Academic Editors: Graham Pawelec, Rosa Alduina and Changsoo Kim
Background: Fusarium wilt and Ascochyta blight are the most important diseases of chickpea. The current study was designed to investigate the individual and combined effect of salicylic acid (SA) with Pseudomonas stutzeri and Pseudomonas putida to suppress Fusarium wilt and promote growth of chickpea varieties: Thal-2006 and Punjab-2008. Methods: At the time of sowing, inoculum of Fusarium oxysporum was applied to the soil and the incidence of Fusarium wilt was recorded after 60 days. The seeds were inoculated with Pseudomonas stutzeri and Pseudomonas putida prior to sowing. Chickpea plants were treated with salicylic acid at seedling stage. Results: The combination of P. stutzeri and SA significantly increased root length (166% and 145%), shoot height (50% and 47%) and shoot biomass (300% and 233%) in cv. Thal-2006 and cv. Punjab-2008, respectively, in infected plants. Similarly, the combined treatment of P. putida + SA, also enhanced the plant growth parameters of chickpea varieties. Maximum reduction in disease severity was observed in both P. stutzeri + SA (90% and 84%) and P. putida + SA (79% and 77%) treatments in cv. Thal-2006 and Punjab-2008, respectively. Both P. putida + SA and P. stutzeri + SA treatments resulted in increased leaf relative water and total protein content, peroxidase, superoxide dismutase, phenylalanine ammonia-lyase and polyphenol oxidase activities in both resistant (cv. Thal-2006) and susceptible (cv. Punjab-2008) cultivars. Both treatments also significantly reduced malondialdehyde (MDA) and proline content in cv. Thal-2006 and Punjab-2008. Cultivar Thal-2006 was more effective than cv. Punjab-2008. Conclusions: The results suggested that, in combination, salicylic acid and P. stutzeri may play an important role in controlling Fusarium wilt diseases by inducing systemic resistance in chickpea.
Chickpea is Pakistan’s third most cultivated pulse crop after bean and peas. It covers an area of approximately more than one million hectares (1,106,800 ha) with an average yield of 429 kg/ha [1]. The average yield of chickpea in Pakistan is lower than in other leading countries [2]. One of the major limiting factor responsible for its low yield is the incidence of diseases, mostly Fusarium wilt caused by F. oxysporum. The yield losses due to this disease may fluctuate from 10–90% [3]. Wilt disease causes 61% damage at seedling stage and 43% at flowering stage [4].
Fusarium oxysporum is a root-infecting fungal pathogen that causes wilt disease in a broad range of plant species [5]. Fusarium wilt is the second most severe disease after Ascochyta blight [6]. The disease is caused by a vascular pathogen either travels in seed and soil [7]. Symptoms are usually more prominent at the beginning of flowering, appearing after 6–8 weeks of sowing. Late wilted plants show sagging of the petioles, rachis and leaflets, yellowing and necrosis of foliage followed by distortions in the vascular tissues of roots and stem [8].
Integrated disease management (IDM) programs are functioning to eradicate soil-borne diseases within the framework of sustainable agriculture. IDM is based on the use of pathogen-free planting material, site selection to avoid planting into high-risk soils, reduction of F. oxysporum inoculum in soil, choice of cropping practices and application of biocontrol agents and resistant varieties [9]. Biological control, which involves using living organisms to combat disease, is suggested to be a practical and eco-friendly approach to reduce crop damage caused by plant pathogens [10]. Plant growth promoting rhizobacteria (PGPR) are free-living bacteria inhabiting the rhizosphere or intracellularly forming an association with plant roots that improve plant growth. PGPR can also be classified as biocontrol agent that limits pathogens’ growth by synthesizing siderophores, antibiotics and antioxidant and defense enzymes that stimulate induced systemic resistance (ISR) in plants [11]. Plant growth promoting strains of Pseudomonas spp., Bacillus spp., fungi belonging to Trichoderma spp. and non-pathogenic isolates of F. oxysporum were effective biocontrol agents that help the plants to get nutrients from soil [12]. Pseudomonas isolates such as P. putida and P. stutzeri used in the present study were reported previously to inhibit soil-borne pathogens’ growth [13]. Pseudomonas stutzeri isolated from ginseng rhizosphere produced extracellular enzymes such as: chitinase and laminarinase that lyse the mycelium of Fusarium solani to prevent the fungus from causing root rot in different crops [14]. Lalucat et al. [15] reported that P. stutzeri had a LysR-type regulator involved in controlling oxidative stresses. P. putida strain AXMP7 exhibited positive effects on wheat plants by producing stress-inhibiting enzymes, including catalase, ascorbate peroxidase and superoxide dismutase [16].
Plant growth promoting rhizobacteria and bioactive substances have effectively controlled soil-borne fungi [17]. Salicylic acid (SA) is a phenolic compound that induces systemic acquired resistance in plants and regulates physiological processes in plants [18]. Therefore, the exogenous application of SA along with PGPR provides the plant with better tolerance against pathogen attack [19]. Salicylic acid activates defense-related genes that induce pathogenesis-related proteins which break down the fungal cell wall [20]. Jendoubi et al. [21] described that exogenous application of SA can induce the production of soluble phenolic compounds in tomato leaves and protect the plants from Fusarium wilt infection. Husien and Yousif [22] reported that the inhibitory effects of SA against Rhizoctonia solani on cucumber seedlings were more significant than that of fungicide treatment (Ficomil MZ72) against F. oxysporum and R. solani.
Plants have developed antioxidant enzyme responses to environmental and biotic stresses such as salinity, osmotic stress, high light intensity, drought and pathogen attack [23]. Antioxidant enzymes include: glutathione peroxidase, superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) among others. Antioxidant enzyme activities in plants were stimulated to resist the damage caused by the accumulation of reactive oxygen species (ROS) due to abiotic stresses and pathogen attack. It restricts pathogen spread and aids the hypersensitive response and cell death through the coordinated down-regulation of ROS-scavenging mechanisms [24]. The current study was designed to evaluate the efficiency of salicylic acid, P. stutzeri and P. putida, when used individually or in combination in controlling Fusarium wilt disease in chickpea. Moreover, the study was aimed to investigate the production of malondialdehyde, antioxidant, and defense enzymes in chickpea plants in response to oxidative stress associated with resistance to Fusarium wilt disease.
The antagonist strains Pseudomonas stutzeri (KX574858) and Pseudomonas putida (KX574857) and pathogen (F. oxysporum) were obtained from Molecular Plant Pathology Laboratory, Department of Plant Sciences, Quaid-i-Azam University, Islamabad, Pakistan. The bacteria were cultured on LB media and F. oxysporum was grown on sabouraud’s dextrose agar (SDA) plates for 5–6 days to obtain pure cultures.
The seeds of two chickpea varieties: i.e., Thal-2006 (resistant) and Punjab-2008 (susceptible), were collected from, Crop Sciences Institute, National Agricultural Research Centre (NARC), Islamabad, Pakistan.
The bacterial isolates: P. stutzeri and P. putida were grown
in tryptone soy broth in a shaking incubator (ECELLA E23, USA) at 28 °C
following the protocol of Deng et al. [25]. The bacterial
cultures were centrifuged at 6000 rpm for 15 min and re-suspended in phosphate
buffer (pH 7.4). The supernatant was discarded and the bacterial pellet was
diluted with distilled water. The bacterial culture’s optical
density (OD) was adjusted to 1 at 600 nm. Surface sterilization of seeds were
performed with 0.1% HgCl
Inoculum of F. oxysporum was prepared by multiplying the pathogen on
millet seeds following the method of Dita et al. [26]. The cell density
of F. oxysporum was recorded as 1.3
The in vitro effects of SA, bacteria and their combined treatments on
F. oxysporum growth were evaluated using the protocol of Liu et al. [28]. Different concentrations of SA were prepared
by serial dilution in distilled water, and then added to the Sabouraud dextrose
agar medium. The culture of P. stutzeri and P. putida was
carried out using a 24 h old culture. To evaluate the effect of the bacteria on
F. oxysporum growth, a circular disc (4 mm in diameter) of filter paper
was placed on both sides of the SDA medium containing 2.5
Where D
Chickpea seeds were sown in 20
Shoot fresh weight and root fresh weight was attained by weighing freshly harvested plant samples in weighing balance. Plant samples were heated in an oven for 70 h at 82 °C for dry weight estimation [30].
Chlorophyll content was measured by chlorophyll meter (SPAD 502 Plus Konica, Minolta SENSING, INC, JAPAN) from fully expanded matured leaves [31].
The leaf relative Water Content (RWC) of each leaf was calculated according to the formula of Yamasaki and Dillenburg [32].
The disease incidence and disease severity was evaluated at the vegetative stage (V3) on the 60th day of sowing following the method of Groth et al. [33] and Mckinney [34], respectively. The DS ranged from 0–4 point scale, according to the percentage of chlorosis, necrosis and wilt, as follows: 0 = healthy, 1 = 1–33%, 2 = 33%–66%, 3 = 66%–100%, 4 = dead plant [35].
The plant tissue (0.2 g) was pulverized at 4 ºC in 4 mL of
phosphate buffer (pH 7.8) solution containing 1 g polyvinylpyrrolidone (PVP) and
0.0278 g Na2EDTA in 100 mL phosphate buffer (pH 7.8). Homogenate was
centrifuged at 4 ºC for 10 min and the volume of the
supernatant was adjusted to 8 mL. The SOD was calculated by modifying the
protocol of Beauchamp and Fridovich [36]. The absorbance was recorded at 560 nm
against the blank (phosphate buffer pH 7). One unit enzyme activity (U) was
defined as the amount of SOD, which reduced the absorbance by 50% as compared to
the control (lacking enzyme). The SOD activity was expressed as U mg
Fresh leaves (1 g) were macerated using 5 mL of ice cold calcium chloride (0.5
M) solution. The mixture was centrifuged at 1000
Fresh leaves (0.5 g) were homogenized in 4 ml chilled phosphate buffer (pH 6.8).
Homogenate was centrifuged at 10,000 rpm for 20 min at 4 ºC.
The PPO activity was determined by incubating a reaction mixture containing 500
Fresh leaves (1 g) were ground at 4 °C in 5 mL of 0.1 M sodium borate
buffer (pH 8.8). Homogenate was centrifuged at 4 ºC
and the resultant supernatant was used as enzyme extract. Enzyme extract (200
The lipid peroxidation estimated as malondialdehyde (MDA) content was recorded
by calculating the amount of MDA formed by thiobarbituric acid (TBA). To measure
lipid peroxidation in the leaves, 2-thiobarbituric acid (TBA), which measures MDA
as an end product of lipid peroxidation, was used. The samples were mixed with
10% trichloroacetic acid (TCA) containing 0.65% TBA, and the absorbance
was determined after heating at 95 °C for 25 min. Then, absorption
wavelengths of supernatants on 450 nm, 532 nm, 600 nm were recorded. The MDA
content was expressed as μmol L
The results were analyzed by covariance (ANCOVA), ANOVA and principal component
analysis (PCA) using XL-STAT (2010, Lumivero, Denver, CO, USA) and
Statistix (8.1, Analytical Software, Tallahassee, FL, USA) respectively. Means
were compared using the least significance test (LSD) at p
All the treatments reduced the growth of Fusarium oxysporum as
compared to the control (Table 1). The growth inhibition rates (IR%) of
F. oxysporum obtained with SA augmented by increasing the concentration
of SA. The growth inhibition rates (%) were significantly affected by the
treatments and incubation periods (after 5 and 10 days). After five days of
incubation, the highest inhibitions were recorded by the treatments SA + S3M4 at
8 mg L
| Treatments | 5 Days of Incubation | 10 days of incubation | ||
|---|---|---|---|---|
| (mg L |
Colony diameter (mm) | IR% | Colony diameter (mm) | IR% |
| Control | 50 |
0 | 78 |
0 |
| 0.5SA | 45 |
4 | 74 |
5.1 |
| 3SA | 41 |
11 | 71 |
9 |
| 5SA | 37 |
16 | 67 |
14 |
| 8SA | 30 |
20 | 64 |
18 |
| S40 | 28 |
25 | 60 |
23 |
| 0.5SA + S40 | 26 |
32 | 55 |
29 |
| 3SA + S40 | 24 |
39 | 50 |
36 |
| 5SA + S40 | 22 |
43 | 45 |
42 |
| 8SA + S40 | 19 |
40 | 40 |
49 |
| S3M4 | 25 |
52 | 36 |
54 |
| 0.5SA + S3M4 | 23 |
57 | 33 |
58 |
| 3SA + S3M4 | 19 |
61 | 27 |
65 |
| 5SA + S3M4 | 15 |
66 | 22 |
72 |
| 8SA + S3M4 | 13 |
73 | 18 |
77 |
| The data are the average of two independent trials with three replicates for
each treatment. The Means followed by the different letters were significantly
different as determined by the LSD test (p | ||||
There was a 30% and 35% reduction in the germination index (GI) of cv. Thal-2006 (resistant) and Punjab-2008 (susceptible), respectively, in infected plants (T10), as compared to T1 (Table 2). The percentage reduction in GI in infected plants (T10) was higher in the susceptible variety than in the resistant variety. A maximum increase (100%) in GI was observed in the infected plants pre-treated with P. stutzeri + SA (T8) and infected plants pre-treated with P. putida + SA (T9).
| No. of Treatments | Germination index (%) | Root length (cm) | Shoot height (cm) | Chlorophyll (mg cm |
Shoot biomass (g) | Root biomass (g) | Relative water content (%) |
| T1 Thal | 100 |
7 |
19 |
21 |
0.9 |
1.2 |
37 |
| T1 Punjab | 100 |
6.9 |
18.5 |
20.2 |
0.9 |
1.2 |
37 |
| T2 Thal | 93 |
11 |
23 |
31 |
2 |
1.8 |
50 |
| T2 Punjab | 93 |
11 |
23 |
31 |
2 |
1.8 |
50 |
| T3 Thal | 88 |
10 |
21 |
28 |
1.7 |
1.6 |
46 |
| T3 Punjab | 88 |
10 |
21 |
28 |
1.7 |
1.6 |
46 |
| T4 Thal | 82 |
8.5 |
20 |
26 |
1.4 |
1.4 |
43 |
| T4 Punjab | 82 |
8.5 |
20 |
26 |
1.4 |
1.4 |
43 |
| T5 Thal | 95 |
14 |
26 |
40 |
2.7 |
2.5 |
65 |
| T5 Punjab | 95 |
13.8 |
26 |
40 |
2.7 |
2.5 |
65 |
| T6 Thal | 90 |
12.9 |
24 |
37 |
2.3 |
2.2 |
62 |
| T6 Punjab | 90 |
12.8 |
24 |
36.3 |
2.3 |
2.2 |
62 |
| T7 Thal | 82 |
9 |
22.5 |
34 |
1.5 |
1.9 |
57 |
| T7 Punjab | 82 |
9 |
22 |
33.2 |
1.5 |
1.9 |
57 |
| T8 Thal | 100 |
16 |
30 |
55 |
4 |
3.5 |
75 |
| T8 Punjab | 100 |
15 |
28 |
53 |
3.7 |
3.3 |
73 |
| T9 Thal | 98 |
14 |
29 |
50 |
3.2 |
2.9 |
71 |
| T9 Punjab | 98 |
12 |
27 |
48 |
2.8 |
2.7 |
69 |
| T10 Thal | 70 |
5 |
16 |
16 |
0.7 |
0.9 |
32 |
| T10 Punjab | 65 |
4 |
14 |
12 |
0.6 |
0.7 |
29 |
| Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). Germination index (%), root
length (cm), shoot height (cm), chlorophyll content (mg cm | |||||||
Infection (T10) resulted in a 28% and 42% reduction in root length as compared to control in cv. Thal-2006 and Punjab-2008, respectively (Table 2). Both SA and PGPRs under uninfected and infected conditions enhanced the root length of chickpea plants. A significant increase (128% and 117%) was observed in the root length of the infected plants treated with P. stutzeri + SA (T8), followed by P. putida and SA (T9) (100% and 73%) in cv. Thal-2006 and Punjab-2008 respectively, over control.
T10 reduced shoot height by 15% and 24% in cv. Thal-2006 and Punjab-2008, respectively as compared to T1 (Table 2). Maximum increase (57% and 51%) in shoot height was recorded in T8 followed by T9 (52% and 45%) in cv. Thal-2006 and cv. Punjab-2008, respectively, as compared to T1. T7 was less effective than T5 and T6 in both varieties
Fusarium wilt reduced chlorophyll content by 23% and 40% in cv. Thal-2006 and Punjab-2008, respectively, as compared to the control (Table 2). Maximum increase (161% and 162%) in chlorophyll content was recorded due to T8 followed by T9 in cv. Thal-2006 and Punjab-2008, respectively, over control.
Fusarium wilt decreased root biomass by 25% and 41% and shoot biomass by 22% and 33% in cv. Thal-2006 and Punjab-2008, respectively, over control (Table 2). T8 showed a maximum increase in root biomass (191% and 175%) and shoot biomass (344% and 311%) in cv. Thal-2006 and cv. Punjab-2008, respectively, followed by T9, as compared to control.
Infection (T10) significantly reduced the relative water content (%) by 13% and 21% in cv. Thal-2006 and Punjab-2008, respectively, compared to T1 (Table 2). A maximum increase of 102% and 97% in relative water content (RWC) was recorded in T8 and followed by T9 (91% and 86%) in cv. Thal-2006 and Punjab-2008, respectively, over control.
Chickpea plants infected with Fusarium wilt (T10) resulted in 88% and 90% increase in disease incidence (DI) in cv. Thal-2006 and Punjab-2008, respectively, over control (Fig. 1A). There was a reduction in DI following treatment with P. stutzeri (T5), P. putida (T6) and salicylic acid (T7) in both varieties. The maximum decrease in DI was due to P. stutzeri and SA (T8) (88% and 77%) followed by the combined treatment of P. putida and SA (T9) (82% and 73%) in cv. Thal-2006 and Punjab-2008, respectively, as compared to infection (T10).
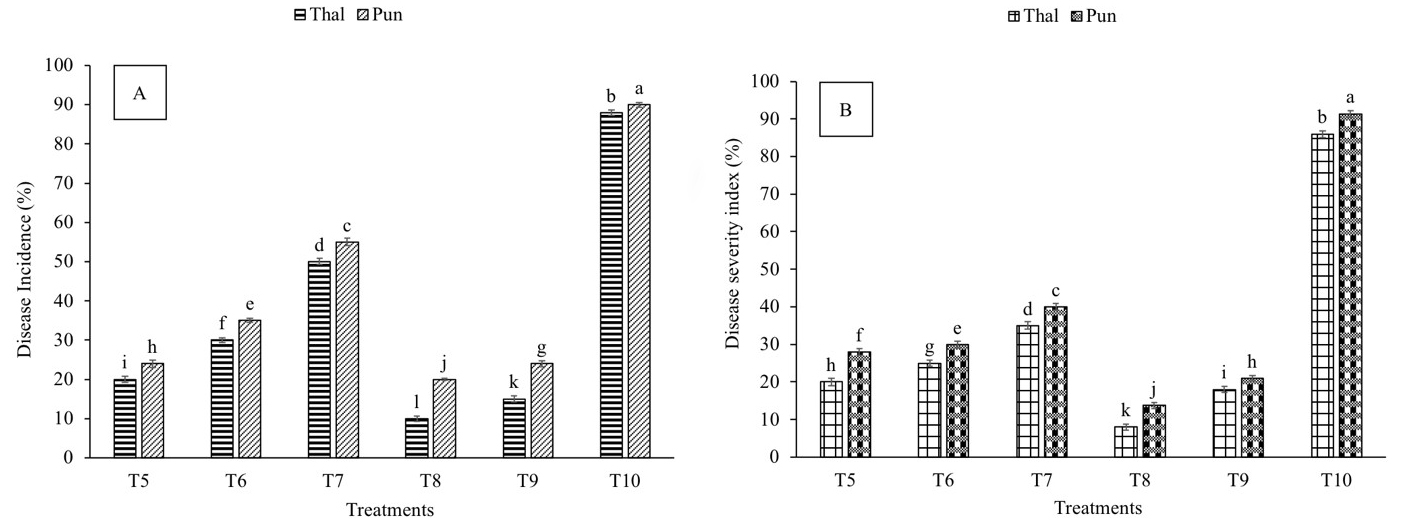 Fig. 1.
Fig. 1.Effect of Fusarium oxysporum on disease incidence and
disease severity of chickpea plants. (A) Disease incidence (B) Disease severity.
Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1 (seeds inoculated with
P. stutzeri and soil infested with F. oxysporum), T2 (seeds
inoculated with P. putida and soil infested with F. oxysporum),
T3 (seedlings sprayed with salicylic acid and soil infested with F.
oxysporum), T4 (plants treated with P. stutzeri + salicylic acid and
soil infested with F. oxysporum), T5 (plants treated with P.
putida + salicylic acid and soil infested with F. oxysporum) and T6
(soil infested with F. oxysporum). Plants were harvested at 60th day of
sowing, infestation with F. oxysporum and inoculation with PGPR.
Salicylic acid (8 mg L
Compared to control, infection (T10) resulted in 86% and 91% increase in disease severity (DS) in cv. Thal-2006 and Punjab-2008, respectively (Fig. 1B). Maximum reduction (90% and 84%) in DS was observed in T8 followed by T9 (79% and 77%) in cv. Thal-2006 and Punjab-2008, respectively, as compared to T10.
Antioxidant and defense-related enzymes such as superoxide dismutase (SOD), peroxidase (POD), polyphenol oxidase (PPO) and phenylalanine ammonia-lyase activities were checked on the 30th and 60th days after infestation.
The SOD activity was increased in all the treatments on the 30th and 60th days after infection as compared to T1 (Fig. 2A,B). A significant difference in SOD activity was observed with the duration of exposure to disease in both varieties. The treatments followed a similar pattern in both varieties, but the percentage increase in SOD activity was higher in T8, T9 and T10 in the resistant variety on the 30th and 60th days after infection. T8 showed a maximum increase in SOD activity in chickpea leaves on the 30th (712% and 600%) and 60th (1650 and 1400%) days after infection, followed by T9 in cv. Thal-2006 and cv. Punjab-2008, respectively, as compared to control.
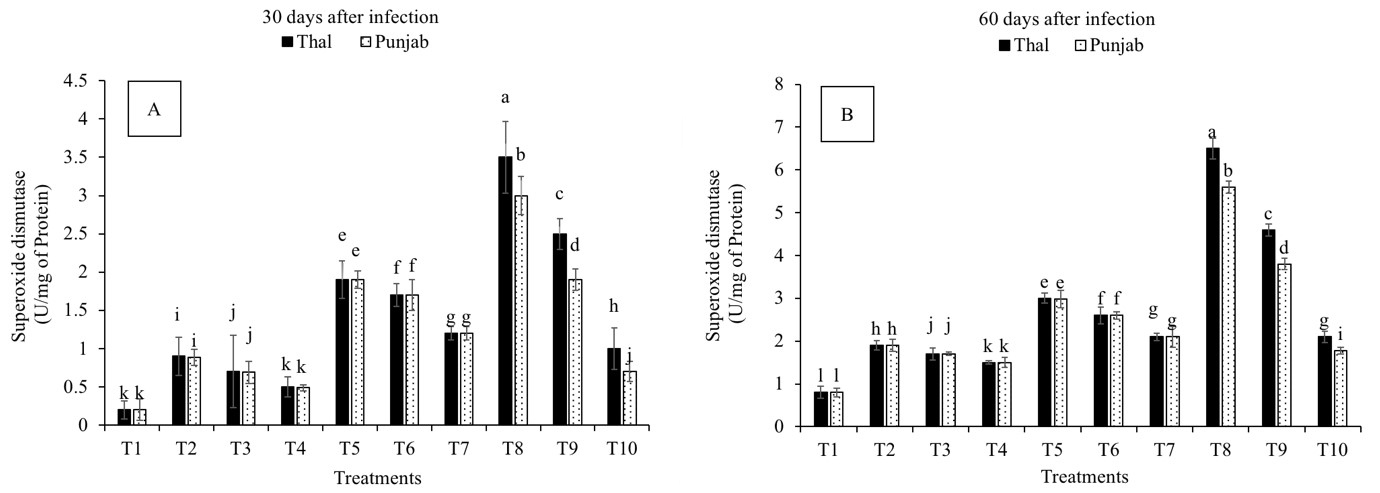 Fig. 2.
Fig. 2.Effect of Fusarium oxysporum on superoxide dismutase
(SOD) activity in chickpea leaves at 30th and 60th days after infestation. (A)
30 days after infection and (B) 60 days after infection.
Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1 (Uninfected uninoculated
control), T2 (Seeds inoculated with P. stutzeri), T3 (Seeds inoculated
with P. putida), T4 (Seedlings sprayed with salicylic acid), T5
(Seeds inoculated with P. stutzeri and soil infested with F.
oxysporum), T6 (Seeds inoculated with P. putida and soil infested with
F. oxysporum), T7 (Seedlings sprayed with salicylic acid and soil
infested with F. oxysporum), T8 (P. stutzeri + salicylic acid
and soil infested with F. oxysporum), T9 (P. putida + salicylic
acid and soil infested with F. oxysporum) and T10 (Soil infested with
F. oxysporum). Plants were harvested at 60th day of sowing, infestation
with F. oxysporum and inoculation with PGPR. Salicylic acid (8
mg L
The peroxidase activity was increased in all the treatments on the 30th and 60th days after infection as compared to T1 (Fig. 3A,B). A significant difference in the POD activity was observed in all the treatments with the duration of exposure to infection. The treatments followed a similar pattern in both varieties, but the % increase in POD was higher in T8 and T9 in the resistant variety at both time points. Among all the treatments, T8 showed maximum increase in POD activity on the 30th (388% and 333%) and 60th (933% and 833%) days after infection, followed by T9 in cv. Thal-2006 and cv. Punjab-2008, respectively, as compared to T1.
 Fig. 3.
Fig. 3.Effect of Fusarium oxysporum on peroxidase (POD)
activity in chickpea leaves at 30th and 60th days after infestation. (A)
30 days after infection and (B) 60 days after infection.
Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1 (Uninfected uninoculated
control), T2 (Seeds inoculated with P. stutzeri), T3 (Seeds inoculated
with P. putida), T4 (Seedlings sprayed with salicylic acid), T5
(Seeds inoculated with P. stutzeri and soil infested with F.
oxysporum), T6 (Seeds inoculated with P. putida and soil infested with
F. oxysporum), T7 (Seedlings sprayed with salicylic acid and soil
infested with F. oxysporum), T8 (P. stutzeri + salicylic acid
and soil infested with F. oxysporum), T9 (P. putida + salicylic
acid and soil infested with F. oxysporum) and T10 (Soil infested with
F. oxysporum). Plants were harvested at 60th day of sowing, infestation
with F. oxysporum and inoculation with PGPR. Salicylic acid (8
mg L
All the treatments increased polyphenol oxidase activity on the 30th and 60th days after infection as compared to T1 (Fig. 4A,B). A significant difference in PPO activity was observed in all the treatments with the duration of exposure to infection. Maximum PPO activity was observed in T8 on the 30th (280% and 328%) and 60th (600% and 566%) days after infection followed by T9, T5 and T6, in cv. Thal-2006 and cv. Punjab-2008, respectively, as compared to T1.
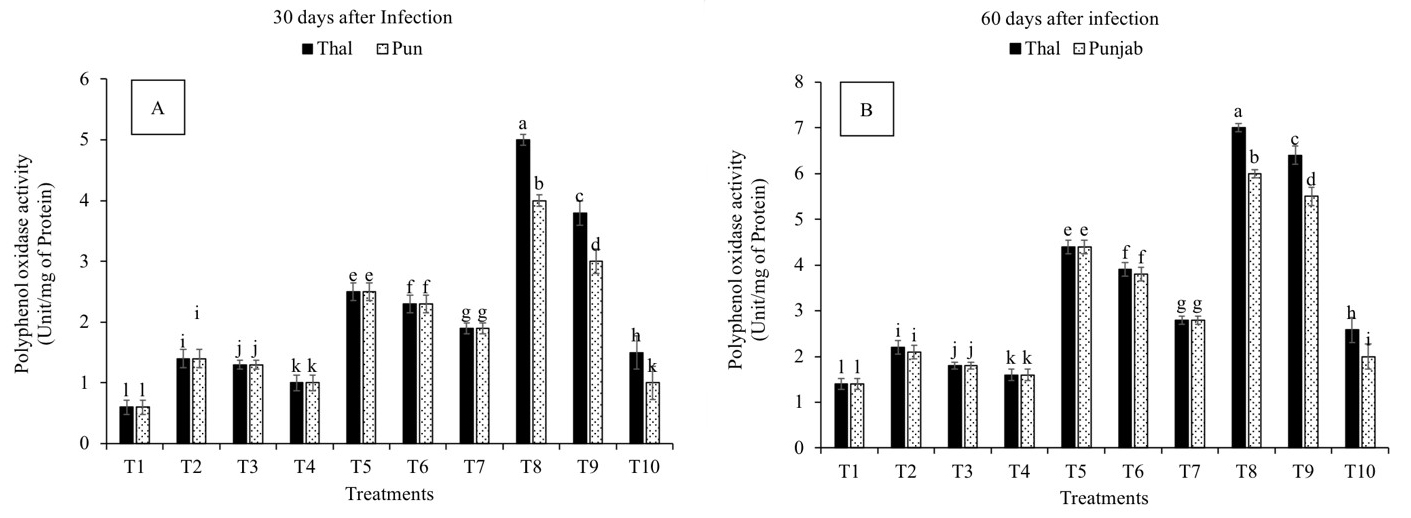 Fig. 4.
Fig. 4.Effect of Fusarium oxysporum on polyphenol oxidase
activity (PPO) in chickpea leaves at 30th and 60th days after infestation. (A)
30 days after infection and (B) 60 days after infection.
Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1 (Uninfected uninoculated
control), T2 (Seeds inoculated with P. stutzeri), T3 (Seeds inoculated
with P. putida), T4 (Seedlings sprayed with salicylic acid), T5
(Seeds inoculated with P. stutzeri and soil infested with F.
oxysporum), T6 (Seeds inoculated with P. putida and soil infested with
F. oxysporum), T7 (Seedlings sprayed with salicylic acid and soil
infested with F. oxysporum), T8 (P. stutzeri + salicylic acid
and soil infested with F. oxysporum), T9 (P. putida + salicylic
acid and soil infested with F. oxysporum) and T10 (Soil infested with
F. oxysporum). Plants were harvested at 60th day of sowing, infestation
with F. oxysporum and inoculation with PGPR. Salicylic acid (8
mg L
The phenylalanine ammonia-lyase activity was increased in all the treatments on the 30th and 60th days after infection as compared to T1 (Fig. 5A,B). A significant difference in PAL activity was observed in all the treatments with the duration of exposure to infection. Measurements recorded on the 30th (400% and 377%) and 60th (542 % and 457%) days after infection revealed that PAL activity was significantly increased in T8, followed by T9, in cv. Thal-2006 and Punjab-2008, respectively, as compared to control.
 Fig. 5.
Fig. 5.Effect of Fusarium oxysporum on phenylalanine
ammonia-lyase activity (PAL) in chickpea leaves at 30th and 60th days after
infestation. (A) 30 days after infection and (B) 60 days
after infection. Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1
(Uninfected uninoculated control), T2 (Seeds inoculated with P.
stutzeri), T3 (Seeds inoculated with P. putida), T4 (Seedlings sprayed
with salicylic acid), T5 (Seeds inoculated with P. stutzeri and
soil infested with F. oxysporum), T6 (Seeds inoculated with P.
putida and soil infested with F. oxysporum), T7 (Seedlings sprayed with
salicylic acid and soil infested with F. oxysporum), T8 (P.
stutzeri + salicylic acid and soil infested with F. oxysporum), T9
(P. putida + salicylic acid and soil infested with F.
oxysporum) and T10 (Soil infested with F. oxysporum). Plants were
harvested at 60th day of sowing, infestation with F. oxysporum and
inoculation with PGPR. Salicylic acid (8 mg L
The MDA content was reduced in all the treatments with the duration of exposure to infection as compared to infected plants (T10) (Fig. 6A,B). Significant reduction in MDA content was recorded in T8 and T9 on the 30th (90% and 81%) and 60th (92 % and 86%) days after infection, in cv. Thal-2006 and Punjab-2008, respectively, as compared to T10.
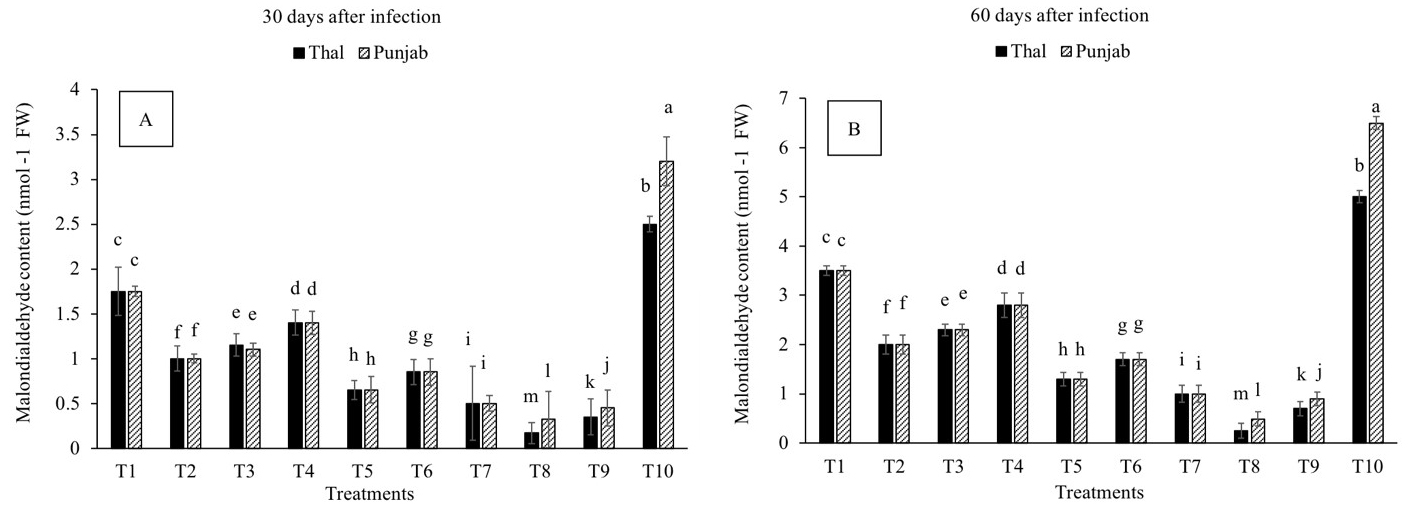 Fig. 6.
Fig. 6.Effect of Fusarium oxysporum on
malondialdehyde content (MDA) in chickpea leaves at 30th and 60th days after
infestation. (A) 30 days after infection and (B) 60 days
after infection Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1
(Uninfected uninoculated control), T2 (Seeds inoculated with P.
stutzeri), T3 (Seeds inoculated with P. putida), T4 (Seedlings sprayed
with salicylic acid), T5 (Seeds inoculated with P. stutzeri and soil
infested with F. oxysporum), T6 (Seeds inoculated with P. putida and
soil infested with F. oxysporum), T7 (Seedlings sprayed with salicylic
acid and soil infested with F. oxysporum), T8 (P. stutzeri + salicylic
acid and soil infested with F. oxysporum), T9 (P. putida +
salicylic acid and soil infested with F. oxysporum) and T10 (Soil
infested with F. oxysporum). Plants were harvested at 60th day of
sowing, infestation with F. oxysporum and inoculation with PGPR.
Salicylic acid (8 mg L–1) was sprayed on the chickpea seedlings 30 days after
sowing. Means followed by the different letters were significantly different as
determined by the LSD test (p
All the treatments increased the total protein content in chickpea plants as compared to the control (Fig. 7A). The treatments followed a similar pattern in both varieties. Still, the percentage increase in protein content was higher in T8, T9 and T10 in resistant variety. Maximum increase in protein content was recorded in T8 (333% and 286%) and T9 (244% and 211%) in cv. Thal-2006 and Punjab-2008, respectively, over control.
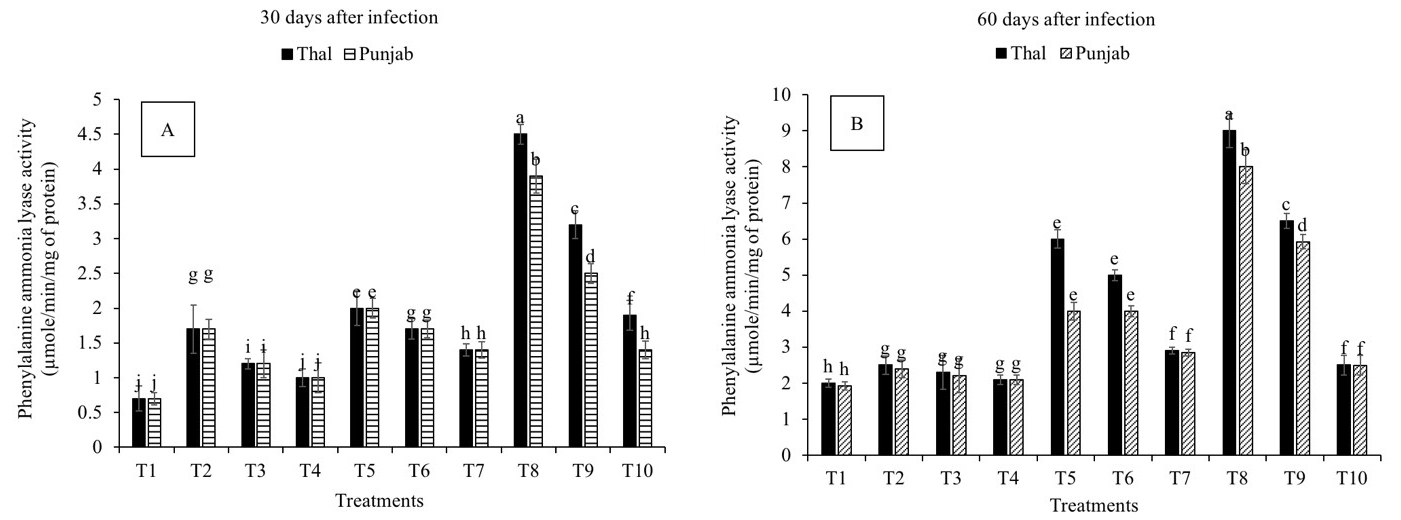 Fig. 7.
Fig. 7.Effect of Fusarium oxysporum on total protein
and proline content of chickpea plants. (A) Protein content and (B) Proline
content. Thal (cv. Thal-2006) and Punjab (cv. Punjab-2008). T1 (Uninfected
uninoculated control), T2 (Seeds inoculated with P. stutzeri), T3 (Seeds
inoculated with P. putida), T4 (Seedlings sprayed with salicylic acid), T5 (Seeds
inoculated with P. stutzeri and soil infested with F. oxysporum), T6
(Seeds inoculated with P. putida and soil infested with F.
oxysporum), T7 (Seedlings sprayed with salicylic acid and soil infested with
F. oxysporum), T8 (P. stutzeri + salicylic acid and soil
infested with F. oxysporum), T9 (P. putida + salicylic acid and
soil infested with F. oxysporum) and T10 (Soil infested with F.
oxysporum). Plants were harvested at 60th day of sowing, infestation with
F. oxysporum and inoculation with PGPR. Salicylic acid (8 mg L
All the treatments reduced the proline content of leaves in both varieties over infection (T10) (Fig. 7B). T10 resulted in a maximum increase (23% and 51%) in proline content in cv. Thal-2006 and Punjab-2008, respectively, as compared to control. Plants treated with T8 significantly reduced the proline content (88% and 80%) followed by T9, T5 and T6 in cv. Thal-2006 and Punjab-2008, respectively, over control.
Disease severity was positively correlated (alpha = 0.05) with SOD, POD and PPO and MDA while negatively correlated with PAL, protein and proline content (Fig. 8A). Germination index, relative water content (RWC), SFW, SDW, root length, shoot height and chlorophyll content were positively correlated with each other and negatively correlated with disease severity as determined by principal component analysis (Fig. 8B).
 Fig. 8.
Fig. 8.8A Correlation between antioxidant and defense enzymes, proline content, protein content and disease severity determined by principal component analysis (PCA). 8B Correlation between plant growth parameters and disease severity evaluated by principal component analysis (PCA). (A) The biplot between axes, F1 and F2, display 93.91% variation in which input of F1 is 76.49% and F2 is 17.42%. The variables positively correlated are present in the same quadrant. Malondialdehyde content (MDA), peroxidase (POD), superoxide dismutase (SOD), polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL). (B) The biplot between axes, F1 and F2, indicate 87.36% variation in which contribution of F1 and F2 are 71.98% and 15.38%, respectively. Shoot biomass (SBM), relative water content (RWC), root length (RL), shoot height (SH), chlorophyll content (Chl. cont.) and germination index (GI).
Inoculation with P. stutzeri showed significant effects on chickpea growth parameters as indicated by principal component analysis (Fig. 9A). The root biomass (RBM), shoot biomass (SBM), shoot height (SH), root length (RL) and chlorophyll content were positively correlated (alpha = 0.05) with phosphate solubilization, IAA production and catalase and protease activities of P. stutzeri. P. stutzeri and P. putida showed significant effects on antioxidant and defense enzyme activities in infected plants as indicated by principal component analysis (Fig. 9B). The SOD, POD, PPO and PAL activities of chickpea leaves were positively correlated (alpha = 0.05) with protease, catalase, HCN and antifungal activity of P. stutzeri and P. putida.
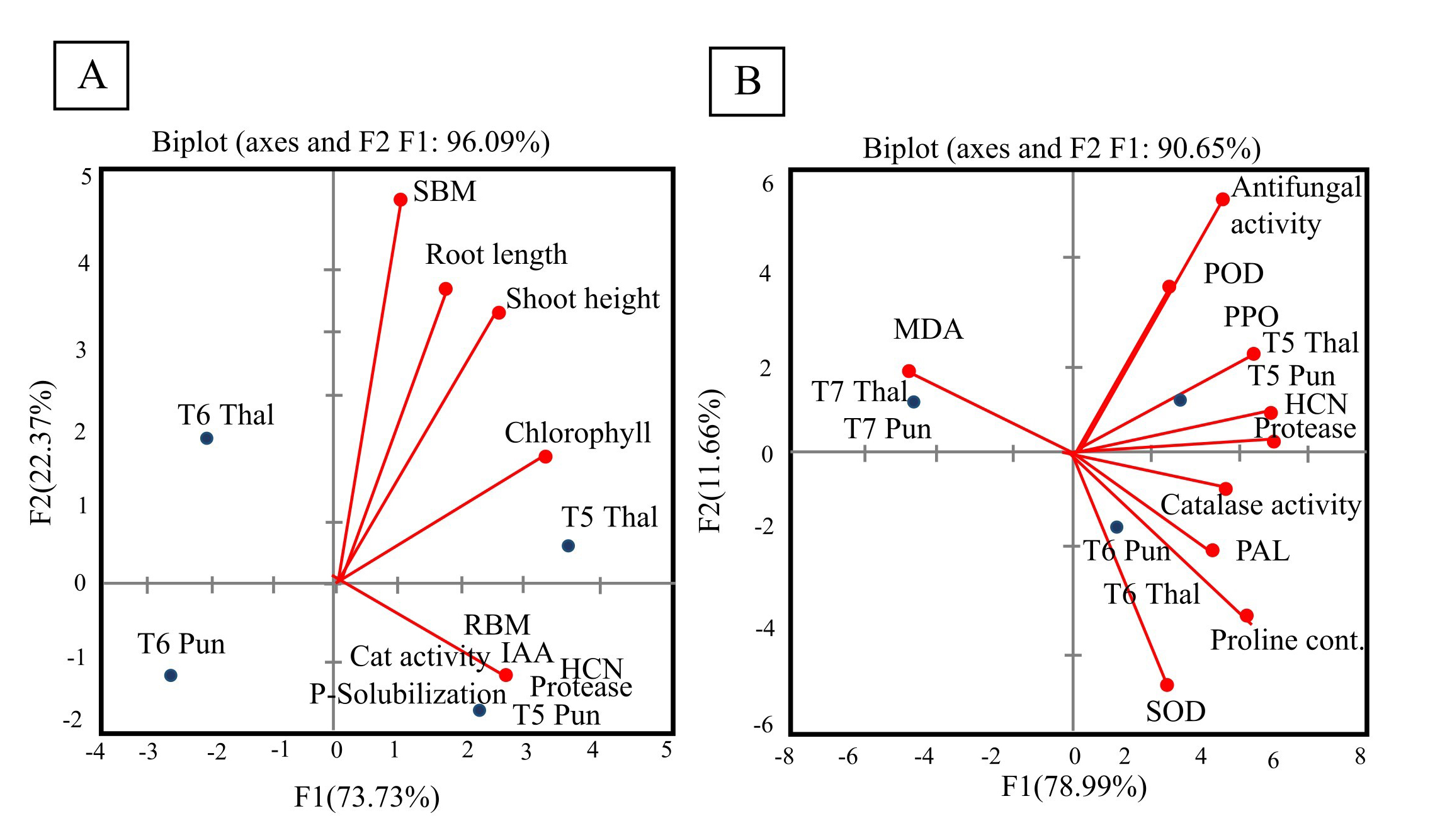 Fig. 9.
Fig. 9.9A Correlation between plant growth parameters and bacterial metabolites evaluated by principal component analysis (PCA). 9B Correlation between plant antioxidant and defense-related enzymes and bacterial metabolites evaluated by principal component analysis. (A) The biplot between axes (F1 and F2) shows 96.09% variation in which contribution of F1 is 73.73% and F2 is 22.37%. The variables positively correlated are present in the same quadrant. Red dots represent correlation between chickpea growth parameters (root biomass, shoot biomass, root length, shoot height and chlorophyll content) and bacterial metabolites (protease, catalase, indole acetic acid, HCN production and phosphate solubilization). Blue dots represent the effect of bacterial treatments on plant growth parameters. RBM (root biomass), SBM (shoot biomass), T5 (P. stutzeri) and T6 (P. putida). (B) The biplot between axes (F1 and F2) shows 90.65% variation in which contribution of F1 is 78.99% and F2 is 11.66%. Red dots represent correlation between chickpea antioxidant and defense-related enzymes and bacterial metabolites (protease, catalase, HCN and antifungal activity). Blue dots represent the effect of salicylic acid and bacterial treatments on plant growth parameters. POD (peroxidase), SOD (superoxide dismutase), PAL (phenylalanine ammonia-lyase) PPO (polyphenol oxidase), CAT (catalase), AFA (antifungal activity), T5 (P. stutzeri), T6 (P. putida) and T7 (salicylic acid).
Results revealed that Fusarium wilt significantly reduced the root growth and biomass of chickpea plants in both the varieties but cv. Punjab-2008 was more affected than cv. Thal-2006. The combined effect of P. stutzeri and salicylic acid enhanced shoot biomass and root length of chickpea plants and significantly alleviated disease severity and incidence in tolerant variety. It was previously demonstrated that the combined treatment of P. fluorescens and chemical inducers effectively reduced the Fusarium wilt in chickpea plants [43]. The role of salicylic acid is complex against necrotrophic pathogens like Fusarium oxysporum. It was observed that the non-expression of pathogenesis-related gene 1 (NPR1) is an essential positive regulator of salicylic acid (SA)-induced pathogenesis-related (PR) gene expression and systemic acquired resistance (SAR) [44]. The combined effect of P. stutzeri and SA has significantly inhibited the linear increase in the growth of F. oxysporum and exhibited higher inhibition rate than the combined effect of P. putida and SA. This further implies that P. stutzeri was more efficient than P. putida. The ameliorative effects of PGPR and SA on disease incidence and severity were lower in cv. Punjab-2008. Noteworthy, cv. Punjab-2008 had a greater incidence of disease with higher severity than cv. Thal-2006. Arshad et al. [45] reported that Thal-2006 is a disease and drought-resistant variety released during the year 2006 for the arid tract, as the imposition of one stress imparts tolerance for other stresses. Disease severity was negatively correlated with germination index, root length, shoot height, leaf relative water content, shoot biomass and chlorophyll content. Rania et al. [46] reported that plant growth parameters like plant height and shoot biomass were negatively correlated with increased severity of Fusarium wilt in tomato plants as demonstrated by Pearson’s correlation analyses.
Both PGPRs act synergistically with SA to enhance the chlorophyll content under infected conditions, P. stutzeri being more efficient. The response of cv. Thal-2006 for P. putida and P. stutzeri + SA was more pronounced. The individual effect of P. stutzeri was greater on all the growth parameters of chickpea plants as compared to SA under infected conditions because growth-promoting bacteria produced phytohormones involved in plant growth and transmitted to the interacting plants [47]. Moreover, a positive correlation existed between phosphate solubilization, IAA production, and catalase and protease activities of P. stutzeri and growth parameters of chickpea plants. Also, both the varieties differed significantly in infection. Khan et al. [48] reported that plant growth regulators (PGRs) exogenously applied in combination with PGPR act synergistically to promote root growth, chlorophyll, protein and sugar contents and lead to drought tolerance. The stimulatory role of PGPR on the growth of Pisum sativum against Fusarium wilt has been reported earlier [49]. El-Khallal [50] demonstrated that the combined application of bioagent (arbuscular mycorrhiza) and salicylic acid significantly enhanced the growth parameters of tomato plants infected with Fusarium wilt.
Plants detoxify ROS by upregulating antioxidant enzymes [51]. The observed higher activity of antioxidant enzymes (SOD and POD) and defense enzymes (PPO and PAL) in the combined treatment of salicylic acid and PGPR under infected conditions in a tolerant variety is possibly attributed to encounter the oxidative stress. The enhanced POD activity renders fast defense against plant pathogens including lignification, suberization and regulation of cell wall elongation [52]. Leiwakabessy et al. [53] reported an increase in peroxidase activity in rice plants by combining salicylic acid and endophytic bacteria after pathogen exposure. Abdel-Monaim [17] stated that guar plants treated with combined treatment of P. fluorescens and SA exhibited the highest level of PPO activity against Rhizoctonia solani. Single treatments of PGPR were more effective than that of SA. A positive correlation existed between the activities of protease and catalase, HCN production and antifungal activities of both PGPRs and activities of antioxidant and defense enzymes in chickpea plants. Also, both varieties (cv. Thal-2006 and cv. Punjab-2008) responded differently to the combined treatment of SA and PGPR (P. stutzeri and P. putida) following infection with Fusarium wilt. The variety Thal-2006 proved to be more resistant. The activities of PPO, PAL, SOD and POD were lower in cv. Punjab-2008 which suggest that defense mechanisms were poor in cv. Punjab-2008. Upadhyay et al. [54] postulated that the combined treatment of SA with P. fluorescens effectively induced PAL against Uromyces viciae-fabae in pea plants. A positive correlation between disease severity and MDA content, SOD, POD and PPO indicated that enhanced activities of defense enzymes may have a role in mitigating pathogen-induced oxidative damage [55]. Infection produced an oxidative stress in the plants followed by enhanced production of reactive oxygen species that have an important role in plant signaling. These ROS species induce systemic acquired resistance in plants and activate the defense enzymes to scavenge the free radicals [56].
The highest increase in protein content in response to infection was detected under the combined treatment of P. stutzeri and SA. The two varieties behave differently in response to combined treatment of PGPR and SA under infected conditions. The variety Thal-2006 being more resistant, showed higher protein content and lower proline content. The suppressive effects of both PGPRs and SA are noteworthy for reducing the lipid peroxidation as measured by the malondialdehyde (MDA) and proline contents of the leaves. Lipid peroxidation is a biomarker for tissue and membrane damage under stress conditions. An increase in lipid peroxidation indicates an increase in oxidative damage [57]. Lastochkina et al. [58] recorded an increase in pathogen-induced proline accumulation and lipid peroxidation in tubers infected with Phytophthora infestans and Fusarium oxysporum. However, inoculation with PGPR+SA significantly reduced the lipid peroxidation and proline content in infected tubers. This decrease in lipid peroxidation with PGPR inoculation may be attributed to the PGPR mediated lowering ROS production generated by the pathogen attack and cell injuries which increase tolerance to biotic and environmental stresses. Ali et al. [59] demonstrated that enhanced accumulation of pathogenesis-related (PR) proteins confers resistance to plant cultivars against pathogens. Salicylic acid plays a vital role in stress responses by modulating osmolytes, including proline, sugars, amines, and glycine betaine [60]. Proline is an osmolyte accumulated under oxidative stress and renders stress against pathogen attack. Zehra et al. [61] reported that defense-related proteins and proline were found to be maximum after 72 h of pathogen inoculation under Trichoderma harzianum and SA combined treatment render better protection against Fusarium wilt as compared to single treatments. Abdel-Monaim et al. [17] described that the combination of biocontrol agent (Trichoderma viride) with chemical inducer (SA) significantly improved the protein content and provide protection against damping off disease.
It is inferred from the results that PGPR enhances the defensive mechanisms of SA. P. stutzeri was more effective for growth promotion and defense against Fusarium infection and its proliferation. Enhanced disease severity in chickpea plants was positively correlated with higher levels of MDA, SOD, POD, PPO and proline accumulation. Response of the susceptible variety, Punjab-2008 to different treatments against Fusarium wilt was lower than that of the resistant variety Thal-2006. It demonstrates that possibly one moisture stress of the Thal area predisposes it to tolerate the Fusarium wilt better [62].
The data presented in this study are available within the article.
All authors listed have made substantial, direct and intellectual contribution to the work and approved it for publication. Conceptualization: AB, RM, UMQ and MFHM, Formal analysis; RM, TA and MFHM; investigation, NK; resources, RM, AB, UMQ and MFHM, writing—original draft preparation, RM, MFHM, UMQ and AB, editing, RM, NK and AB, supervision, AB; Project administration, AB. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research has received no external funding.
The authors declare no conflict of interest. Given his role as Guest Editor and Editorial Board Member, Naeem Khan had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Graham Pawelec, Rosa Alduina and Changsoo Kim.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
