Cite this article
50
Downloads
1
Citations
223
Views
- Academic Editor
-
-
-
Announcements
Open Access
Opinion
Formation of the Native Topology of a Protein is due to the “Conserved but Non-Functional” Residues: A Case of Apomyoglobin Folding
Valentina E. Bychkova1,*,†, Dmitry A. Dolgikh2,3, Vitalii A. Balobanov1, Alexei V. Finkelstein1,3,*,†
Show Less
Affiliation
1
Institute of Protein Research, Russian Academy of Sciences, 142290 Pushchino, Moscow Region, Russia
2
Shemyakin–Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, 117871 Moscow, Russia
3
Biology Department, Lomonosov Moscow State University, 119192 Moscow, Russia
*Correspondence:
vbychkova@mail.ru (Valentina E. Bychkova);
afinkel@vega.protres.ru (Alexei V. Finkelstein)
†These authors contributed equally.
†These authors contributed equally.
Front. Biosci. (Landmark Ed) 2024, 29(11),
379;
https://doi.org/10.31083/j.fbl2911379
Submitted:
17 July 2024 |
Revised:
9 September 2024 |
Accepted:
12 September 2024 |
Published:
8 November 2024
Copyright: © 2024 The Author(s). Published by IMR Press.
This is an open access article under the
CC BY 4.0 license.
Abstract
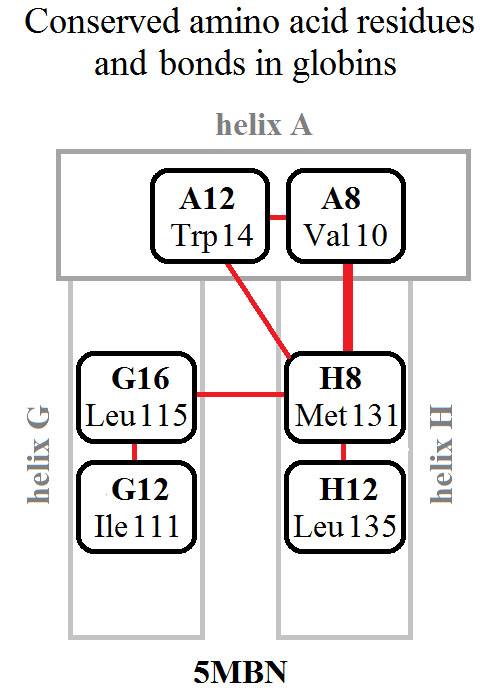
This paper is dedicated to the memory of Oleg B. Ptitsyn (1929-1999) and presents an answer to his question: “What is the role of conserved non-functional residues in protein folding?”. This answer follows from the experimental works of three labs. The role of non-functional but conserved residues of apomyoglobin (apoMb) in the formation of the native protein fold in the molten globule state has been experimentally revealed. This research proves that the non-functional but conserved residues of apoMb are necessary for the formation and maintenance of the correct topological arrangement of the main elements in the apoMb secondary structure already in the early folding intermediate.
Keywords
globin
apomyoglobin
conserved residues
non-functional residues
protein stability
protein folding
folding intermediate
native fold topology
Graphical Abstract
Figures

Fig. 1.

