Academic Editor: Graham Pawelec
Background: Kidneys are among the vital organs of the human body; therefore,
damage from any exogenous/endogenous agent may put human life at risk. Arachis
hypogaea (AH) contains different free radical scavenging flavonoids, stilbenes,
and tannins. This research aimed to elucidate the possible nephroprotective
mechanism of AH methanolic crude extract (AHcr) and n-hexane oil fraction (AHO)
against gentamycin-induced nephrotoxicity. Methods: After the extraction of the
crude oil of the plant, they were tested against a Gentamycin (GM)-treated group of Swiss
Albino mice for their nephroprotective action. Animals were divided into six
(6) equal groups with five (5) animals in each group. These groups were: control
group (0.5 mL normal saline via intraperitoneal -i.p), gentamycin group
(gentamycin 100 mg/kg i.p), Silymarin + gentamycin group (Silymarin 50 mg/kg and
gentamycin 100 mg/kg i.p), plant extract (AHcr1) and gentamycin group (AHcr1 250
mg/kg and gentamycin 100 mg/kg i.p), AHcr2 + gentamycin group (AHcr2; 500 mg/kg
and gentamycin 100 mg/kg i.p) and the hexane oil fraction (AHO) + gentamycin (AHO
1 mL/kg and GM 100 mg/kg i.p). After completion of doses, animals were sacrificed
for the collection of blood to further investigate biochemical changes and
histopathological changes in kidney tissues. Results: Serum creatinine, urea, and
blood urea nitrogen significantly increased (p
The kidney is one of the vital organs in the human body, performing various life-supporting functions. Therefore, any damage to it can put human health at risk. With each passing day, courtesy changing lifestyles and food patterns of modern humans, the number of cases of kidney damage is increasing [1, 2].
Kidney diseases have been increasing all over the world. They are expected to become the fifth leading cause of death by 2040 [3]. There are various reasons for it, few of them include but are not limited to the consumption of artificial components, i.e., additives in the form of preservatives etc. in the food have increased dramatically. As kidneys are designed to excrete wastes produced by the natural components of food only, therefore, consumption of such artificial additives may cause damage and ultimately badly affect their normal function [4]. Besides this, environmental factors may also damage kidneys, e.g., a mixture of heavy metals like arsenic in water from industrial wastes, inhaling polluted air and irrigating farms with polluted water may result in the accumulation of unwanted wastes in our bodies [5]. Last but not the least, medicines which have become an essential part of our life, is also a major source of kidney damage, as almost all medicines are exogenous synthetic chemicals in nature; they may, therefore, result in kidney injuries. Examples of drugs causing kidney damage are cisplatin, aminoglycosides, amphotericin and Non Steroidal Anti Inflamatory Drugs (NSAIDs) and aminoglycosides etc. [6, 7].
Traditionally its seeds are used for the extraction of oil which is further used for cosmetological purposes. Major constituents of the Arachis oil are oleic acid and linoleic acid [8, 9]. Whether the damage to the kidneys is endogenous or exogenous- the major mechanism behind nephrotoxicity is the oxidative stress that leads to the generation of free radicals that caused damage to the nephron [10, 11]. Due to the presence of high quantities of antioxidants in different parts of different plants, they can be expected to have protective effects against toxicities that are caused by oxidative stress. Various studies have reviewed several such plants having antioxidant effects against oxidative stress-related toxicity [12, 13]. Manifestation of kidney disorders is in the form of change of various, accumulation of wastes, urea creatinine increases in the blood, or a high Blood Urea Nitrogen (BUN). Blood is a complex tissue which contains many biomarkers that can be used to assess the functioning of the body. These biomarkers are either by-products of some metabolites or some normal enzymes. Any violation of the normal homeostatic process may upgrade or degrade the quantities of these indicators. They can, therefore, be labelled as biomarkers or indicators. As the function of kidneys is to remove wastes from the body, therefore, any rise in the number of wastes in the blood will indicate a drop in kidney function. Some of these wastes serve as biomarkers for assessing kidney function, such as Urea, Creatinine and (BUN).
A. hypogae belongs to the family Fabacae. It is cultivated in different regions of the world, with China, India, Nigeria, and United States as the top producers. It has been used not only for its edible properties but also for its various pharmaceutical properties [14]. Here, we examined the nephroprotective potential of A. hypogea methanolic extract and n-hexane oil fraction using both in vitro (histopathological studies) and Swiss albino mice models. The scientific basis was to first induce the damage in animal kidneys and then study the repair capability of the tested compound. The difference in readings of the normal control group and the groups that received the doses of the extract and oil tested the hypothesis of its nephroprotective effect. After completion of doses to the animals, they were sacrificed for the isolation of blood and kidney tissues.
1.5 kg of fresh A. hypogaea seeds were purchased from a certified supplier located in the city of Mardan, Pakistan. Their shells and skins were removed, and kernels were weighed again. These kernels were ground into a fine powder using a grinder machine (Panasonic, Tokyo, Japan), resulting in the production of a viscous oily paste which was later dried to form a dry powder.
The plant extract was obtained by macerating the dried powder into a 90% methanol solution. Out of the ground powder of peanut, 900 mg was separated and taken into a well closable container which had been poured 5 L of methanol into. It was left in methanol (Sigma-Aldrich, Dorset, UK) for 14 days with a closed lid, during which time; it would be stirred 5 times daily. After 14 days, the macerated mixture was filtered through muslin cloth and then through commercially available filter paper (Whatman paper No. 1) (Fischer Scientific, Loughborough, UK). After that, the components of A. hypogaea that had been dissolved in methanol, were collected as filtrate. To optimise the yield, the same procedure was repeated. The filtrate collected was dried by using a rotary evaporator (GWSI, Zhengzhou China) (for 3 days). The total weight of the final extract obtained was 103 gm, which was then stored in a tightly closed lid for further activity.
It was obtained by taking 500 mg of peanut ground powder was taken in a container and 2.5 mL of the n-Hexane solution was poured into it, which was then left for 14 days in a tightly closed container (to prevent the evaporation of the oil). During these 14 days, it was constantly stirred at least 5 times daily for maximum oil yield. After the course of 14 days, the mixture was filtered through a fine muslin cloth followed by filtration through Whatman filter paper. The filtrate obtained was a white solution (1.8 L in volume). The solvent was removed by using a rotary evaporator (for 24 h). The final product (oil) obtained was yellowish translucent liquid (41 mL).
For the phytochemical screening of A. hypogaea, a methanol extract of it was used, which was tested for the presence of different phytoconstituents utilising the method of Harborne, 1973 [15].
1 mL of the methanolic extract was taken in a test tube and mixed with 2 mL of distilled water. To this, two drops of ferric cyanide (Sigma-Aldrich, Dorset, UK) were added, and the mixture was observed for dark green or blue-green colouration [15].
5 mL of crude methanol extract solution was taken in a test tube, and a few drops of concentrated sulphuric acid were added to it along with 500 mg of magnesium. The mixture was observed for dark red or pink colouration [15].
5 mL of distilled water was mixed with crude extract in a test tube and shaken vigorously. To this, a few drops of olive oil were added, and the mixture was observed for forming foam, a confirmatory indicator for the presence of saponins [15].
Dilute KOH (Fischer Scientific, Loughborough, UK) was added to 1 mL of crude extract. Blue-green or red colouration indicates the presence of quinones.
1 mL of sample solution was taken in a test tube, and a few drops of Dragendorff’s reagent (potassium bismuth iodide solution) (Fischer Scientific, Loughborough, UK) were added. It was observed for a reddish-brown precipitate that confirmed the presence of alkaloids [15].
The sample was taken in a test tube, and a few drops of concentrated Sulphuric acid were carefully added. The mixture was observed for the appearance of a red colour [15].
5 mL of the extract was taken in a test tube, and 2 mL of chloroform was added.
After mixing, 3 mL of concentrated H
5 mL of the extract was treated with 2 mL of glacial acetic acid containing one
drop of ferric chloride solution. This was underplayed with 1 mL of concentrated
H
International guidelines handled animals used in this activity and for this purpose, were approved by the ethical committee of Abdul Wali Khan University Mardan (AWKUM) (ethical approval ID: AWKUM/pharm-19). A total of 30 8-week-old Swiss Albino mice were obtained and were grouped into six groups. Before starting the activity, all the animals were weighed and physically examined. They were healthy and weighed 28–36 g, approximately. These animals were procured 24 h before starting the activity and were kept in ideal environmental conditions in the animal house of the Pharmacy Department, University of Peshawar. Handling of animals was carried out according to the standard operating procedures set up by the Department of Pharmacy, University of Peshawar. All animals successfully made it to the start of the experiment.
Before the selection of doses, both the extract and oil were first tested for their potential acute toxicity. For this purpose, OECD guideline No. 423 was followed [16]. These animals were first acclimatised for 24 h in the animal house environment. After that, four groups were selected, with four animals in each group (n = 4). Their behavioral pattern was noted and labeled as time 0 behavioral patterns. They have given doses per the following schedule: Group 1: Received 500 mg intraperitoneally (i.p); Group 2: Given 1 g of extract i.p; Group 3: Received 1.5 g of extract i.p; Group 4: Received 2 g of extract i.p. The same protocol was carried out for fixed oil as well. Three groups with four animals in each group (n = 4) were taken, and oil was administered as follows: Group 1: Received 3 mL intraperitoneally (i.p); Group 2: Given 6 mL of oil i.p; Group 3: Received 10 mL of oil i.p. After dosing, the subsequent change in behavior was observed and noted at time intervals as 30 minutes, one h, 2, 3 and 24 h, respectively (after dosing).
The procured animals (30 in number) were divided into six equal groups, each containing five animals. The detail of the dosing of these groups is:
Group I: Received 0.5 mL Normal Saline (NS) (i.p) for ten days.
Group II: Received Gentamicin in a dose of 100 mg/kg/day (i.p) for ten days with 0.5 mL 0.9% Saline Solution.
Group III: Received Silymarin dose of 50 mg/kg/day (i.p) for ten days as a standard nephroprotective agent an hour before giving Gentamicin 100 mg/kg/day and 0.5 mL 0.9% Saline Solution.
Group IV: Received 250 mg/kg/day crude extract of A. hypogaea (i.p) an hour before giving gentamicin for ten days at 100 mg/kg/day and 0.9% of 0.5 mL 0.9% Saline Solution.
Group V: Received 500 mg/kg/day crude extract of A hypogaea (i.p) an hour before giving Gentamicin for ten days at 100 mg/kg/day and 0.5 mL 0.9% Saline Solution.
Group VI: Received 1 mL/kg/day crude oil of A. hypogaea (i.p) an hour before giving Gentamicin for ten days at 100 mg/kg/day and 0.5 mL 0.9% Saline Solution.
The blood was collected in blood tubes for onward investigations of biochemical changes, and tissues were preserved in different bottles of 10% formalin solution for future histopathological examination [16, 17].
The nephroprotective action of A. hypogaea was investigated by using kidney tissues and blood of experimental animals. Blood was tested for biochemical changes, and tissues were examined for morphological changes.
The biomarkers for assessing kidney function are Urea, Creatinine and Blood Urea Nitrogen (BUN) [18]. In this activity, these biomarkers were used as indicators of nephrotoxicity or nephroprotection per these principles. To carry out this procedure, animals were prepared for sacrifice 24 h after completion of all doses. They were first anaesthetised by using chloroform. After anaesthetising, the animal’s heart was located and punctured using a syringe needle. The blood that came through the hand was collected in gel tubes. After collecting blood in gel tubes, these tubes were centrifuged for the collection of serum which was used to determine BUN, Urea and Creatinine using standard auto-analyser techniques (Brukes Company Ltd, Munich, Germany).
The supposed histopathological changes expected to occur in these groups were also observed. To carry out this procedure, the kidneys of all animals were separated, washed through NS solution, and taken in separate jars for each group, half full of 10% formalin. For observation of tissues, individual slides of each tissue of all groups were made. These tissues were first immersed in concentrated alcohol to prepare slides to remove water and formalin. Then it was washed through xylene solution and engrafted onto a block using molten paraffin wax. From these blocks, fragile sections were removed and stained on a slide using standard lab staining chemicals (haematoxylin and eosin) and then glued. They were similarly labelled according to their dosing groups. These prepared slides were then observed for supposed histopathological changes. To ensure unbiasedness in the study results, the dosing category of these groups was concealed from the said doctor. Nephrotoxic features of the tissues were subjectively graded from 0 to 3, where 0 means no nephrotoxicity of tubules and three means that almost all the tubules are dead [19].
The results obtained from biochemical observations were finally presented in
statistical terms. To carry out this, the data obtained was presented as + mean
standard error or SEM. The significance level of statistical data was determined
using one-way ANOVA (Analysis of Variance). The result was considered
statistically significant at p
Tests Results from the phytochemical screening tests of A. hypogaea extract have been tabulated in Table 1.
| Secondary metabolites | Crude extract of Arachis hypogaea |
|---|---|
| Tannins | +++ |
| Flavonoids | +++ |
| Saponins | +++ |
| Quinones | – |
| Alkaloids | +++ |
| Glycoside | + |
| Steroles | + |
| Cardiac glycosides | – |
| Key: +++ Indicate strong presence, ++ Indicate presence, + Indicates only trace of presence, – Indicates absence. | |
Preliminary phytochemical screening of A. hypogaea showed the presence of different biochemically active groups, including alkaloids, tannins, and flavonoids. Flavonoids have biological activities like antioxidant, anti-microbial, anti-inflammatory, and anti-cancer activities [19]. Moreover, alkaloids and tannins are also present in high quantities, which are the most bioactive compounds. They are known for their analgesic, antipyretic activities antioxidant and antimicrobial activities [20, 21].
Table 2 shows the results from acute toxicity tests of all groups treated with an extract of A. hypogaea. They were observed for obvious behavioural indicators like change in respiration, irritability, myo-tonicity, convulsions, mobility, sedation, and motor coordination. Determining the acute toxicity of a product before administering it to experimental animals is very important. It helps us select a dose that is less toxic to these animals. Behavioural modifications that resulted after administering doses were noted [22]. No lethality was recorded as an adverse effect. Based on the obtained results, doses of extract of 250 mg/kg/day and 500 mg/kg/day were considered safe.
| Dose | Time interval | Behavior | ||||||
|---|---|---|---|---|---|---|---|---|
| Respiration | Irritability | Myotonicity | Convulsions | Mobility | Sedation | Motor coordination | ||
| 0.5 gm | 30 min | Normal | NF | Normal | NF | Normal | NF | Normal |
| 1 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 2 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 3 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 1 gm | 30 min | Normal | NF | Normal | NF | Slightly down | NF | Normal |
| 1 hr | Slightly higher | NF | Normal | NF | Down | NF | Normal | |
| 2 hr | Slightly higher | NF | Normal | NF | Down | NF | Normal | |
| 3 hr | Normal | NF | Normal | NF | Slightly down | NF | Normal | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 1.5 gm | 30 min | Higher | Slightly | Normal | NF | Sluggish | NF | Normal |
| 1 hr | Faster | Irritable | Normal | NF | Immobile | NF | Diminished | |
| 2 hr | Higher | Irritable | Normal | NF | Sluggish | NF | Diminished | |
| 3 hr | Higher | Slightly | Normal | NF | Sluggish | NF | Normal | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 2 gm | 30 min | Fast | Moderately Irritable | Normal | NF | Immobile | NF | Normal |
| 1 hr | Faster | Irritable | Stiffness | NF | Immobile | NF | Diminished | |
| 2 hr | Higher | Irritable | Normal | NF | Sluggish | NF | Diminished | |
| 3 hr | Higher | Slightly | Normal | NF | Sluggish | NF | Diminished | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| NF, Not found; Min, Minutes; hr, Hours. | ||||||||
The toxicity of the AHO was studied in test mice at various doses, i.e., 3, 6 and 10 mL/kg/day (of peanut oil). No toxic effects were observed with the oil, except bulging at the injection site, which slowly disappeared. The reason for swelling may be the poor absorption or allergens present in peanut oil, as literature reports that peanut has 19 allergenic proteins [23]. The acute toxicity test gives an overview of the potentially toxic effects of peanut extract and oil, which clarifies that the doses employed in this activity are, by no means, lethal, as shown in Table 3.
| Dose | Time interval | Behavior | ||||||
|---|---|---|---|---|---|---|---|---|
| Respiration | Irritability | Myotonicity | Convulsions | Mobility | sedation | Motor coordination | ||
| 3 mL/kg/day | 30 min | Normal | NF | Normal | NF | Normal | NF | Normal |
| 1 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 2 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 3 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 6 mL/kg/day | 30 min | Normal | NF | Normal | NF | Normal | NF | Normal |
| 1 hr | Slightly higher | NF | Normal | NF | Down | NF | Normal | |
| 2 hr | Slightly higher | NF | Normal | NF | Slightly down | NF | Normal | |
| 3 hr | Normal | NF | Normal | NF | Slightly down | NF | Normal | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| 10 mL/kg/day | 30 min | Slightly higher | NF | Normal | NF | Normal | NF | Normal |
| 1 hr | Higher | Moderately Irritable | Normal | NF | Down | NF | Normal | |
| 2 hr | Slightly higher | NF | Found | NF | Down | NF | Normal | |
| 3 hr | Slightly higher | NF | Normal | NF | Slightly down | NF | Normal | |
| 24 hr | Normal | NF | Normal | NF | Normal | NF | Normal | |
| NF, Not found; Min, Minutes; hr, Hours. | ||||||||
Serum urea plays an important role in nephrotoxicity [24]; an increase in serum
level of urea is mainly attributed to a compromised kidney function [25]. Here,
the nephroprotective activity of the extracts (at the concentration of 250 and
500 mg) and the oil was studied. Our studies indicate that the extract and oil of
A. hypogaea have got nephroprotective effects, i.e., the serum
urea level of the gentamycin (GM) treated group was significantly higher
(p
 Fig. 1.
Fig. 1.Effect of methanol extract of A. hypogaea on serum urea
level of mice. Data are presented as mean
Creatinine is a by-product of creatine metabolism, which is constantly cleared from our body by the kidneys at a relatively constant rate. The level of creatinine in serum remains relatively stable. Due to its inverse relationship to the Glomerular Filtration Rate (GFR), serum creatinine level is used as a biomarker of kidney function. As the only pathway of creatinine excretion is GFR, a rise in serum creatinine may generally be attributed to diminished kidney function [26]. Thus, an increase in the creatinine level of the GM-treated group indicates GM toxicity to the nephrons.
The results in Fig. 2 show that the extracts (250 and 500 mg) showed superior activity compared to the oil in treating the damage caused by the GM. It was seen that the sections (250 and 500 mg) showed similar pharmacological activity to the standard drug (silymarin). This can be due to the presence of the antioxidants in the plant extract, main stilbenes like resveratrol and flavonoids and procyanidin trimers and tetramers (tannins), as all of these have got significant antioxidant activity [27].
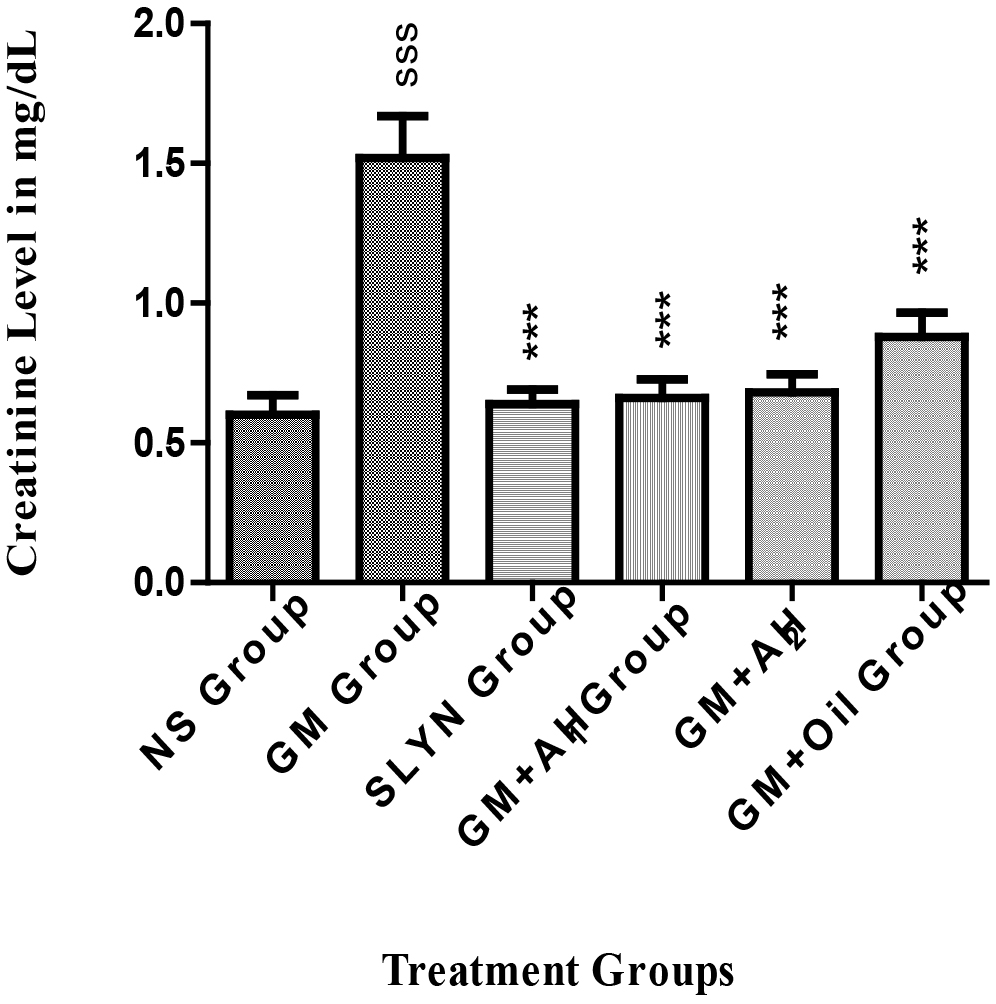 Fig. 2.
Fig. 2.Effect of methanol extract of A. hypogaea on serum
creatinine level of mice. Data are presented as mean + SEM with n = 5.
The Blood Urea Nitrogen (BUN) level of GM treated group was significantly higher
than the NS group (p
 Fig. 3.
Fig. 3.Effect of methanol extract of A. hypogaea on
serum BUN level of mice. Data are presented as mean
Aronson et al. [27] have shown BUN to be a sensitive biomarker for kidney changes; high BUN signifies a more significant shift in kidney hemodynamics that results mainly from toxicities. Narasimhan et al. [28] have further investigated that a rise in the blood level of BUN is an obvious denotation of renal impairment, which may lead to damage to other organs. A drop in BUN will, therefore, show nephroprotection. It may be assumed that the extract and oil of A. hypogaea have some healing effect on the harm caused by GM.
Histopathological changes in renal tissues in the treatment and inducer groups were determined. Change in GM treated group was noted relative to the NS group. Other groups were compared to GM treated group. The difference in these findings has been summarised in Table 4.
| Groups | Glomeruli loss | Tubular Harm | Vessels Loss | Fibrosis | Widespread Necrosis | Inflammation | |
| G-I | Nil | Nil | Nil | Nil | Nil | Nil | |
| G-II | Hydropic change | Widespread tubular loss | Immoderate perivascular loss | High | Immoderate | Acute and chronic | |
| G-III | UR | UR | UR | Nil | Nil | Nil | |
| G-IV | S1 | Unremarkable (UR) | Focal Minimal Tubular Loss | UR | Nil | Nil | Nil |
| S1 | UR | UR | Minimal perivascular Inflammation | Nil | Nil | Focal at places of tubular loss | |
| G-V | S1 | UR | Focal Minimal tubular loss | Minimal perivascular | Nil | Nil | Focal at places of tubular loss |
| S1 | UR | Focal Minimal tubular loss | Mild focal perivascular chronic inflammation | Nil | Nil | Mild Chronic focal inflammation | |
| G-VI | S1 | Mild Hydropic change | Moderate tubular necrosis | Mild chronic perivascular | Nil | Moderate | Mild focal chronic |
| S1 | Mild Hydropic change | Moderate loss | Moderate acute and chronic perivascular | Nil | Mild focal | Mild chronic | |
| The S1, S1 segment of the proximal tubule. | |||||||
An estimated 25% of all patients treated with aminoglycosides have developed nephrotoxicity in some form. These toxicity problems cannot be traced to a single cause. There are three general mechanisms through which aminoglycosides appear to exert their nephrotoxic effects: necrosis of nephron tubules, GFR shrinkage, and dwindling renal blood flow [29, 30]. These are:
Necrosis: It is thought to be the primary mechanism of aminoglycosides nephrotoxicity. Some active metabolic products of these parent anti-biotics enter vide endocytosis in the proximal tubule of the nephron cell, where they concentrate in lysosomes, the Golgi body, and the endoplasmic reticulum. Once this concentration crosses a baseline limit, they attack mitochondria in the cell’s cytosol. Damaging mitochondria further results in necrosis and apoptosis of the whole cell.
Shrinking glomerular filtration: In the glomerulus, there are tiny capillaries called mesangial cells, which perform the same function as that smooth muscle cells. When they stiffen due to some reason, it results in a reduction of GFR. Aminoglycosides induce the same effect, which results in the accumulation of waste products in the blood. These wastes don’t just reside inside blood; they will adversely affect the delicate internal nephron bed and lead to renal toxicity.
Reduction in Renal Blood flow: Like all other body cells, the nephron needs constant blood circulation. Aminoglycosides are thought to cause a decrease in renal blood flow—some metabolic by-products of aminoglycosides deposit in the renal vascular bed, leading to increased vascular resistance. Due to a higher-than-normal resistance in the renal vascular bed, blood filtration by the nephrons is severely compromised. This reduction accumulates metabolic wastes in the blood, primarily toxic to nephrons before any other body cell.
As discussed in the introduction section, currently, there is no data to support the difference in mechanism and degree of toxicity of aminoglycosides [31]; an investigation by Juan et al. [32] that GM causes toxicity through oxidative stress can also be linked to these mechanisms. All these mechanisms lead to metabolic disturbances that chemically increase oxidative stress and the formation of Reactive Oxygen Species (ROS), thereby harming renal tissues. They, in turn, will lead to hydropic changes of glomeruli, tubular loss, vascular inflammation, fibrosis, necrosis etc., of the renal tissues. Therefore, histopathological studies focused on observing these parameters, which were noted and compared. There were no histopathological changes in the regular saline-treated group (Group I). Fig. 4a,b shows the images taken from the slide by microscope. All cells in the tissue gave a uniform outlook, like the same tiles spread on the floor. Therefore, loss in glomerulus, tubules, and vessels; fibrosis, necrosis, and inflammation, and thus all were scored as zero.
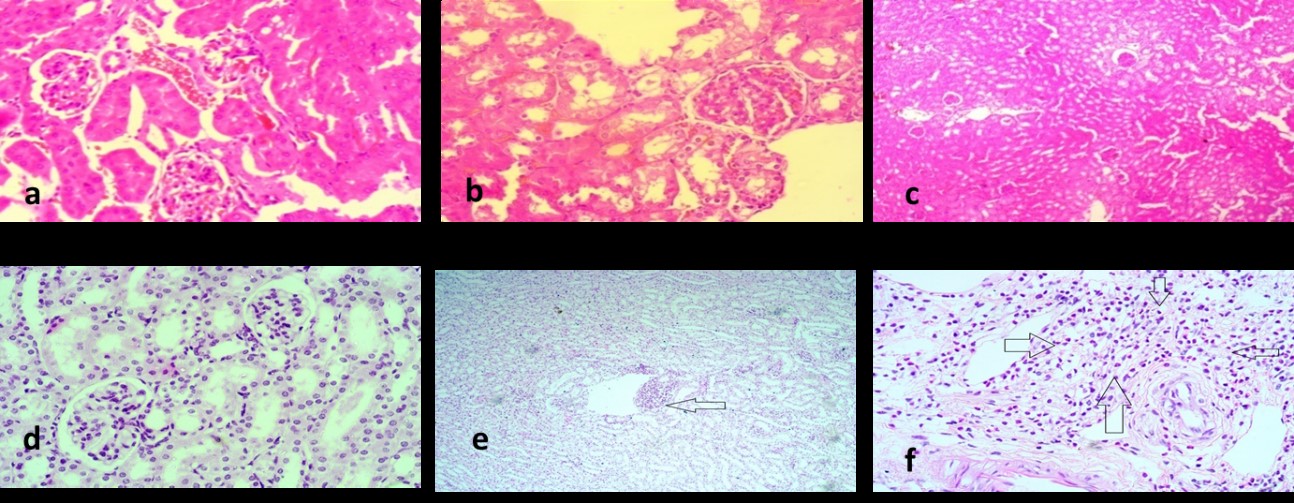 Fig. 4.
Fig. 4.Histopathological images. Figures showing histopathological images of (a) Normal saline group, (b) Gentamycin treated group, (c) Silamyrin treated group, (d) Ahcr1 treated group, (e) Ahcr2 treated group and (f) AHO treated group. Arrow indicates the lesions, the greater the nephrotoxicity more prominent the lesion.
Tissue slides of GM-treated group mice (Group II) showed major histopathological modifications compared to what was expected. Cells gave an uneven outlook. In some places, the nuclei of the cells had clustered together, while other sites remained empty. These slides recorded central tubular necrosis, changes in glomeruli and vascular inflammation. Fig. 4a showed tubular necrosis with intense glomerular degeneration. Fig. 4b showed grade III vacuolation, a common cause of the decline. Fig. 4c showed fibrosis of grade 3, which is a sign of significant inflammation. Cells contained no noticeable cytoplasm, and their morphology had been completely damaged. It showed that GM had induced maximum harm in the renal tissues.
Silymarin was given as a standard nephroprotective agent. In Group III, the animals given GM and Silymarin, the damage was significantly lower than that of the GM-treated group. No noticeable changes from normal were observed. Tubules and glomerulus remautterlyt and fibrosis and necrosis were utterly absent. A small number of cells, however, still gave a cluster shape. Fig. 4 shows the image from the group dosed with Silymarin and GM. A comparison of the GM-treated group and GM and Silymarin-treated group shows that Silymarin has prevented harm to renal tissues. Silymarin is an established hepatoprotective and nephroprotective, so it was used as a standard.
Treatment groups of A. hypogaea extract markedly reduced the toxicity
caused by GM. AH
The drop-in protective activity of 500 mg/kg/day dose against 250 mg/kg/day can be assumed to be due to a higher concentration of cytotoxic stilbenes of A. hypogaea. Abbott et al. [33] have investigated the antioxidant and cytotoxic activity of stilbenes of peanut plants which include resveratrol, arachidin-1 & 3. They found the least antioxidant and significant cytotoxic activity with arachidin-1 & 3 [33].
AHO-treated group or Group VI slides were observed with mild hydropic changes in the glomeruli, moderate loss of tubules and peri-vascular inflammation. It implies that oil treated group saw some nephrotoxicity due to simultaneous dosing of GM. However, in comparison to GM treated group, prevention of toxicity in histological specimens was still significant. The possible reason for greater harm in oil-treated tissues may either be lesser nephroprotective contents in oil chemistry, the presence of a higher concentration of arachidin-1 & 3 and other toxic contents in oil or due to lesser dose.
Co-administration of peanut extract with GM slowed down the worsening of kidney biomarkers compared to the GM-only treated group. Histopathological variations of these groups’ tissues also agree with the results. However, peanut oil did not record the same effects. The difference in the effects of extract and oil of A. hypogaea is assumed to be due to many reasons. First and foremost, the higher concentration of cytotoxic chemicals like arachidin-1 & 3 in oil. As discussed earlier, arachidin-1 & 3 are cytotoxic in nature, even in a small dose. Another reason may be that the amount of the oil that may have been chosen is less than enough to incite minimum effective concentration. In animals of the oil-treated group, it was found that oil takes some time to diffuse into the blood from the injection site. A dose of 1 mL/kg was selected for the activity to save animals from any associated problems.
Moreover, previous literature on the 1 mL/kg/day dose is also available. In this view, the experiment was carried out with the same amount. But results of this group showed less nephroprotective activity than the extract groups.
Another reason for the lesser nephroprotective effect of AHO may be less content of antioxidants. However, Sobolev et al. [34] have given a detailed log of resveratrol in peanut products but are mainly focused on peanut extracts. As explained in the introduction chapter, the nephroprotective effect is principally presumed due to the presence of resveratrol in peanut extract [35]. Contents of resveratrol in oil may be lesser than extract. Comparing the graphs and histopathological images are self-explanatory concerning the nephroprotective effect of oil and extract of A. hypogaea.
The results obtained from this proved the validity of the tested hypothesis. However, for future researchers on this plant for the same effect, the following suggestions are recommended:
(1) Due to less blood volume, mice are not recommended for this type of research. A more suitable research animal will be rabbits or guinea pigs with a higher blood volume.
(2) In the case of other oils, the dose may be even higher for the animal chosen to verify its nephroprotective effect.
(3) The content of resveratrol in oil and extract may be compared in particular.
Although hard work has been done, this study has some limitations, including but not the possibility of human error, especially in handling animal tissues. Moreover, this study lacks advanced analytical methods (HPLC etc.) for screening the phytochemical constituents.
The study had been designed to avert nephrotoxicity induced by GM in particular. Results of kidney biomarkers and histopathological observations of the treatment groups of the study indicated the promising nephroprotective effects of peanut extract and oil. These results (based upon the semiquantitative analysis) were further validated by the presence of chemical compounds with free radical scavenging activities. Thus, this study’s findings could pave the pathway for the commercial use of A. hypogaea extract and oil as a potential nephroprotective agent if further research is done.
All data generated or analyzed during this study are included in this published article.
Conceptualisation—HK and LCM; Data curation—AG, KFA and IUR; Formal analysis—AG, HK and SIS; Funding acquisition—HK and CA; Investigation—AG, SIS, SHQ and CA; Methodology—HK, SHQ and LCM; Resources—KFA and SHQ; Software—KWG; Supervision—HK; Validation—KWG; Visualization—KWG and LCM; Writing – original draft—AG and HK; Writing – review & editing—SIS, SHQ, IUR, CA and LCM.
International guidelines handled the ethical committee-approved animals used in this activity and for this purpose of Abdul Wali Khan University Mardan (AWKUM) (ethical approval ID: AWKUM/pharm-19).
Taif University Researchers Supporting Program (Taif University Saudi Arabia) supports this work (Project Number: TURSP-2020/153).
This research received no external funding.
The authors declare no conflict of interest. HK is serving as one of the Editorial Board members and Guest editors of this journal. We declare that HK had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to GP.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
