MicroRNAs (miRNAs) have been regarded as modulators in vascular pathologies,
including hypertension. Dysregulated proliferation and migration of VSMCs
(vascular smooth muscle cells) contributes to vascular remodeling during
hypertension. miR-634 was reported to be dysregulated in hypertensive patients.
The involvement of miR-634 in hypertension and the role of miR-634 on VSMCs
proliferation and migration were then evaluated. Firstly, HASMCs (human aortic
smooth muscle cells) were incubated with 2
Hypertension causes cardiovascular diseases and is considered as a world-wide disease [1]. The etiology of hypertension is complicated with various pathogenic factors. Environmental and genetic factors could lead to increased blood pressure through affecting physiological processes [2], thus leading to hypertension [3]. Overreaction of renin-angiotensin-aldosterone system, dysfunction of vascular endothelial cells, cardiac hypertrophy and platelet function injury have been widely regarded as pathogenesis of hypertension [4]. Meanwhile, vascular remodeling, associated with dysregulated proliferation and migration of VSMCs, is the central mechanism for hypertension [5]. Therefore, inhibition of VSMCs proliferation and migration represents an effective therapeutic strategy for hypertension [6].
miRNA, or microRNAs, are small non-coding RNAs with about 19–23 nucleotides in
length in a class of organisms [7]. It has been reported that miRNAs could be
involved in multiple pathological processes, including VSMCs proliferation and
migration, through targeting 3
Wnt/
All procedures involved in human subjects were approved by the Ethics Committee
of the First Affiliated Hospital of Xi’an Jiaotong University according to those
of the 1964 Helsinki Declaration and its later amendments for ethical research
involving human subjects. Forty-one health people with systolic blood pressure
| Healthy | Hypertension | P | |
| (n = 41) | (n = 68) | ||
| Gender (F/M) | 22/19 | 31/37 | 0.414 |
| Age, years | 44.93 |
46.29 |
0.175 |
| Disease duration (years) | 0 | 6.25 |
|
| BMI (kg/m |
22.98 |
22.73 |
0.447 |
| Overall SBP (mmHg) | 118.21 |
150.30 |
|
| Overall DBP (mmHg) | 69.97 |
89.31 |
|
| Daytime SBP (mmHg) | 121.58 |
149.43 |
|
| Daytime DBP (mmHg) | 78.13 |
99.60 |
|
| Glucose (mg/dL) | 84.74 |
86.15 |
0.102 |
| Total cholesterol (mg/dL) | 174.79 |
179.19 |
0.226 |
| HDL (mg/dL) | 55.31 |
52.87 |
0.236 |
| LDL (mg/dL) | 95.97 |
100.86 |
0.238 |
| Triglyceride (mg/dL) | 93.08 |
97.55 |
0.414 |
| CrCl (mL/min per 1.73 m |
106.02 |
110.40 |
0.416 |
| MAU (mg per 24 h) | 15.42 |
17.25 |
0.177 |
| CIMT (mm) | 0.54 |
0.78 |
|
| CRP (mg/L) | 1.47 |
2.13 |
0.001 |
| miR-634 expression | 1.05 |
0.56 |
|
| Pearson chi-square test is for Gender and T test for others. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; CrCl, creatinine clearance; MAU, microalbuminuria; CIMT, carotid intima media thickness; CRP, C-reactive protein. | |||
HASMCs (human aortic smooth muscle cells) or human vascular smooth muscle cells
(HVSMCs) were acquired from Lonza (Rockland, ME, USA) and cultured in Smooth
Muscle Cell Medium (SciencCell, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (Lonza) at 37
Mimic (5
HASMCs (4000 cells/well) were seeded and then treated with or without different
treatment or transfection. After the different treatment, cultured medium of
HASMCs were changed to fresh medium containing 50
HASMCs (30000 cells/well) in serum-free medium were seeded to the top of the
chamber with 8
The 3
Plasma RNAs, miRNAs or RNAs extracted from HASMCs were isolated and
reverse-transcribed into cDNAs. qRT-PCR analysis was conducted with QuantiTect
SYBR Green PCR Master Mix (Qiagen, Valencia, CA, USA). GAPDH or U6 were used as
endogenous controls. The primer sequences were showed as below: miR-634 (F:
5
For isolation of cytoplasm and nuclear fractionation, HASMCs were harvested and
suspended in isolation buffer A containing protease inhibitors (HiScript II First
Strand cDNA Synthesis Kit; Vazyme Biotech, Nanjing, Jiangsu, China). After
rotating for 1 minute and centrifuging at 12000 g for 5 minutes, supernatant with
cytoplasm fraction was collected. The debris was suspended in isolation buffer B
containing protease inhibitors, and then rotated for the collection of nuclear
fractionation. For western blot analysis, proteins (30
All the experiments were performed at least in triplicates. Data were expressed
as mean
To establish cell model of hypertension, HASMCs were incubated with culture
medium containing 2
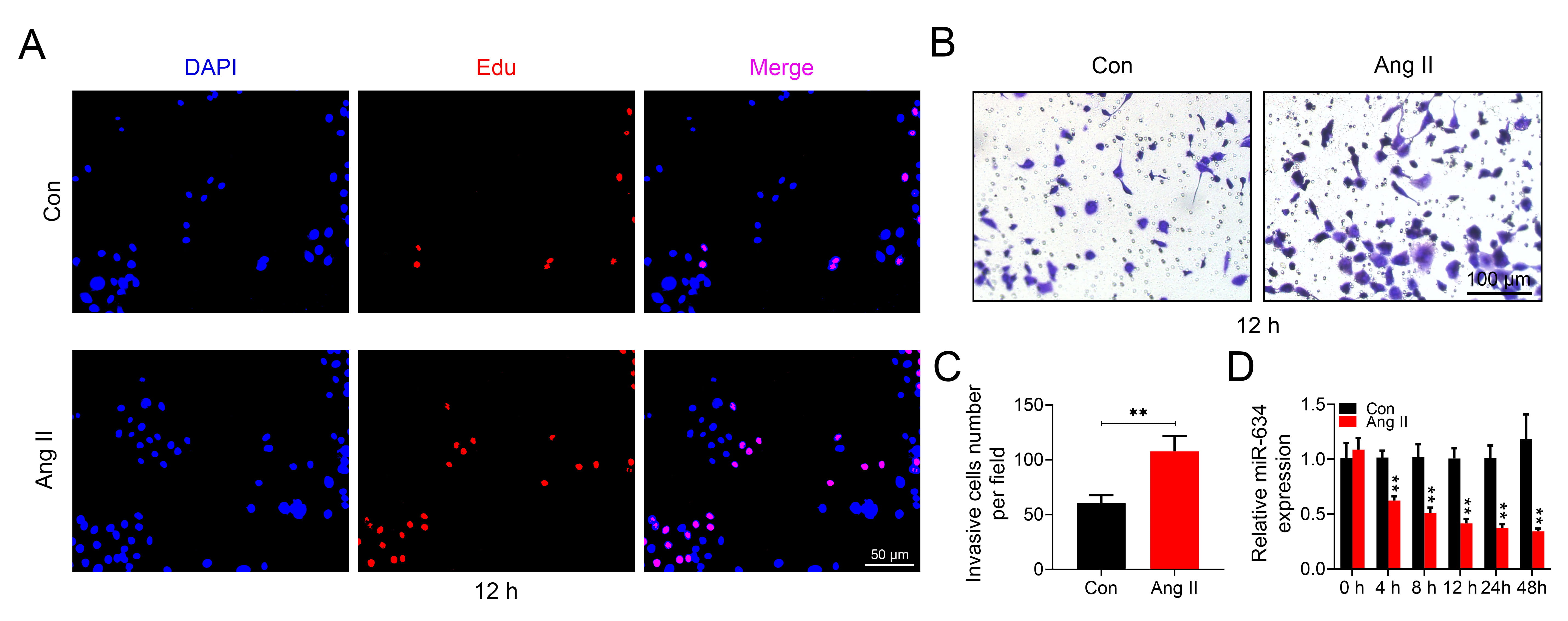 Fig. 1.
Fig. 1.miR-634 was reduced in Ang II-induced HASMCs.
(A) The effect of 2
To evaluate the role of miR-634 in HASMCs proliferation and migration, HASMCs or HVSMCs were transfected with miR-634 mimic and then treated with Ang II. qRT-PCR confirmed the up-regulation of miR-634 in HASMCs (Fig. 2A) and HVSMCs (Supplementary Fig. 1A) transfected with miR-634 mimic. Over-expression of miR-634 suppressed cell proliferation (Fig. 2B) and migration (Fig. 2C,D) of HASMCs, as evidenced by the decreased EdU incorporation (Fig. 2B) and invasion cells number (Fig. 2D) in HASMCs transfected with miR-634 mimic compared with cells transfected with negative control. Moreover, transfection with miR-634 mimic suppressed the cell proliferation (Fig. 2B) and migration (Fig. 2C,D) of Ang II-induced HASMCs, suggesting that miR-634 might contribute to amelioration of hypertension. The cell proliferation (Supplementary Fig. 1B) and migration (Supplementary Fig. 1C,D) of HVSMCs were also promoted by Ang II treatment, while reduced by over-expression of miR-634.
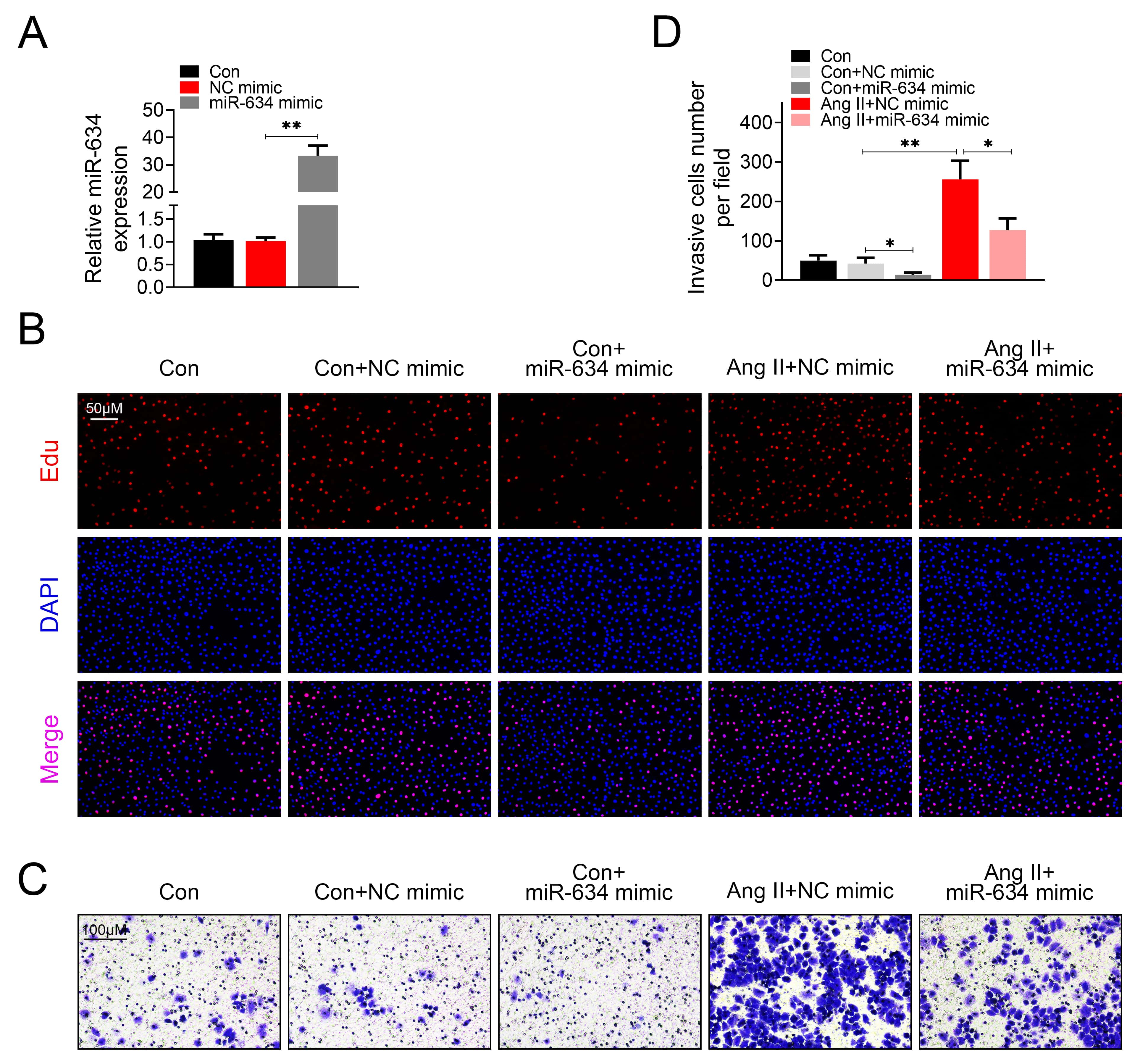 Fig. 2.
Fig. 2.miR-634 suppressed Ang II-induced HASMCs proliferation and
migration.
(A) Transfection efficiency of miR-634 mimic in HASMCs was detected by qRT-PCR.
(B) Effect of miR-634 on cell proliferation of HASMCs with or without Ang II
treatment. (C) Effect of miR-634 on cell migration of HASMCs with or without Ang
II treatment. (D) Effect of miR-634 on invasion cells number of HASMCs with or
without Ang II treatment. *, **, p
To investigate the mechanism of miR-634 in HASMCs, the target gene of miR-634 was predicted as Wnt4 via Targetscan (http://www.targetscan.org/vert_72/) (Fig. 3A). Luciferase activity of pGL3-WT-Wnt4 was decreased in HASMCs transfected with miR-634 mimic compared with the negative control (Fig. 3B), while activity of pGL3-MUT-Wnt4 was not affected by miR-634 mimic compared with the negative control (Fig. 3B), suggesting that miR-634 could target Wnt4 in HASMCs. To validate effect of miR-634 on Wnt4 expression, HASMCs were transfected with miR-634 mimic or inhibitor (Fig. 3C). Results revealed that mRNA (Fig. 3D) and protein (Fig. 3E) of Wnt4 were reduced by miR-634 mimic, while enhanced by the inhibitor. Moreover, Wnt4 was up-regulated in HASMCs after 12 hours of Ang II treatment (Supplementary Fig. 2A,B). These results indicated that miR-634 could target Wnt4 and repress its expression.
 Fig. 3.
Fig. 3.Target gene of miR-634.
(A) Potential binding target of miR-634 was predicted as Wnt4. (B) The effect of
miR-634 on luciferase activities of pGL3-WT-Wnt4 and pGL3-MUT-Wnt4. (C)
Transfection efficiency of miR-634 mimic or inhibitor in HASMCs was detected by
qRT-PCR. (D) Effect of miR-634 on mRNA expression of Wnt4 in HASMCs. (E) Effect
of miR-634 on protein expression of Wnt4 in HASMCs. ** p
To investigate the role of miR-634/Wnt4 axis in HASMCs, Ang II-induced HASMCs
were transfected with pcDNA-Wnt4 or cotransfected with miR-634 mimic and
pcDNA-Wnt4. The transfection efficiency of pcDNA-Wnt4 was shown in
Supplementary Fig. 3. Results showed that over-expression of Wnt4
aggravated the promotive effects of Ang II on cell proliferation (Fig. 4A) and
migration (Fig. 4B,C). However, cotransfection with miR-634 mimic and
pcDNA-Wnt4 attenuated the promotive effects of Wnt4 on Ang II-induced HASMCs
proliferation (Fig. 4A) and migration (Fig. 4B,C). These results showed that
miR-634/Wnt4 axis participated in regulation of Ang II-induced HASMCs
proliferation and migration. As a downstream target of Wnt4, nuclear distribution
of
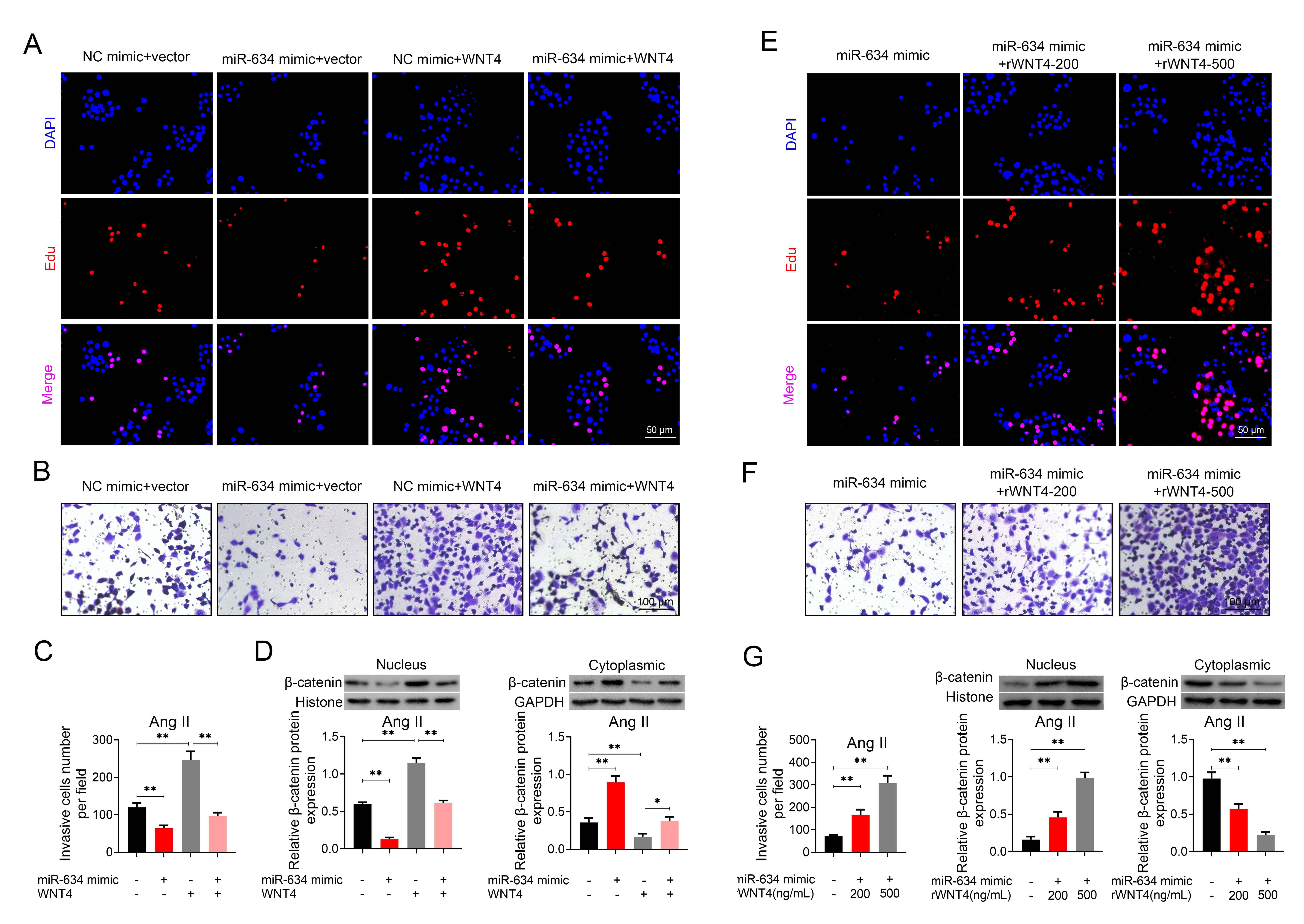 Fig. 4.
Fig. 4.Over-expression of Wnt4 counteracted the suppressive effects of
miR-634 on Ang II-induced HASMCs proliferation and migration.
(A) Effect of miR-634 mimic and pcDNA-Wnt4 on cell proliferation of HASMCs with
Ang II treatment. (B) Effect of miR-634 mimic and pcDNA-Wnt4 on cell migration of
HASMCs with Ang II treatment. (C) Effect of miR-634 mimic and pcDNA-Wnt4 on cell
migration of HASMCs with Ang II treatment. (D) Effect of miR-634 mimic and
pcDNA-Wnt4 on nucleus and cytoplasmic distribution of
Expression level of miR-634 in hypertensive patients was evaluated by qRT-PCR.
Plasma samples from healthy individuals or hypertensive patients were analyzed
and the result showed a significant decrease in miR-634 in hypertensive patients
compared with health individuals (p
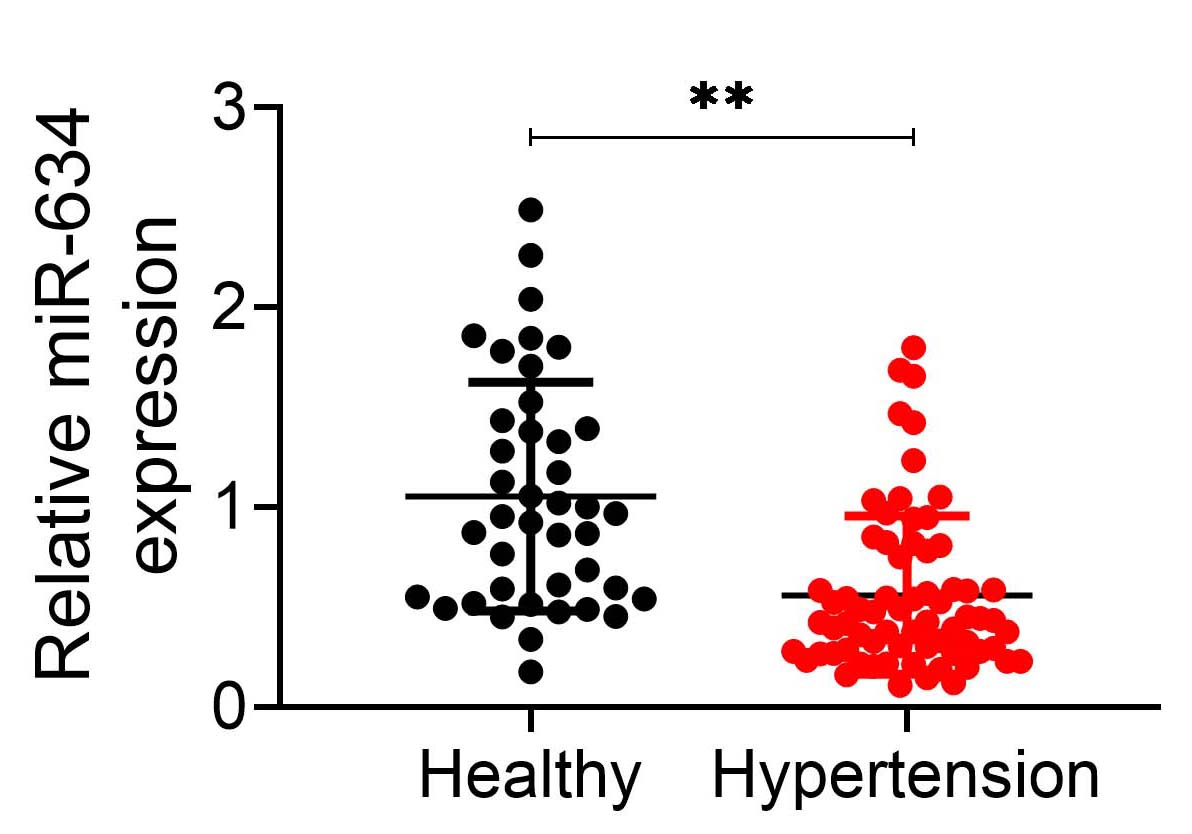 Fig. 5.
Fig. 5.miR-634 was reduced in hypertensive patients.
Expression level of miR-634 in hypertensive patients and healthy individuals. **
p
miRNA regulate renal function to maintain normal blood pressure [21]. Extracellular vesicles secreted by miR-27a promoted blood pressure and led to hypertension [22]. miRNA target VSMCs to mediate vascular resistance or remodeling for the development of hypertension [23]. For example, reduction of miR-34a resulted in increased proliferation of human pulmonary artery smooth muscle cells and contributed to pulmonary arterial hypertension development [24]. Over-expression of miR-17-5p regulated cell proliferation of human pulmonary artery smooth muscle cell through targeting arginase II [25]. Abrogation of pulmonary artery smooth muscle cells proliferation and migration by miR-233 led to the attenuation of vascular remodeling and pulmonary arterial hypertension [26]. However, whether miR-634 is associated with VSMCs proliferation and migration, thus regulating hypertension, remains unclear.
Previous study has indicated that miRNAs could be dysregulated in plasma samples or renal tissues of hypertensive patients, thus representing biomarkers of hypertension [27]. Down-regulation of miR-634 in idiopathic pulmonary hypertension patients [12] or hypertensive patients [13] have also been reported. Data from the present study revealed that miR-634 was down-regulated in the plasma of hypertensive patients. However, the smaller sample size, as well as devoid of clinicopathological correlation between miR-634 expression and hypertensive patients, restricted the use of circulating miR-634 as biomarker for hypertensive patients.
Ang II, a peptide hormone with vasoconstrictor effect, was elevated in plasma of essential hypertension [28]. Ang II could induce phenotypic transformation of VSMCs [29], and promote proliferation and migration of VSMCs, leading to vascular remodeling for the development of hypertension [30]. Therefore, Ang II-induced HASMCs has been widely used as a cell model of hypertension [31]. Our results also showed increased HASMCs proliferation and migration by Ang II treatment. Moreover, miR-634 was reduced in Ang II-induced HASMCs in a time-dependent manner, suggesting the potential role of miR-634 in VSMCs.
Inhibition of Ang II-induced VSMCs proliferation and migration could facilitate the amelioration of hypertension [32, 33]. miR-634 has been reported to demonstrate anti-tumor activity by inhibition of cancer cell proliferation and migration [34, 35, 36]. Data from EdU staining and transwell assay showed that miR-634 suppressed Ang II-induced HASMCs proliferation and migration, suggesting that miR-634 might be a potential target for the intervention of hypertension. Moreover, regulation of VSMCs differentiation [37] or contraction [38] participate in development of hypertension. The effect of miR-634 on VSMCs differentiation and contraction needs to be further investigated. Tissue macrophages play an important role in the pathogenesis of hypertension [39]. Macrophage-derived exosomes promoted the inflammation of endothelial cells, thus participating in hypertension [40]. Antibodies against endothelial cells mediated pulmonary arterial hypertension through regulation of endothelial cell apoptosis [41]. Since endothelial cells have been shown to release hyperpolarizing factors to modulate VSMCs and implicated in the pathophysiology of hypertension [42]. The regulatory role of miR-634 on other cells (endothelial cells, macrophages and immune cells) involved in hypertension should be investigated.
Prediction via Targetscan 7.2
(http://www.targetscan.org/vert_72/) and
validation by luciferase activity assay further confirmed that miR-634 could
target Wnt4 in HASMCs. Moreover, miR-634 could decrease the expression of Wnt4.
As reported before, Wnt4 was increased during proliferation process of VSMCs
[43], and activation of Wnt pathway could contribute to VSMC proliferation and
migration [44]. Therefore, inhibition of Wnt pathway could be useful for
suppression of VSMCs proliferation and migration, thus attenuating hypertension
[45]. Here, our results indicated that over-expression of Wnt4 promoted VSMCs
proliferation and migration, and counteracted the suppressive effects of miR-634
on Ang II-induced HASMCs proliferation and migration. These results suggested
that miR-634 could attenuate hypertension through inhibition of Wnt pathway. Wnt
could activate Disheveled and rescue
In general, our study for the first time demonstrated that miR-634 functioned as
a novel regulator of VSMCs proliferation and migration via inhibiting
Wnt4/
LN and YK designed the study, supervised the data collection, analyzed the data, NS interpreted the data and prepare the manuscript for publication, LK and YX supervised the data collection, analyzed the data and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Ethical approval was obtained from the Ethics Committee of the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (approval number: XJTU1AF2017LSL-001).
Not applicable.
This work was supported by the National Natural Science Foundation of China (Grant No. 81770426).
The authors declare no conflict of interest.
All data generated or analyzed during this study are included in this published article.
miRNAs, microRNAs; Ang, angiotensin; UTR, untranslated region.
