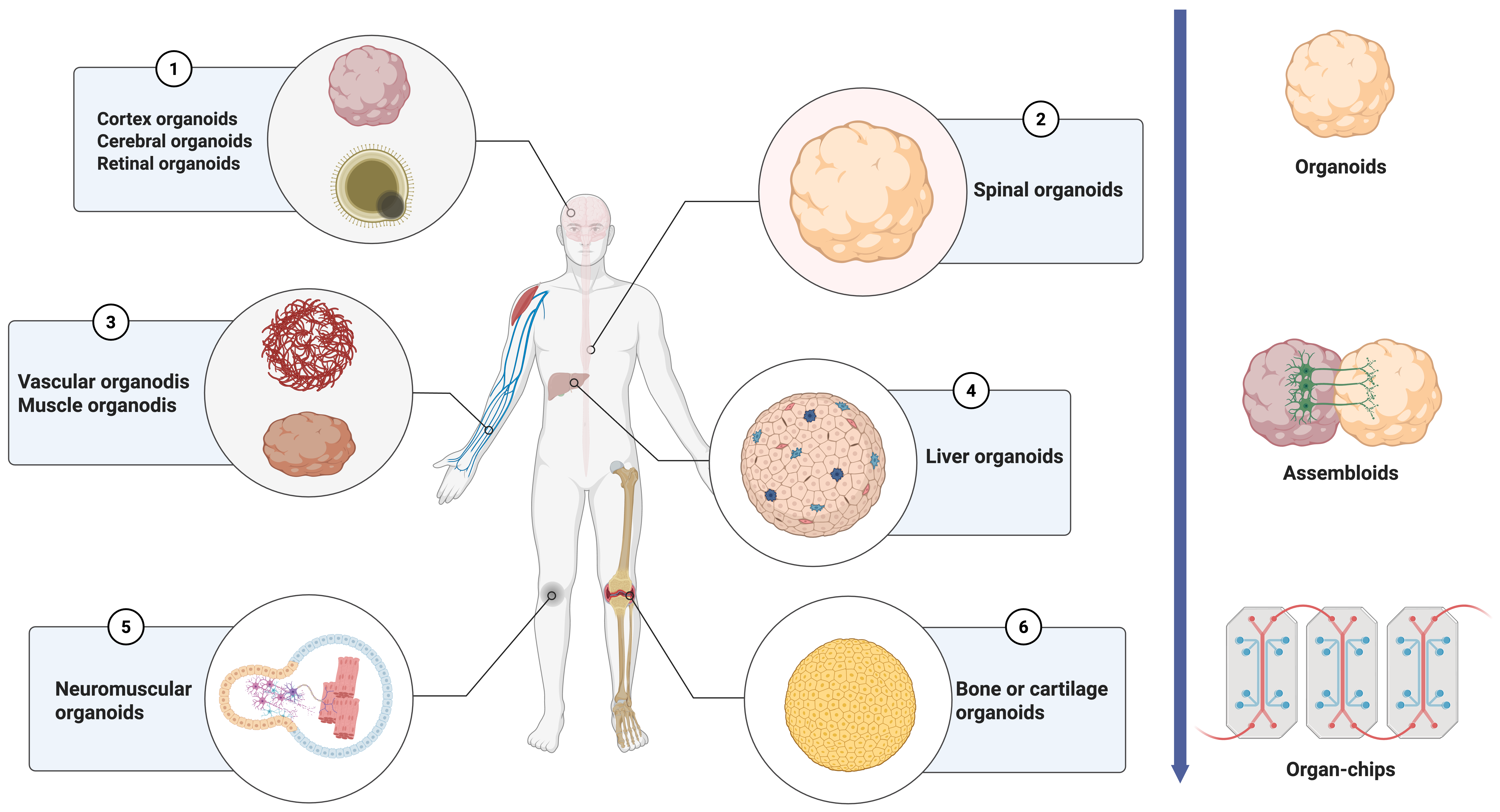 Fig. 1.
Fig. 1.
Summarize possible organoid types for assessing exercise effects in vitro. The diagram describes different types of organoids specifically for human organs or systems. Considering the multiple effects of exercise on human body regulation, organoids for a single system would be insufficient to model in vitro exercise effects. Assembloids mirroring the conjunction organs and organ chips to assess the interaction of multiple distal organs would provide a systematic tool for exercise modeling. Created with BioRender.com.
Organoids derived from iPSCs of athletes may inherit genetic signatures and
epigenetic imprints reflective of exercise adaptation. Mitochondrial dysfunction
and inflammatory responses linked to ATXN2 (encoded by ataxin 2 gene)
polyQ expansions form a pathogenic cycle [48], while exercise remodels skeletal
muscle mitochondria via the PGC-1
The rationale enlightens the application of iPSCs-induced functional cells or organoids to represent the disease phenotypes of the donors’ [51]. Induced motor neurons (iMNs) from iPSCs of sporadic amyotrophic lateral sclerosis (ALS) patients presented a rapid neural degradation compared with control [52]. This study suggests that the functional cells induced from iPSCs of different donors may faithfully recapitulate their inherent in vivo. Herein, athlete-derived iPSCs would be a possible resource to induce organoids to model exercise benefits in vitro. Considering the DNA mutation accumulation during the process of age and iPSCs passages, donors of control iPSCs require comparable age and somatic cell types (fibroblasts, T-cells, or non-T cell PBMCs), together with athlete donors. Such strategies may help to explore the mechanisms of the sport-induced regenerative capacity of the interested tissue. Regular exercise creates a somatic microenvironment conducive to mitigating or preventing the accumulation of DNA mutations [54, 55]. Such a mechanism may result in the improved quality of the iPSCs and thereby show the correlated phenotypes during organoid induction. The improved microenvironment resulting from exercise can also be investigated by transplanting organoids into a rodent host. Healthy or patient-derived organoids transplanted into a host administered with treadmill running or freewheeling may be employed to explore the effects of exercise on body microenvironments. Transplantation of the organoids has been widely reported for the liver, brain, intestine, etc. [56, 57, 58]. Take cortical organoids as an example, extrinsic neurogenesis, axonal growth, and functional neural projection could be used as the readout for evaluating the histocompatibility of organoids for transplanting into different exercise intensity models in comparison with the control test. Enlightened by serum pharmacology, organoids could also be treated with the serum collected from athletes or animals after physical exercise [59]. A recent study found that co-transplantation of autologous regulatory T (Treg) cells with midbrain dopamine neurons derived from human induced pluripotent stem cells suppresses surgical injury-induced neuroinflammation, enhances survival of transplanted dopamine neurons, reduces non-target cell proliferation, and improves motor function in Parkinson’s disease rodent models [60]. Collectively, these advances underscore that athlete-derived iPSC organoids enable multimodal profiling of exercise-adapted epigenomic signatures and their translational potential in regenerative medicine.
Skeletal muscle (SKM) plays a pivotal role in almost all types of exercise. Modeling the habituated muscular movement in organoids would provide a model to investigate the effects of exercise in vitro. Several studies have shown the methodologies to generate the SKM organoids from iPSCs [61, 62, 63]. Maffioletti et al. [64] created SKM organoids from iPSCs with a scaffold to track the SKM contraction and length of SKM from patients with muscular dystrophy. The use of the bioengineering scaffold manufactured with 3D printing approaches would provide a control or sensory device for the strength of SKM [65]. In addition to the scaffold-based approach, optogenetics offers an alternative strategy for modulating SKM movement with precise temporal control. Moreover, the rhythm of the movement signals could be generated from the neurons projecting to SKM in vivo. Andersen et al. [66] produced assembloids that conjunct the SKM organoids with spinal cord organoids and cortical organoids to mimic the cortical-spinal-SKM axis. Via induction of the neuromesodermal progenitors, neuromuscular organoids to duplicate the trunk spinal neural projection to SKM could be generated for evaluating neuromuscular junction diseases [46, 67]. The same approaches were used in the self-organized neuromuscular junction organoids at a 2D level, which facilitated the image-based phenotypic screening with high content system (HCS) [68]. Notably, retroviral tracing of the neural projections from the central nervous system (CNS) to SKM provides a method to evaluate the projection of the CNS neural to SKM. Electrical stimulation by microelectrode array (MEA) platforms would also provide stimulation to the SKM movement. The parameters of the MEA for stimulation require a reference to the inner rhythm of the SKM, whether it is quiescent or in motion. Current studies focus on assessing the oscillation of brain organoids in vitro [69, 70, 71]. Those studies provide an important reference for selecting the readout to mimic exercise-regulated neural activities in the CNS.
Assembloids or SKM organoids connect neurons and can only provide a tool for understanding the direct effects of exercise on neural and SKM. In contrast to systematic cross-organ functions of exercise, combining multiple organoids into a chip with a 3D-printed microfluidic instrument may be an alternative solution. This organ chip helps to establish a systematic system to assess the impact of the metabolism of SKM exercise on other organs [72].
Treating serum from athletes or animals after exercise training on wild-type organoids could be another way to develop an in vitro exercise organoid model. Proteomics and metabolomics offer insights into the bioactive components stimulated by ongoing exercise training. Single-cell transcriptomics provides new insights into how neural mechanisms are regulated [11]. Task-driven neural network modeling algorithms that use motion capture data, biomechanical simulation, and representational learning for training introducthletes or animals after exercise training on wild-type organoids could be another way to develop an in vitro exercise organoid model.
Fundamental research on the biochemical mechanisms of exercise provides an in vitro inducer to mimic exercise. These biochemical changes include oxidative stress, metabolites, hormones, inflammatory components, and neurotransmitters, which could alter the biological functions of organoids [11, 12, 13, 14]. Therefore, to replicate the effects of exercise on organoids, the causality between exercise and the aforementioned inducers should be confirmed as highly relevant. For organoids containing SKM tissue, directly adding acetylcholine can induce increased SKM contraction, thus modeling physical exercise in vitro [15]. Elevated oxidative stress is another key effect of physical activity [16]. It has been shown that increased reactive oxygen species (ROS) in neural stem cells promotes neural differentiation, which may relate to the role of exercise in enhancing adult neurogenesis [17]. Other hormones, such as insulin-like growth factor 1 (IGF-1), adiponectin, and glucagon-like peptide-1 (GLP-1), are well-established for their roles in linking exercise to their effects on multiple organs [11, 12, 72]. Exercise mimetics could serve as a method to induce exercise effects in vitro. Through integrated multi-omics analysis, Geng et al. [18] discovered that exercise-driven betaine enrichment confers geroprotection against age-related health decline in mice. Consequently, treating organoids with these components may offer a partial biological model of exercise in vitro.
Organoids serve as a groundbreaking platform for exploring the anti-aging effects of exercise at the cellular and molecular levels. By combining relevance to humans with experimental flexibility, they can fill critical gaps left by animal models and speed up the development of exercise-based therapies for aging. Future efforts should focus on increasing organoid complexity, integrating multi-omics data, and confirming findings in clinical groups.
Exercise is a complex regulation of the whole-body system. An in vivo exercise model may provide an overview of the biological underpinnings at the whole-body level. Human-derived tissue types are highly required for a deep understanding of the dual nature of the exercise. Most human-primary tissue, particularly the brain, spinal cord, liver, and kidney, is difficult to harvest. The advantage of the organoids from iPSCs provide a 3D in vitro model for most organ systems. More importantly, iPSCs from sportsmen or normal subjects could be biobanked for further induction and analysis. Systematic analysis of the sportsmen’s serum by multiple omics approaches may obtain an innovative component triggered by exercise (Fig. 2). Consecutive passage of iPSCs could gain a sufficient sample number of organoids for high-throughput screening, which can select bioactive components in serum from the subject of exercise.
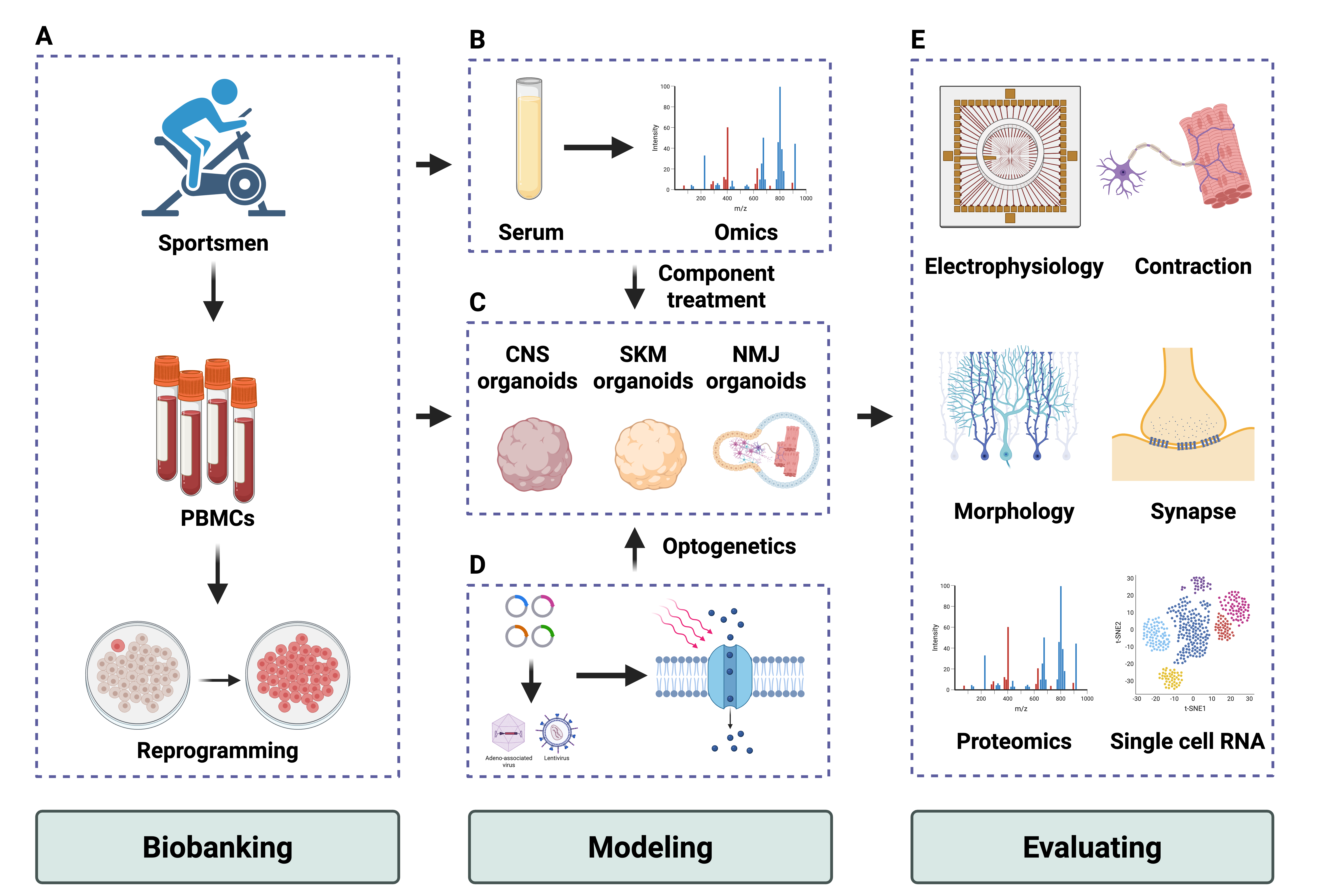 Fig. 2.
Fig. 2.
Strategy to establish the exercise model in vitro with organoids. The diagram illustrates the possible strategies for developing the in vitro organoids exercise model. (1) PBMCs or fibroblasts from healthy sportsmen could be reprogrammed into iPSCs and biobank (A). Meanwhile, bulk serum or bioactive compounds from sportsmen could be a trigger for modeling exercise effects (B). (2) iPSCs could generate organoids, including CNS, SKM, and NMJ-specific models (C,D). etc. (3) Benchmarks of the organoids, including electrophysiology, contraction functions, morphologies, and multiple omics, can provide the readouts for evaluating exercise effects (E). Created with BioRender.com. PBMC, Peripheral blood mononuclear cell; iPSCs, induced pluripotent stem cell; CNS, central nervous system; SKM, skeletal muscle; NMJ, neuromuscular junction.
Single-cell RNA and spatial transcriptome provide an overview of biological events correlating with exercise, including cell diversity and in situ signaling. Comparison of the bioinformatic profiles with the in vivo exercise model may enhance the validation of the organoids for modeling exercise. Moreover, the transplantation of organoids into exercise subjects may also provide insight into how exercise enables the microenvironment regulation of human tissue. Organ chips with microfluidic support could be used to assess the precision of the exercise’s impact on organ-specific levels. With the development of multiple omics for deep decoding of the bioactive compounds induced by exercise in vivo and the optimization of the organoid’s induction, the in vitro exercise model field would mature as a tool in precise and alternative medicine.
LMX, YW, CG, and LNS provided the conception of the review. LMX and YW wrote the manuscript, designed the table, and the figures. CSX, JQL, WJX, MTH, JHW, and JYP participated in the article search work. LNS and CG provided funding support and supervised the manuscript writing. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was funded by Open Research Fund of the State Key Laboratory of Cognitive Neuroscience andLearning (CNLZD2104 to LS), the Natural Science Foundation of China (82474336 to GC), the Shenzhen Medical Academyof Research and Translation (Grant No. D2401026).
The authors declare no conflict of interest.
In this manuscript, AI-assisted technology was used for checking grammatical and spelling errors. The corresponding author takes full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
