1. Introduction
Tumor ablation is one of the minimally invasive techniques and is preferred for
the treatment of tumors of the lung, kidney and liver. It provides an alternative
for failed chemotherapy or radiotherapy or for non-surgical candidates. Ablation
is also preferred as a first-line treatment in patients suffering from benign
tumors of liver or small hepatocellular carcinoma [1]. Thermal ablation is
performed by either heating or cooling of targeted tissues to cytotoxic
level. Tumor cells are basically more vulnerable to heat as compared to normal
cells because of differential sensitivity to hypoxia [2] and hydrogen ion
concentration [3]. Interstitial laser ablation is yet another hyperthermic
ablation procedure. The light generated by neodymium:yttrium aluminum garnet
laser (1064 nm) is focused to the target tissue; the light is absorbed by the
tissue and converted to heat for therapeutic purpose [1].
Photodynamic therapy (PDT) is a technique in which the cancer cells are exposed
to light of specific wavelength after administration of nontoxic photosensitizers
[4, 5]. The excitation of photosensitizers by light exposure causes the emission
of fluorescence as well as the generation of potentially toxic free radicals
which impart photosensitizers the properties of both imaging and therapeutic
agents [6, 7, 8]. One of the major disadvantages of PDT for combined imaging and
treatment applications is the limited tissue penetration by visible light, for
the activation of photosensitizers [9]. Therefore, there is a need to develop
agents for PDT that can be activated by light at of 620–750 nm which is called
as ‘visible red optical window’ [10]. At these wavelengths, body tissues are
transparent, and the visible red radiation can be utilized to activate
photosensitizers in deep tumors without causing any appreciable phototoxicity to
normal tissues. On the other hand chemotherapy is associated with systemic
toxicity [11], whereas radiotherapy can damage adjacent normal tissue if an
appropriate dose is not selected [12].
Graphene-based nanomaterials, in contrast to other types of carbon materials,
possess a large surface area, are easy to functionalize and have improved
solubility due to their unique optical, physicochemical and biomedical properties
which enhance their applications in nanomedicine [13, 14, 15]. Graphene oxide (GO)
nanoparticles functionalized with other materials have shown theranostic
properties for cancer diagnosis and therapy [16, 17]. Usman et al. [18]
synthesized a GO-based delivery system for magnetic resonance imaging (MRI) using
gadolinium nitrate as a contrast agent and naturally occurring protocatechuic
acid as an anticancer compound. Yang et al. [19] have shown that
manganese ferrite (MnFeO) nanoparticles deposited on GO show intense
optical absorbance in the near infrared (NIR) region and high photothermal
stability, which makes them highly efficient in photothermal ablation of cancer
cells.
Manganese oxides, viz., MnO, MnO, MnO, and MnO,
are attractive candidates for novel MRI contrast agent due to their inherent
properties based on electronic configuration that can produce a large magnetic
moment and cause nearby water protons relaxation [20]. Therefore, manganese
oxides are one of the most widely investigated nanomaterials for image-guided
therapeutic purposes [21, 22]. In this study, we synthesized hybrid nanoparticles
containing highly reduced graphene oxide and MnO
(HRG-MnO) and studied their biocompatibility as well as therapeutic
potential for PDT of cancer, using a cellular model.
2. Materials and Methods
2.1 Chemicals and Reagents
All chemicals including solvents used for the synthesis of nanoparticles were
procured from Sigma Aldrich (St. Louis, MO, USA). Graphite powder (99.999%) was
obtained from Alfa Aesar, Kandel, Germany. Deionized water was prepared from a
Millipore Milli-Q system and used in all experiments.
2.2 Preparation of MnO Nanoparticles
In a three-necked flask (100 mL capacity), a slurry of manganese (II)
acetylacetonate was dissolved in oleylamine, keeping their molar ratio as 1:25,
respectively. After heating the mixture at 162 °C for 11 h under nitrogen cover,
the resultant mixture was allowed to cool to ambient temperature resulting in the
formation of a brown suspension. The contents were centrifuged at 9000 rpm for 15
min, to collect a brown precipitate after removal of supernatant. Pure
MnO was acquired after multiple washings of the brown precipitate
with ethanol. The synthesized MnO has the tendency to be readily
dispersed in typical organic solvents including dichloromethane, toluene and
hexane. The synthesized MnO nanoparticles were vacuum dried before
their usage.
2.3 Preparation of Highly Reduced Graphene Oxide (HRG)
Initially graphite oxide (GO) was synthesized from graphite powder and then
using a modified Hummers method [23, 24] and then it was converted to graphene
oxide (GRO) following several steps of centrifugation, washing and finally
sonication. GRO was reduced according to a previously reported method [25].
Briefly, GRO was dispersed in water and sonicated for 30 min. The resulting
suspension was allowed to heat up to 100 °C and subsequently 3 mL of hydrazine
hydrated were added. The temperature was slightly reduced (98 °C), and the
suspension was kept under stirring for 24 h. Finally, a black powder was obtained
which was filtered and washed several times with water. The resultant suspension
was centrifuged at 4000 rpm for several 3 min, and the final product was
collected via filtration and dried under vacuum.
2.4 Preparation of HRG-MnO Hybrid Nanoparticles
Approximately, 200 mg of MnO nanoparticles and 200 mg of highly
reduced graphene powder were subjected to milling process using Fritsch
Pulverisette P7 planetary ball mill (Idar-Oberstein, Germany) and stainless steel
beads of 5 mm diameter. The nanocomposite powder and steel balls in the equal
weight proportion (ratio 1:1) were introduced into the stainless steel container.
The milling process of the components was allowed to continue for 16 h with
intermittent pauses at regular intervals.
2.5 Characterization of Nanoparticles
The synthesized nanoparticles were characterized for size, elemental
composition, physicochemical properties and stability using high resolution
transmission electron microscopy (JEM-2100F, JEOL, Japan), energy-dispersive
X-ray spectroscopy (EDX), UV–Vis spectroscopy (Perkin Elmer lambda 35, Waltham,
MA, USA), FT-IR spectroscopy (Perkin Elmer 1000 FT-IR spectrometer), X-ray
diffraction analysis (D2 Phaser X-ray diffractometer, Bruker, Germany) and
thermogravimetric analysis (TGA/DSC1, Mettler Toledo AG, Analytical,
Schwerzenbach, Switzerland).
2.6 Cytotoxicity Assay
The cytotoxicity of MnO and HRG-MnO nanoparticles was
performed by 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT)
method. A549 cells were seeded in the 96-well plate (4 10 cells
per well) in RPMI medium and incubated in the atmosphere of 5% CO at 37 °C
for 24 h. Different concentrations (6.25, 12.5, 25, 50 and 100
g/mL) of MnO and HRG-MnO were added to the
respective wells of micro plate followed by 4 h incubation. Phosphate buffer
saline (PBS) and triton X-100 were used as control and negative control,
respectively. For laser-induced phototoxicity analysis, the cells were treated
with a 670 nm laser irradiation at 0.1 W/cm for 5 min and further incubated
for 24 h. Aqueous solution of MTT (50 L) was added to the wells of
micro plate, 4 h before the termination of incubation period (24 h). After
discarding the upper layer, MTT solubilization solution, DMSO (100
L) was added to all the wells of the micro plate for dissolving the
formazan crystals followed by measuring the absorbance at 590 nm, which was
converted to cell viability based on absorbance of dissolved formazan. The
percent cell viability was calculated using the following equation:
2.7 Fluorescence Microscopy of Live and Dead Cells
The live/dead assay kit containing fluorescein diacetate (FDA) and propidium
iodide (PI) to visualize live and dead cells, respectively was used and cells
were visualized under fluorescence microscope. A549 cells (2 10
cells per well) were seeded on a 24 well plate and incubated in the atmosphere of
5% CO at 37 °C for 24 h. MnO and HRG-MnO
nanoparticles (50 g/mL) were added to the 24 well plate. PBS was
used as a control and the plate was incubated for 4 h. Then cells were exposed to
a 670 nm laser irradiation at 0.1 W/cm for 5 min and further incubated for
24 h. FDA and PI were added to treated cells and incubated for 5 min. Then the
cells were washed with PBS thrice to remove excess FDA/PI and fluorescence images
were acquired by a fluorescence microscope with 490 nm excitation and emission at
525 nm.
2.8 Detection of Intracellular Reactive Oxygen Species (ROS)
For intracellular ROS detection, A549 cells (2 10 cells per
well) were incubated in 24-well plate with 5% CO at 37 °C for 12 h.
HRG–MnO nanoparticles diluted in media to yield a final
concentration of 50 g/mL, were added to the cells and incubated for
4 h. The incubated cells were irradiated with 670 nm laser (0.1 W/cm) for 5
min and cells were washed with PBS. Serum free medium containing
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) (20 M) was added
into the wells and the plate was incubated for another 15 min. Then the cells
were washed with PBS thrice to remove excess DCFH-DA and fluorescence images were
acquired by fluorescence microscope with 485 nm excitation and emission at 530 nm
wavelengths. DCFH-DA has been used extensively for total ROS detection. After
cellular uptake, DCFH-DA is cleaved off the acetyl groups by cellular esterase,
resulting in the formation of DCFH, which is oxidized by ROS to produce
2,7-dichlorofluorescein (DCF), which emits green fluorescence at excitation and
emission wavelengths of 485 nm and 530 nm, respectively [26].
2.9 Statistics
All the cell based analyses were performed in triplicate and the results
reported as means standard deviation. The data were analyzed by one-way
analysis of variance (ANOVA) followed by Dunnett’s test. p values
0.05 were considered as statistically significant.
3. Results
3.1 Characterization of Nanoparticles
The shape of hybrid nanoparticles appeared as round with the average diameter of
12 2.21 nm (Fig. 1A). In EDX analysis, the intense signals at 0.65, 5.88,
and 6.62 keV strongly suggests that ‘Mn’ was the major element, which has an
optical absorption in this range owing to the surface plasmon resonance (SPR).
The other signals found in the range 0.0–0.5 keV signify the absorption of
carbon and oxygen, confirming the formation of HRG-MnO nanocomposite
(Fig. 1B). UV-Vis spectrum of HRG-MnO nanoparticles showed respective
absorption bands at ~220 (MnO)
and ~270 nm (HRG) indicating the formation of
HRG-MnO (Fig. 1C). FT-IR spectrum of HRG-MnO displayed
the graphene oxide bands at ~1630 cm (for C=C stretching),
~1209 cm (for C–O–C stretching),
~1050 cm (for C–O stretching), and a broad band at around
3440 cm for hydroxyl groups indicated the presence of various
oxygen-containing functional groups, such as carbonyl, carboxylic, epoxy, and
hydroxyl groups in graphene oxide. The removal of oxygen-containing groups of
graphene oxide in HRG was indicated by the disappearance of some of the bands
such as the band at ~1740 (which is present in HRG only; spectrum
not shown). Also the relative decrease in the intensity of some of the bands,
like the decrease in intensity of broad band at 3440 cm points towards the
reduction of graphene oxide. The existence of other absorption bands of Mn at 624
and 525 cm clearly indicated the formation of HRG-MnO
nanocomposite (Fig. 1D). The XRD patterns of MnO nanoparticles such
as 18.2° (101), 29.1° (112), 31.2° (200),
32.5° (103), 36.3° (211), 38.2° (004), 44.6°
(220), 50.8° (105), 53.8° (312), 58.7° (321),
60.0° (224), and 64.8° (314) indicated the formation of
manganese oxide. XRD pattern of HRG-MnO with the appearance of a
broad peak at ~22.4° (002) confirmed the reduction of
graphene oxide in the form of HRG. The existence of all these reflections
indicates the formation of HRG-MnO nanoparticles (Fig. 1E). TGA
analysis of HRG-MnO nanoparticles displays the weight loss of about
20% after heating up to 800 °C indicating the presence of substantial oxygen
functionalities despite appreciable stability of hybrid nanoparticles at high
temperatures (Fig. 1F).
 Fig. 1.
Fig. 1.
Characterization of HRG-MnO nanoparticles using.
(A) Transmission electron microscopy. (B) Energy-dispersive X-ray spectroscopy.
(C) UV-visible spectroscopy. (D) FT-IR. (E) X-ray diffraction analysis. (F)
Thermogravimetric analysis.
3.2 Cytotoxicity and In-Vitro PDT
The cytotoxicity analysis using MTT assay showed that more than 98% of A549
cells survived even after the exposure of a high concentration (100
g/mL) of nanomaterials indicating the biocompatibility of both
MnO and HRG-MnO nanoparticles (Fig. 2). Almost 100%
cells were viable when treated with phosphate buffered saline (PBS) or
MnO nanoparticles in presence of 670 nm laser irradiation (0.1
W/cm) for 5 min (Fig. 3). However, laser irradiation resulted in
significant and concentration-dependent cellular damage by HRG-MnO
nanoparticles (Fig. 3).
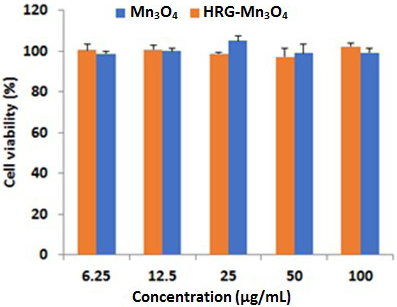 Fig. 2.
Fig. 2.
Cytotoxicity analysis showing cell viability of A549 cells
treated with different concentrations of MnO and HRG-MnO
nanoparticles.
 Fig. 3.
Fig. 3.
Cell viability of A549 cells incubated with different
concentrations of PBS (control), Triton X100 (negative control), MnO
and HRG-MnO nanoparticles in presence of 670 nm laser irradiation
(0.1 W/cm) for 5 min. Data are represented as mean standard
deviation (n = 3). * p 0.05, ** p 0.01 and ***
p 0.001 versus respective control groups.
3.3 Live/Dead Cell Analysis
To study the interactions between cells and the nanoparticles, we used the
visible red optical imaging of A549 cells after incubation in PBS,
MnO nanoparticles, HRG-MnO nanoparticles and
HRG-MnO nanoparticles with and without laser irradiation for 5 min
(Fig. 4). After 5 min, propidium iodide (PI) (red emission for dead cells)
fluorescent dots were observed in HRG-MnO nanoparticles plus laser
treated group when compared to HRG-MnO nanoparticles treated cells.
However, no red fluorescent dots were observed in PBS and MnO treated
A549 groups of cells. On the other hand, A549 cells incubated in PBS,
MnO nanoparticles, HRG-MnO nanoparticles showed abundant
green emission indicating the presence of live cells (Fig. 4).
 Fig. 4.
Fig. 4.
Fluorescence microscopy images of A549 cells co-stained with
fluorescein diacetate (green emission for live cells) and propidium iodide (red
emission for dead cells) with PBS (control), HRG-MnO nanoparticles
with/without laser irradiation (670 nm, 0.1 W/cm) for 5 min.
3.4 Intracellular ROS Generation
The intracellular ROS production was investigated by fluorescence microscopy
with cell permeable green fluorescence ROS indicator DCFH-DA. As shown in Fig. 5D, HRG-MnO nanoparticles increased the intracellular ROS generation
in A549 cells in presence of laser irradiation. A remarkable green fluorescence
of DCF was observed with HRG-MnO in presence of laser irradiation
whereas the green fluorescence is negligible for control cells (Fig. 5A).
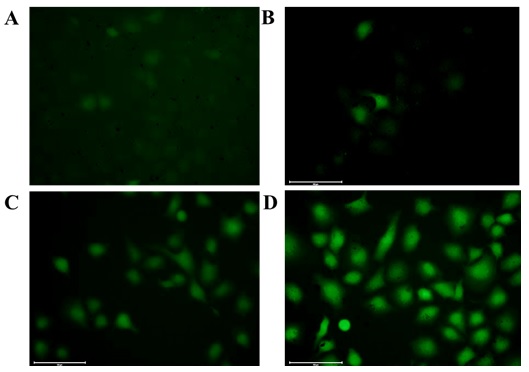 Fig. 5.
Fig. 5.
Fluorescence microscopy images of A549 cells under different
treatments. (A) Incubated with only DCFH-DA. (B) Incubated with DCFH-DA +
MnO. (C) Incubated with DCFH-DA + HRG-MnO. (D) Incubated
with DCFH-DA + HRG-MnO+ laser irradiation (670 nm, 0.1 W/cm) for
5 min (scale bar: 100 m).
4. Discussion
In this study, we synthesized hybrid nanoparticles containing the equal amounts
of two moieties, highly reduced graphene (HRG) and MnO. Both these
constituents have specific properties; HRG is effective for optical imaging and
PDT whereas MnO possesses MRI imaging property. Manganese oxide
nanoparticles have been shown to be a
promising T-weighted contrast agent with high
magnetization spins and fast water proton exchange rates [27]. Therefore,
manganese oxide nanoparticles are emerging as potentially useful MRI contrast
agents for biomedical imaging and tumor diagnosis [28]. Although, manganese oxide
nanoparticles with good crystallinity can easily be synthesized on a large scale
under mild and ambient reaction conditions, it is difficult to design and
synthesize highly stable Mn complexes with high sensitivities for clinical
applications [29]. This drawback can be overcome by building manganese-based
nanoparticulate systems, such as MnO, MnO, MnO-SiO [30]. Combination of optical and MRI imaging has emerged as an attractive
technique for both in-vivo animal and clinical cancer diagnosis [31]. In
recent years, there is a trend of theranostic nanoparticles possessing the
capability of imaging and therapy together [8, 13, 32].
We performed in-vitro cytotoxicity assay to investigate the toxicity
profile of newly synthesized HRG-MnO hybrid nanoparticles.
In-vitro cytotoxicity studies of nanoparticles are preferred as they are
simple, cost-effective and faster than in-vivo models [33]. These
results confirmed that the HRG-MnO nanoparticles are nontoxic and
biocompatible under physiological conditions (Fig. 2). The commonly used
contrasting agents which are based on gadolinium (Gd) cause kidney fibrosis in
some cases necessitating the search for alternative agents. Xiao et al.
[34] synthesized MnO nanoparticles that showed high relaxivity, twice
higher than that of commercially used contrasting agents [35]. MnO
NPs coated with polyethylene glycol (PEG), designed as MRI contrasting agent,
have shown good biocompatibility after intravenous injection in mice [20].
Previous studies have shown that graphene oxide nanoparticles are less toxic to
different cell lines with a survival rate exceeding 80% at a high concentration
of 200 g/mL [36, 37]. Wang et al. [38] observed that graphene
oxide is nontoxic at low and medium doses whereas high doses cause significant
toxicity, both in-vitro and in-vivo, with a strong tendency to
affect lung, liver, spleen and kidney.
The cell viability analysis of newly synthesized nanoparticles was investigated
in A549 cells treated with MnO or hybrid HRG-MnO
nanoparticles. After laser irradiation, a significant and concentration-dependent
cytotoxicity of HRG-MnO was observed as compared to MnO
nanoparticles (Fig. 3). These findings were confirmed by fluorescence microscopy
imaging of live/dead cells after exposure to various treatments (Fig. 4). We
observed that hybrid nanoparticles produced cytotoxicity only after laser
irradiation suggesting their potential for PDT of cancer. The results of DCFH-DA
fluorescence microscopy showed excessive generation of ROS in A549 cells exposed
to HRG-MnO nanoparticles and laser irradiation (Fig. 5). Because of
the limited migration of O from its formation site [39], the location
of cellular and tissue damage by PDT are mainly related to the localization of
the photosensitizer [40]. Those photosensitizers which are not taken up by cells
have been found to be extremely inefficient even their ability of producing high
yield of O [41]. Moreover, since most PDT sensitizers do not
accumulate in cell nuclei, PDT has generally a low potential of causing DNA
damage, mutations, and carcinogenesis [42]. Photosensitizers that preferentially
localize in mitochondria usually induce apoptosis whereas the photosensitizers
that localized in plasma membrane tend to cause necrosis during the exposure of
light [41]. Another important parameter that can affect cytotoxicity is the
availability of oxygen within the tissue receiving PDT treatment. The rates of
O generation and hence tissue oxygen consumption are high when both
photosensitizer level and the exposure of light are high [43, 44].
Graphene based materials are excellent photosensitizers [45] and showed improved
anticancer PDT effects compared to the conventional photosensitizers [46]. The
photo-activation of a photosensitizer initially enables its excitation to a
triplet state through a transient intermediate called ‘singlet state’. The
electron and energy transfer to surrounding free oxygen produces potentially
toxic reactive oxygen species (ROS), including superoxide anion radical, hydroxyl
radical, and hydrogen peroxide. Excessive generation of toxic ROS causes tumor
cell death by oxidative stress, as schematically presented in Fig. 6. Although,
GO in a low concentration (10 g/mL) did not enter A549 cells and had no
obvious toxicity, the higher concentration of GO (200 g/mL) caused
oxidative stress and induced a slight loss of cell viability [37]. Enhancement of
killing of cancer cells exposed to HRG-MnO nanoparticles followed by
laser irradiation is associated with enhanced generation of ROS resulting in
lipid peroxidation and disruption of cellular membranes causing cell death [16].
 Fig. 6.
Fig. 6.
Schematic presentation of mechanism of HRG-MnO
induced cytotoxicity under laser irradiation.
5. Conclusions
The newly synthesized HRG-MnO hybrid nanoparticles do not pose any
cytotoxicity at normal physiological conditions and therefore they are
biocompatible. However, exposure of laser light of specific wavelength resulted
in massive cellular damage by HRG-MnO nanoparticles, which was
directly related to generation of intracellular ROS. These findings suggest a
great potential of HRG-MnO nanoparticles for photodynamic therapy.
Further studies are warranted to explore their MRI imaging property and
in-vivo anticancer activity using animal models of cancer.
6. Limitations
In this study, we compared HRG-MnO hybrid nanoparticles with
MnO nanoparticles whereas the PDT potential of HRG alone was not
evaluated. Although, it is the HRG moiety in HRG-MnO hybrid
nanoparticles that is mainly responsible for killing the cancer cells under laser
irradiation however it is important to find out whether the presence of
MnO in HRG-MnO hybrid nanoparticles affects the PDT
potential of HRG or not, by testing the effect of HRG alone. Another limitation
of this study is the use of only one type of cancer cells (A549); use of more
than one cell line would certainly result in broader implications.
Availability of Data and Materials
Data contained within the article.
Author Contributions
Conceptualization—HAK; Methodology—HAK, YKL, MRSha, ASA; Formal analysis—MRSha, STA, AAE, ASA; Investigation—HAK, YKL, MRSid; Resources—HAK, YKL; Data curation—HAK, YKL, MRSid, AAE; Original draft preparation—HAK, NJS, YKL, MRSha; Writing, review and editing—HAK, NJS, MRSha; Supervision—HAK; Project administration—HAK, STA; Funding acquisition—HAK.
Ethics Approval and Consent to Participate
The study protocol was approved by Institutional Review Board (Approval No.
KSU-SE-21-23).
Acknowledgment
We acknowledge excellent technical support from laboratory staff.
Funding
National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz
City for Science and Technology, Kingdom of Saudi Arabia, Award Number
(14-NAN-862-02).
Conflict of Interest
Given his role as Guest Editor and member of Editorial Board, Haseeb Khan had no
involvement in the peer-review of this article and has no access to information
regarding its peer-review. Full responsibility for the editorial process for this
article was delegated to Peter Brenneisen.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5. Fig. 6.
Fig. 6.