- Academic Editor
As an evolutionarily conserved timekeeping system, the circadian clock orchestrates physiological adaptations to diurnal environmental cues through transcriptional-translational feedback loops (TTFLs). Accumulating evidence reveals that circadian regulation governs immunological processes, with the immune system—a critical host defense mechanism—exhibiting robust circadian rhythmicity in functional organization. This review synthesizes recent advances in circadian modulation of pathogen-host interactions, immune cell trafficking, effector functions, circadian light hygiene—gut immune crosstalk, and tumor immunobiology. We examine the bidirectional crosstalk between circadian oscillators and immune pathways while addressing the clinical implications for immune-related pathologies. Significantly, we advocate chrono-immunotherapy as a transformative paradigm that leverages circadian principles to optimize therapeutic timing, enhancing efficacy while minimizing adverse effects. Future research directions aimed at elucidating mechanistic foundations and accelerating clinical translation are outlined. A comprehensive understanding of circadian-immune system dynamics not only provides fundamental insights into biological regulation but also establishes a chronobiological framework for precision medicine in immune-mediated disorders.
The circadian rhythm constitutes an endogenous ~24-hour biological oscillator that enables organisms to entrain physiological processes to periodic environmental cues (e.g., photic and thermal fluctuations), thereby optimizing adaptive behaviors and maintaining metabolic homeostasis—a fundamental biological mechanism critical for health maintenance and disease prevention [1]. This phylogenetically conserved timekeeping mechanism is ubiquitously observed across taxonomic kingdoms, from prokaryotic cyanobacteria to complex mammalian systems [2].
At the molecular level, mammalian circadian regulation operates through a hierarchically organized network of cellular oscillators. The suprachiasmatic nucleus (SCN) of the hypothalamus functions as the central pacemaker, coordinating peripheral clocks through integrated neural and humoral signalling pathways [3, 4]. The core molecular mechanism involves transcription-translation feedback loops (TTFLs), wherein clock gene products autoregulate their expression through sequential post-translational modifications, including phosphorylation and regulated nuclear translocation [4]. Notably, while circadian systems arose through convergent evolution in diverse taxa, they exhibit conserved architectural principles characterized by autoregulatory genetic networks mediated by Per-Arnt-Sim (PAS) domain-containing transcriptional regulators [5]. The groundbreaking work of Nobel laureates Hall, Rosbash, and Young, utilizing Drosophila melanogaster models, identifying period gene-encoded components as essential for establish the autonomous circadian oscillator [6].
Accumulating evidence demonstrates that circadian rhythms represent a fundamental regulatory layer governing virtually all biological processes [7]. Circadian disruption has been etiologically linked to numerous metabolic and endocrine disorders, including obesity, metabolic syndrome, obstructive sleep apnea (OSA), and type 2 diabetes mellitus [8, 9, 10, 11, 12]. This pervasive regulatory influence highlights the circadian system’s integral role in maintaining cellular homeostasis, coordinating physiological functions, and integrating metabolic-endocrine signaling, thereby revealing novel therapeutic targets for associated pathologies [7].
Of particular relevance, circadian oscillations exert precise temporal control over immune system function, modulating host defense mechanisms against inflammatory processes and pathogenic challenges from bacterial, parasitic, and viral agents [13, 14, 15]. Notably, viral replication cycles exhibit distinct temporal regulation patterns, with certain pathogens employing chronobiological strategies to establish either acute or persistent infections [14]. These findings underscore the critical importance of elucidating circadian-immune system interactions in contemporary biomedical research.
This review synthesizes current knowledge regarding circadian regulation of immunological processes. We systematically evaluate: (1) circadian-mediated modulation of host-pathogen interactions; (2) temporal control of leukocyte functional dynamics; (3) circadian light hygiene and gut-immnue crosstalk; and (4) chronobiological regulation of anti-tumor immunity. Furthermore, we examine the clinical implications of circadian biology in immunotherapeutic strategies and chronotherapeutic interventions through a translational medicine perspective. Finally, we identify key research directions to advance understanding of circadian-immune crosstalk, with particular emphasis on mechanistic studies of temporal regulation and its therapeutic applications in precision chrono-immunology.
The mammalian circadian timing system comprises three functionally integrated components: (1) input pathway that transduce environmental signals (e.g., light-dark cycles) to the central pacemaker; (2) a central pacemaker generating self-sustained oscillations; and (3) output pathway that regulate rhythmic physiological, metabolic, and behavioral processes [16]. While the suprachiasmatic nucleus (SCN) of the hypothalamus functions as the dominant circadian pacemaker in mammals, autonomous circadian oscillators exist in peripheral tissues [4]. The SCN synchronizes these peripheral clocks through coordinated neuroendocrine and autonomic nervous signaling [6]. Environmental zeitgebers (“time-givers”), particularly photic stimuli and feeding cycles, entrain the central oscillator, which in turn phase-locks peripheral oscillators via coupled transcriptional-translational feedback loops (TTFLs) [17] (Fig. 1).
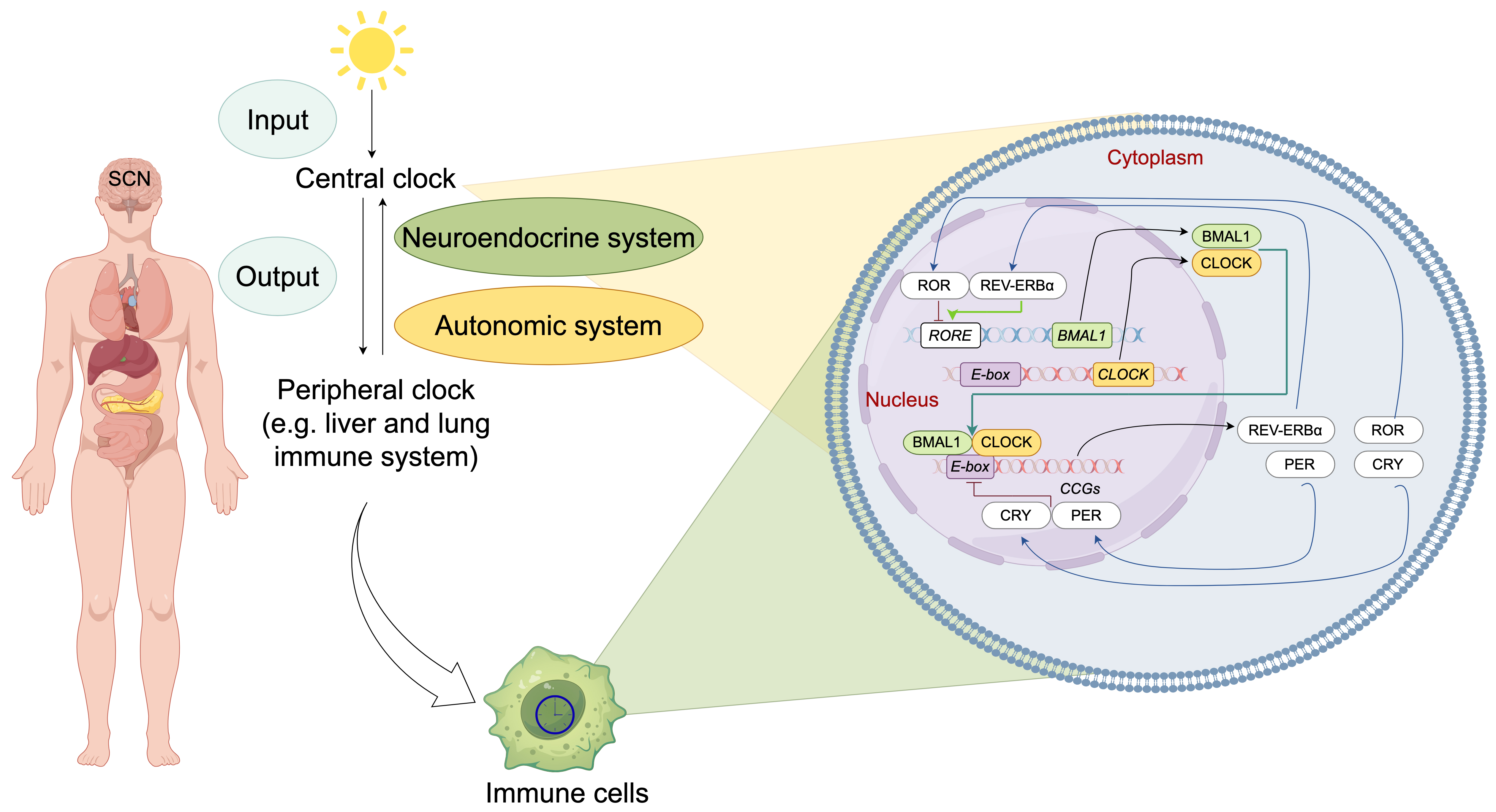 Fig. 1.
Fig. 1.
Molecular architecture and systemic organization of the
mammalian circadian clock system. The mammalian circadian timing system operates
through a hierarchically organized network, with the central oscillator in the
suprachiasmatic nucleus (SCN) serving as the master pacemaker. The SCN integrates
photic input and synchronizes peripheral oscillators through dual efferent
pathways: (1) autonomic neural signaling and (2) neuroendocrine regulation. At
the molecular level, cell-autonomous circadian rhythms are generated by
evolutionarily conserved transcriptional-translational feedback loops (TTFLs)
comprising core clock components: CLOCK-BMAL1 heterodimers function as primary
transcriptional activators by binding to E-box enhancer elements (CACGTG) in
promoter regions, thereby initiating the expression of core clock repressors
Period [PER1-3] and Cryptochrome [CRY1-2] and numerous
clock-controlled genes (CCGs). Accumulated PER-CRY protein complexes form
repressive complexes that inhibit CLOCK-BMAL1 transcriptional activity, thereby
inhibit their own transcription. Concurrently, CLOCK-BMAL1 activates
transcription of nuclear receptors (REV-ERB
At the molecular level, cell-autonomous circadian oscillators in both SCN
neurons and peripheral cells share a conserved core mechanism centered on
interlocking TTFLs [4] (Fig. 1). In the primary transcriptional loop, the core
transcriptional activators circadian locomotor output cycles kaput (CLOCK) and
brain and muscle ARNT-like 1 (BMAL1) form heterodimers that bind E-box regulatory
elements, driving transcriptional activation of numerous clock-controlled genes
(CCGs) [11, 18]. Period (PER1-3) and Cryptochrome
(CRY1-2) genes, transcripted by CLOCK and BMAL1 heterodimers,
accumulate progressively to repress their own transcription, establishing a
negative feedback mechanism [19]. Additionally, the CLOCK-BMAL1 complex regulates
nuclear receptor superfamily members, including reverse orientation c-erbA gene
(REV-ERB
The circadian-immune axis exhibits bidirectional crosstalk that profoundly modulates host defense mechanisms against bacterial, parasitic, and viral infections, with significant implications for inflammatory pathogenesis [21].
Experimental studies using Salmonella enterica serovar Typhimurium
infection mice models demonstrate circadian gating of immune responses, with
enhanced antibacterial activity during the active phase (zeitgeber time [ZT]
0–12) and attenuated responses during rest (ZT12–24) [13]. Notably,
infection-responsive circadian oscillations in antimicrobial peptide genes (e.g.,
Reg3
BMAL1 deficiency in macrophages exacerbates LPS-induced sepsis in mice [22]. Mechanistically, BMAL-deficient macrophages exhibit upregulatiton of C-X-C motif chemokine ligand 2 (CXCL2), which enhances polymorphonuclear neutrophils recruitment and neutrophil extracellular traps (NETs) formation via binding to the chemokine receptor C-X-C motif chemokine receptor 2 (CXCR2), thereby exacerbating lung injury in sepsis model [22]. Additionally, BMAL1 deficiency has been linked to aggravated Porphyromonas Gingivalis-induced atherosclerosis via promotion of oxidative stress [23], and BMAL1 dysfunction in circadian-disrupted animal model intensifies inflammatory response to Helicobacter pylori infection [24]. Furthermore, BMAL1 regulates coagulation factor biosynthesis in the mouse liver during Streptococcus oralis infection [25].
Melatonin, an indoleamine with circadian rhythmicity in pineal gland synthesis and secretion, orchestrates immune modulation via pleiotropic pathways and exhibits broad-spectrum antibacterial activity [26, 27]. For instance, melatonin treatment has been shown to protect to hippocampal neurons in rats infected with Klebsiella pneumoniae [28]. Its immunomodulatory adjuvant effects have been investigated in patients with Helicobacter pylori [29, 30], Mycobacterium tuberculosis [31], and Clostridium perfringens infections [32]. Melatonin administration also attenuates sepsis-induced spleen apoptosis and reduces inflammatory infiltration in multiple tissues [26].
Host circadian regulation of immune functions plays a decisive role in modulating the progression and outcome of parasitic infections [33]. For example, circadian rhythmicity of immune system has been shown to influence developmental cycles of Plasmodium species within erythrocytes by temporally controlling parasite maturation [34]. Similarly, infection kinetics of the helminth Trichuris muris exhibit time-of-day-dependent variations in worm expulsion rates, a phenomenon mediated by circadian regulation of dendritic cell [35]. In Leishmania major infections, macrophage- and neutrophil-expressed BMAL1 orchestrates infection susceptibility through dual mechanisms: modulating cellular recruitment via chemokine expression rhythms and establishing circadian control over parasitic invasion efficiency [15].
Experimental evidence indicates that pinealectomy or the administration of the melatonin receptor inhibitor luzindole desynchronizes the cell cycle of Plasmodium falciparum and reduce parasitemia [36]. Erythrocytes exposed to melatonin or related indolic compounds exhibit an increase in P. falciparum multinucleated forms [37], whereas high doses melatonin treatment prevents hepatocyte apoptosis induced by malaria infection in mice [38]. Melatonin administration has also been shown to reduce hepatic necrosis areas in Entamoeba histolytica infected hamsters [39], decrease blood trypomastigotes counts in Trypanosoma cruzi infected rats [40], suppress cholangiocarcinoma and liver injury in Opisthorchis viverrine-infected hamsters [41], and enhance cellular immunity by stimulating CD4+ and CD8+ cell production in Toxoplasma gondii-infected rats [42].
The replication, transcription, and translation of viral particles are inherently contingent upon host cellular machinery [14]. Experimental studies have established that CLOCK histone acetyltransferase complex is integral to transcriptional regulation of herpes simplex virus (HSV) throughout its replicative cycle, whereby CLOCK knockdown significantly attenuates viral gene expression [43]. Circadian regulatory systems exert dual mechanistic effects on viral pathogenesis: they not only govern the expression of host factors essential for viral replication [44, 45], but also modulate host inflammatory responses that dictate influenza infection outcomes [46].
In bone marrow-derived macrophages (BMDMs), BMAL1 has been identified as a
critical regulator of cell-specific innate immune defenses against respiratory
syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) [47].
Bmal1-deficient embryonic fibroblasts exhibit altered viral
susceptibility, with enhanced replication efficiency of RSV and PIV3 [47].
Accumulating evidence shows that NLRP3 inflammasome activation during SARS-CoV-2
infection is significantly associated with COVID-19 disease severity [48, 49].
Noably, Rev-erb
Melatonin modulates immunometabolic processes through multi-target mechanisms: enhancing BMAL1-mediated regulation of mitochondrial bioenergetics [52], activating Sirtuin-3/SOD2 axis to optimize tricarboxylic acid cycle flux and oxidative phosphorylation efficiency [53]. Thereby supporting antiviral responses against Venezuelan equine encephalitis (VEE), respiratory syncytial virus (RSV), and Ebola virus infections [54, 55, 56]. This indoleamine may also reduce the risk of and aid in the treatment of COVID-19 and other RNA viral infections [57].
The circadian modulation of pathogen-host interactions highlights its systemic impact on immune regulation. Beyond infections diseases, the circadian clock further orchestrates immune cell dynamics, as discussed below.
The circadian clock system not only orchestrates the body’s defense response to
pathogenic invasions but also exerts profound regulatory effects on both innate
and adaptive immune responses by temporally controlling immune cell populations
and their functional dynamics (Table 1, Ref. [13, 15, 19, 22, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101]).
Emerging evidence indicates that circadian rhythmicity governs immune cell
trafficking, spatial organization, clonal expansion, lineage commitment, and
functional competence through integrated mechanisms involving intrinsic cellular
oscillators and extrinsic microenvironmental zeitgebers [21, 58, 59, 102]. At the
molecular level, core clock components (e.g., BMAL1 and CLOCK) directly regulate
the transcriptional activity of immunologically relevant genes, including those
involved in NF-
| Gene | Model | Tissue/cells | Immune function | References |
| Bmal1 | Bmal1-/- | Peripheral blood, spleen | Reduced B cell numbers, impaired B cell development | [60] |
| Neutrophils, macrophages | Loss of time-dependent neutrophil and macrophage infiltration after Leishmania infection | [15] | ||
| Bmal1fl/fl; Lyz2-Cre | Neutrophils, macrophages | Upregulated CXCL2 expression, increased recruitment of polymorphonuclear neutrophils and the formation of neutrophil extracellular traps (NETs) after Sepsis-induced acute lung injury | [22] | |
| Macrophages | Increased IL-6, TNFα after LPS stimulation | [61, 62, 63] | ||
| Increased ROS, HIF1a, Il1β, and Il6 and after LPS stimulation | [64, 65] | |||
| Increased proinflammatory miR-155 cluster | [63] | |||
| Increased atypical inflammasome-mediated pyrodeath and lethality | [66] | |||
| Increased the expression of PD-L1 | [67] | |||
| Increased mitochondrial dysfunction | [68] | |||
| Monocytes | Increased proinflammatory cytokines after L. monocytogenes infection | [59] | ||
| Neutrophils | Enhanced April expression; immature of neutrophils when treated with TLR7/8 agonist | [69] | ||
| Bmal1fl/fl; hMRP8-cre | Neutrophils | Impaired clearance of aged neutrophils | [70] | |
| Bmal1fl/fl; CCSP-iCre | Club-cell | Loss of rhythmic expression of CXCL5 and enhanced neutrophil responses to LPS | [58] | |
| Bmal1fl/fl; ERCre+ | Lung fibroblasts | Increased CXCL5 expression, exacerbated neutrophil recruitment and elevated NF-κB signaling activity | [71] | |
| Bmal1fl/fl; Cd4-Cre | Lymphocytes | Ablated the overall rhythmicity of T cell and B cell numbers in lymph nodes (LNs); | [72] | |
| Bmal1fl/fl; xCd19-Cre | Loss of oscillation in CCR7 and impeded lymphocyte homing; | |||
| Loss of oscillations in S1pr1 and lymphocyte egress | ||||
| Rorc-Cre | ILC3s | Reduced ILC3 subsets and IL-17- and IL-22-producing ILC3; | [73, 74] | |
| Altered diurnal patterns of Proteobacteria and Bacteroidetes; | ||||
| Reduced expression of Reg3b, Reg3g, Muc3 and Muc13 | ||||
| Clock | ClockΔ19/Δ19 | T cells | Loss of the circadian rhythm of T-cell proliferation following T-cell receptor stimulation | [75] |
| Macrophages | Reduced secretion of IL-6 and TNF-α and induction of Il-6, Il-1β, Tnfα, Cxcl-1, Ifn-β, and Che-mokine (C-C motif) ligand 2 (Ccl2) in response to LPS stimulation or Salmonella infection | [13] | ||
| Expressed higher levels of scavenger receptors and impaired cholesterol metabolism and enhanced atherosclerosis | [76] | |||
| Hepatocytes, MEFs | Reduced NFκB activity | [77] | ||
| MEFs | Reduced inflammatory cytokines after LPS | [78] | ||
| BMMCs | Loss of the circadian rhythm of IL-33-mediated IL-6, IL-13, and TNF-α production | [79] | ||
| Loss of time-dependent variation in IgE-mediated allergic reactions | [80] | |||
| Skin γ/δ T-cell | Reduced expression of IL-23R | [81] | ||
| Per1 | Per1-/- | Splenic NK cells | Loss of the circadian rhythm of IFN-γ, perforin, and granzyme B | [19] |
| Monocytes/macrophages | Increased Ccr2 expression levels; Increased TNFα, IL-1β, IL-6, MCP-1 after LPS treatment | [82] | ||
| siRNA | T cells | Increased the protein secretion of Th1 cytokines, such as IFN-γ and IL-2 and partially rescued the hydrocortison-induced effects on Th1 differentiation | [83] | |
| Per2 | Per2Brdm1 | Macrophages | Decreased levels of Tlr9 mRNA and reduced TNFα, IL-12 expression after CpG ODNs (TLR9 ligand) induction | [84] |
| Increased IL-6 after lipoteichoic acid treatment and loss of IL-6 rhythms | [85] | |||
| BMMCs | Loss of diurnal anaphylactic cutaneous reaction | [86] | ||
| Per2-/- | Splenocytes | Reduced expression of IFN-γ, IL-1β after LPS treatment | [87] | |
| Cry | Cry1-/-; Cry2 -/- | Fibroblasts | Increased expression of IL-6, TNF-α, and iNOS and activated NF-κB and PKA signaling | [88] |
| Macrophages | Increased expression of IL-6, Cxcl1, and iNOS | |||
| MEFs, Splenocytes | Increased T cell cellularity; | [89] | ||
| Increased TNFα levels; | ||||
| Increased LPS hypersensitivity | ||||
| Rorc | Rorc-/- | T cells | Reduced severity of experimental autoimmune encephalomyelitis (EAE) and absence of tissue-infiltrating Th17 Cells | [90] |
| Lymphoid tissue | Thymocyte development and lymphoid organogenesis | [91, 92, 93] | ||
| Increased apoptosis of thymocytes and lack lymphnodes and Peyer’s patches; | [94, 95] | |||
| Impaired TCRα repertoire | ||||
| Rorα | Rorα-/- | Lymphocytes | Defective T and B cell development; | [96] |
| Increased IFN-γ production after TCR stimulation | ||||
| BMMCs | Increased amount of TNF-α and IL-6 upon activation | [96] | ||
| Macrophages | Increased amount of TNF-α and IL-6 upon activation | [96] | ||
| Rorα-/-; Rag2-/- | Serum | Elevated serum IgG levels were | [96] | |
| Rev-erbα | Rev-erbα-/- | T cells | Increased expression of Nfiil3, reduced intestinal Th17 cell frequencies and increased susceptibility to inflammatory disease | [97] |
| Macrophages | Activation of the NF-κB/NLRP3 axis and increased the severity of colitis | [98] | ||
| Increased the expression of Ccl2 | [99] | |||
| Diminished LPS-evoked IL-6 response rhythm | [62] | |||
| Rorc-Cre | ILC3s | Perturbed enteric ILC3 subsets | [73, 74] | |
| Agonists | Macrophages | Inhibited IL-6 protein secretion; Reduced expression of Il-6, Cxcl11, Ccl2, Cxcl6, Il19 | [62] | |
| Suppressed IL-1β production | [100] | |||
| Attenuates DSS-induced colitis | [98] | |||
| Suppressed the expression of Ccl2 | [99] | |||
| BMMCs | Inhibited IgE and IL-33 mediated activation | [101] |
Notes: ILC3, Group 3 innate lymphoid cells; MEF, mouse embryonic fibroblast; BMMCs, one marrow–derived cultured mast cell.
Neutrophils represent 40–70% of human leukocytes and function as key effectors in innate immune defenses against bacterial and fungal pathogens [104]. During Leishmania infection, the host cellular circadian clock modulates expression of CD206 and CD11b—surface markers mediating pathogen adherence and phagocytosis—thereby orchestrating the circadian-dependent recruitment of neutrophils and proinflammatory CD206+ peritoneal macrophages to infection sites [15]. In pulmonary inflammation, neutrophil infiltration exhibits circadian periodicity driven by rhythmic CXCL5 secretion from lung epithelial cells [58, 71, 105].
Sepsis models reveal that macrophage-specific Bmal1 deficiency upregulates CXCL2 expression, promoting CXCR2-mediated polymorphonuclear neutrophil recruitment and neutrophil extracellular trap (NET) formation, which exacerbates pulmonary damage [22]. Bmal1-deficient neutrophils exhibit impaired senescence and dysregulated proinflammatory responses, potentially contributing to the pathogenesis of systemic lupus erythematosus [69]. Additionally, hypothalamic regulation of neutrophil phagocytic activity depends on both endogenous melatonin rhythms and exogenous photoperiod cues [106, 107]. Melatonin protects neutrophils from oxidative stress-induced apoptosis and restores functions such as phagocytosis, degranulation, and NETosis in glutathione (GSH)/glutathione reductase (GR)-deficient neutrophils by regulating reactive oxygen species (ROS) levels [108].
Monocytes, the largest leukocytes in peripheral circulation, originate from hematopoietic stem cell-derived progenitors in bone marrow and serve as precursors for tissue macrophages and dendritic cells. These phagocytic cells play critical roles in eliminating cellular debris and senescent cells via efferocytosis. The inflammatory Ly6C+hi monocyte subset exhibits circadian-regulated trafficking patterns mediated by chemotactic signaling pathways [59]. Specifically, CCR2 receptor interactions with its ligands CCL2/CCL8 drive monocyte trafficking to inflammatory loci. Following Listeria monocytogenes peritoneal infection, the BMAL1-CLOCK heterodimer orchestrates circadian recruitment of polycomb repressive complex 2 (PRC2)—a transcriptional repressor of Ccl2—thereby modulating Ly6C+hi monocyte peritoneal infiltration into peritoneum [59].
Experimental models demonstrate that LPS-induced endotoxic shock exhibits marked
temporal variation in severity [109], with macrophages serving as primary
effectors in innate immune activation [62]. Mechanistic studies show that
TLR4-expressing splenocytes (predominatly monocytes/macrophages) exhibit
circadian-regulated cytokine secretion of TNF-
Bmal1 deficiency induces a pro-inflammatory metabolic shift in
macrophage, characterized by enhanced glycolysis and mitochondrial respiration.
The BMAL1-HIF-1
Lymphocytes display circadian-regulated trafficking patterns between lymph nodes
through coordinated entry and egress mechanisms. The circadian-regulated egress
of lymphocytes reaches its peak during species-specific behavioral rest phases
(diurnal period in mice and nocturnal phase in humans) [72, 115], resulting in
maximal lymph node cellularity during each organism’s active phase [72, 115, 116]. Current evidence identifies two principal regulatory pathways governing
this rhythmic trafficking: (1) Lymphocyte homing mediated by circadian
oscillations in chemokine receptor-ligand interactions (CCR7/CCL21 and
CXCR4/CXCL12), and (2) Lymphocyte egress controlled by rhythmic
sphingosine-1-phosphate receptor 1 (S1PR1) expression [72]. Circadian
coordination occurs through synchronized peak expression of CCR7 on T/B
lymphocytes and its ligand CCL21 in high endothelial venules (HEV) [72]. Genetic
ablation of Bmal1 in CD4+ T cells abolishes both mRNA expression
and surface protein oscillations of CCR7 [72]. Notably,
Experimental evidence shows that Mop3-/- (Bmal1 knockout) mice exhibit significant reductions in B-cell populations within peripheral blood, splenic, and bone marrow compartments, whereas T-cell numbers remain unaffected [60]. Notably, lethally irradiated BALB/c Rag2-/- recipients reconstituted with Mop3-/- bone marrow cells (BMCs), as well as B-cell-specific Bmal1-deficient murine models, display no significant defects in B-cell differentiation or functional capacity [60, 119]. Conversely, transplantation of wild-type BALB/c BMCs into irradiated Mop3-/- recipients impairs B-cell developmental progression [60]. Notably, BMAL1 expression peaks during the early light phase in murine bone marrow, temporally coinciding with enhanced proliferation and differentiation signals in B-cell progenitors. This rhythmic BMAL1 expression may serve as a physiological gatekeeper, aligning bone marrow niche activity with systemic cues to optimize B cell output. Thus, the reduction in B cell numbers observed in Bmal-knockout models not only reflects a loss of gene function but also signifies the disruption of this tightly regulated temporal framework [59, 60, 72].
Naive CD4+ T lymphocytes differentiate into Th17 effector cells,
specialized producers of interleukin-17 (IL-17) that mediate proinflammatory
immune responses [120]. The nuclear receptor ROR
Circadian regulation is manifested through reciprocal expression patterns:
Nfil3 transcription oscillates with nocturnal predominance, whereas
Rorc expression peaks during photophase, establishing an anti-phase
relationship [97]. This temporal control is mechanistically linked to circadian
clock components: REV-ERB
Host circadian rhythms are intricately intertwined with the gut microbiome, which exhibits diurnal oscillations in community composition, gene expression, and metabolite production [124, 125]. These microbial rhythms-particularly in the biosynthesis of short—chain fatty acids (SCFAs) like butyrate and acetate—modulate host immune regulation by altering histone acetylation, promoting Treg differentiation, and influencing dendritic cell activation.
Environmental light cues, especially photoperiod length and nocturnal light pollution, are critical for synchronizing both host and microbial circadian clocks. Disruption in light hygiene (e.g., shift work, screen light exposure, irregular sleep) has been shown to reduce microbiota diversity, decrease SCFA levels, and compromise intestinal epithelial integrity [126, 127]. Such disturbances may exacerbate mucosal inflammation and increase susceptigility to infections or autoimmune disorders.
During the COVID-19 pandemic, reduced outdoor light exposure, social isolation, and disrupted sleep cycles led to widespread circadian misalignment. Concomitantly, melatonin suppression, gut dysbiosis, and increased intestinal permeability were observed in numerous patients and convalescents [128]. Emerging evidence further indicates that post-COVID individuals exhibit heightened sensitivity to light cues, highlighting long-term vulnerability of the light–microbiome–immune axis [129].
Notably, melatonin serves as a key mediator in this tripartite relationship. Beyond its role in regulating sleep–wake cycles, melatonin modulates gut immune responses by preserving tight junction protein expression, mitigating ROS-induced damage, and restoring microbial eubiosis [130]. Chronobiological interventions designed to maintain microbial rhythmicity—such as timed light exposure, scheduled meal timing, and melatonin supplementation—hold promise for alleviating post-infectious or stress-induced immune dysregulation.
The circadian clock acts as a pivotal regulator of systemic homeostasis, and its dysregulation promotes tumorigenesis and malignant progression through chronoimmunological perturbations, thereby increasing cancer risk [131, 132]. Murine models exemplify the empirical validation of circadian oncology principles, where the timing of tumor implantation dictates neoplastic growth dynamics, underscoring the temporal dependency of oncological processes [133].
Circadian rhythm disruption (CRD) induces multilevel dysregulation of tumor-associated immune cells while fostering a protumoral and immunosuppressive tumor immune microenvironment (TIME) (Fig. 2). This pathological niche, enriched with dysregulated cytokine/chemokine networks, establishes a self-reinforcing loop that accelerates malignant transformation [134, 135]. CRD-mediated erosion of circadian macrophage polarization rhythms significantly reduces the M1/M2 index (proinflammatory/anti-inflammatory) in splenic and tumoral compartments, creating an immunosuppressive landscape conducive to tumor expansion [136]. Furthermore, CRD disrupts transcriptional-translational coordination of perforin in natural killer (NK) cells, impairing circadian cytotoxic rhythms and accelerating tumor immune evasion [137].
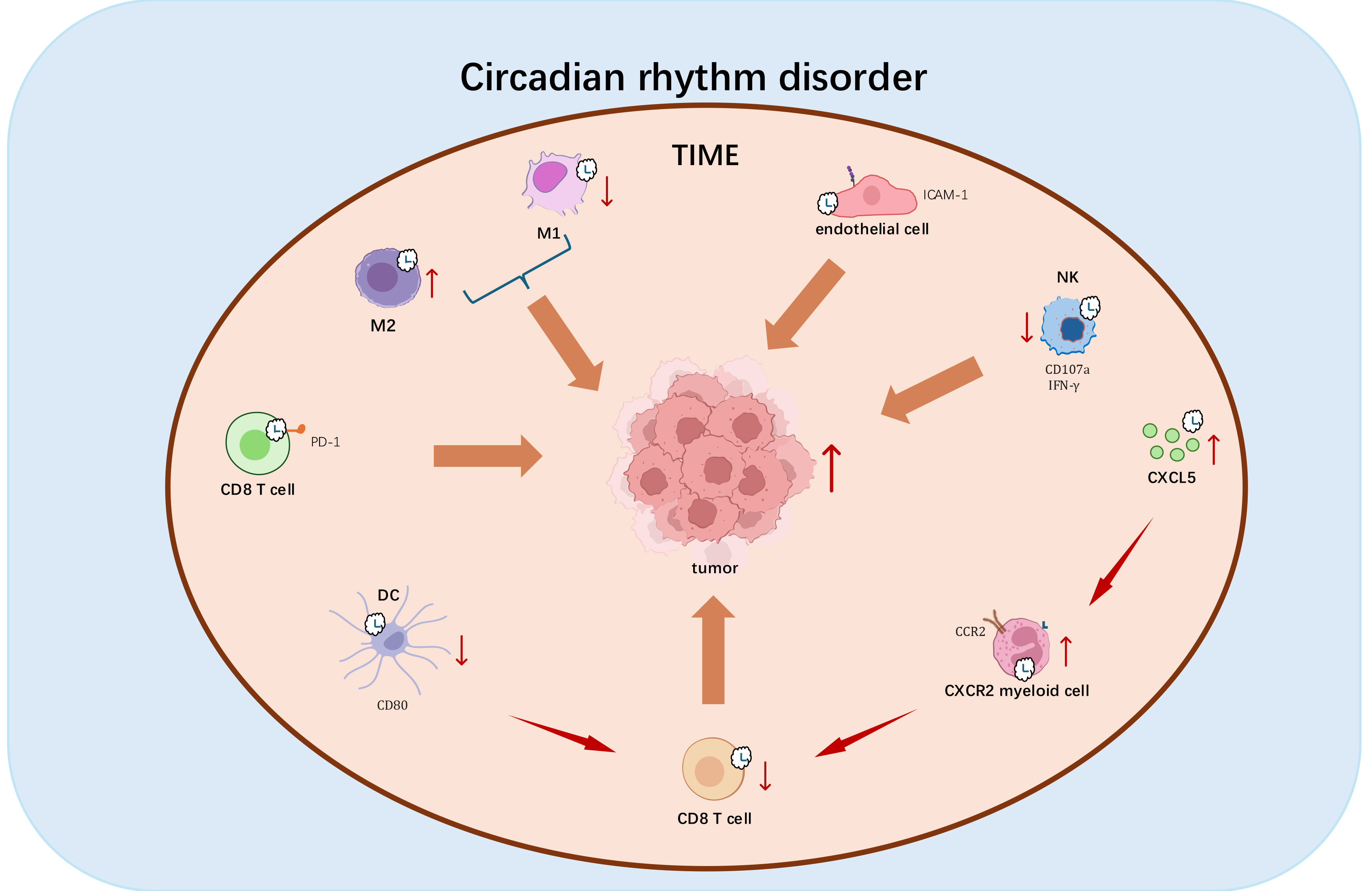 Fig. 2.
Fig. 2.
Mechanistic impact of Circadian rhythm disruption (CRD) on tumorgenesis. CRD exerts multifaceted immunosuppressive effects that promote tumor progression through the following mechanisms: (1) Disrupting M1/M2 macrophage polarization toward tumor-promoting anti-inflammatory phenotypes; (2) Disrupting circadian-regulated perforin expression in natural killer cells, compromising their tumoricidal capacity; (3) Impairing rhythmic ICAM-1 expression, thereby disrupting leukocyte trafficking; (4) Dysregulating circadian surface expression of CD80 and PD-1, attenuating CD8+ T cell activation and potentiating immune checkpoint pathways; (5) Inducing tumor-specific CXCL5 overexpression that facilitates CXCR2+ myeloid-derived suppressor cell recruitment, establishing an immunosuppressive niche; (6) Directly inhibiting cytotoxic T lymphocyte infiltration. These coordinated perturbations in chronoimmunological regulation establish a permissive microenvironment for multifactorial immunosuppression and malignant progression. Red vertical upward arrows indicate cell activation or gene upregulation; red vertical downward arrows indicate cell activity inhibition or gene downregulation; brown arrows indicate promotion. Diagram was assembled in Microsoft PowerPoint.
Emerging evidence highlights TIME modulation and immune infiltration dynamics as circadian-regulated hallmarks of cancer. Endothelial cells exhibit circadian ICAM-1 expression patterns that govern leukocyte trafficking rhythms into tumors [138]. CD8+ T cells demonstrate time-of-day-dependent functional oscillations, transitioning from immunosuppressed morning states to enhanced anti-tumor activity in the evening [138]. BMAL1-mediated circadian control of CD80 in dendritic cells coordinates rhythmic CD8+ T cell priming in tumor-draining lymph nodes, establishing temporal windows for optimal anti-tumor immunity [133]. CRD-induced cytokine network perturbations disrupt leukocyte circadian trafficking, reducing circulatory immune cell numbers and tumor infiltration capacity [139]. Mechanistically, CRD-driven TIME reprogramming involves activation of CXCL5-CXCR2 axis, which recruits immunosuppressive myeloid cells that suppress T cell responses and block CD8+ T cell infiltration [139]. These findings establish the clinical relevance of clock genes and their transcriptional targets (e.g., CXCL5, CD80) as prognostic chronotherapeutic biomarkers in oncology [140, 141, 142, 143].
Collectively, circadian regulatory networks represent a fundamental axis in cancer biology. Deciphering circadian-cancer crosstalk may unveil chronotherapeutic strategies for precision oncology, leveraging temporal biology to optimize immune activation and tumor suppression.
The circadian clock acts as a central regulator of immune homeostasis, exerting
both direct cellular and systemic modulatory effects. Beyond the established
crosstalk between core clock components and immune cells, emerging evidence
highlights circadian-mediated immunomodulation through neuroendocrine, metabolic,
and hypoxic signaling axes. For example, glucocorticoids (GCs) rhythmically
control T-cell trafficking via GR-mediated binding to glucocorticoid response
elements (GREs) in the IL-7R
Chronoimmunological research has uncovered significant temporal variations in immune regulation with direct clinical relevance. The CXCL12-CXCR4 axis has emerged as a therapeutic target in strategies combining CXCR4 inhibitors with immunotherapy to overcome treatment resistance [139]. Circadian synchronization studies reveal robust oscillatory expression patterns of the Pdcd1 gene (encoding PD-1) in activated CD8+ T cells, with corresponding diurnal variations in the efficacy of anti-PD-1 and CAR T-cell therapies [138]. Vaccination timing has been shown to influence tumor growth modulation in preclinical models [133, 138], while circadian dysregulation is implicated in severe COVID-19 pathogenesis [150]. Melatonin administration holds therapeutic potential for mitigating cytokine storms in critical cases [151], and nocturnal bright light therapy—by suppressing plasma melatonin—has been proposed as adjuvant for malaria eradication [152]. These findings underscore the transformative potential of temporally optimized therapies for immune-related disorders.
Substantial mysteries remain regarding the bidirectional circadian-immune system regulation. While core clock components like BMAL1 are established modulators of immune cell rhythms, the role of auxiliary clock proteins in functional attenuation require systematic investigation. Methodologically rigorous approaches—such as cell-type-specific circadian disruption models—are essential to map clock protein domains to immunoregulatory phenotypes. Single-cell transcriptomics should be prioritized to dissect cell-type-specific circadian-immune crosstalk, including how tissue-resident macrophages maintain local rhythms independent of SCN signals control. Development of small-molecule chronotherapeutics (e.g., BMAL1 stabilizers or CRY degraders) may offer precision strategies for autoimmune diseases. Elucidating these mechanisms could advance our understanding of disease pathogenesis and identify novel therapeutic targets.
Recent studies highlight the importance of nocturnal pineal melatonin and
cortisol profiles in resetting immune cells for diurnal function, with
implications for cancer and neurodegenerative conditions [153]. Melatonin peaks
during early sleep, followed by a cortisol rise during the cortisol awakening
response. Like gut-derived butyrate, melatonin inhibits nuclear translocation of
glucocorticoid receptor-alpha (GR-
Understanding how environmental zeitgebers (e.g., light exposure, gut microbial rhythms) integrate into circadian immune networks—as discussed in Section 5—will be essential for developing holistic chrono-immunological interventions. Such insights may bridge basic chronobiology with translational medicine, fostering temporally optimized strategies to enhance immune health.
YJ, CL, and YZ contributed to the conceptualization and writing of the manuscript. YJ and YZ assembled the figures and tables. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to thank the peer reviewers for their constructive feedback, which greatly improved the quality and clarity of this manuscript.
This work was supported by grants from the National Key R&D Program of China (2019YFA0800400), the National Natural Science Foundation of China (NSFC) (31871186), the Hui-Chun Chin and Tsung-Dao Lee Chinese Undergraduate Research Endowment (CURE), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (YX13400214).
The authors declare no conflict of interest.
During the preparation of this work the authors used DeepSeek in order to check spell and grammar. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
