† These authors contributed equally.
Oncolytic adenovirus has been applied in cancer therapy because of several advantages such as cost-effective production, high transduction efficiency and low toxicity. Recent efforts have been focused on the modification of oncolytic adenovirus by encoding transgenes within the viral genome to efficiently and selectively replicate within cancer cells, destroy cancerous cells, induce tumor cell apoptosis, and stimulate the recruitment of immune cells to the tumor site. Nevertheless, there are still big challenges for translational research of oncolytic virotherapy in clinical cancer management. Therefore, here we summarize current status on the design and application of oncolytic adenovirus vectors for prostate cancer therapy. In particular, we describe the main receptors associated with the tropism and transduction of oncolytic adenovirus vectors, and propose new directions in future studies for prostate cancer virotherapy.
Prostate cancer (PCa) is a common cancer in the male worldwide, and most of PCa can be cured by surgery or radiotherapy in the early stage [1]. However, up to 15% of initial patients are diagnosed to develop metastatic lesions, and recurrence rate of patients after conventional radical therapy is more than 40% [2]. Androgen deprivation therapy (ADT) has been developed for recurrent PCa, but some patients still relapse because of the progression of castration-resistant prostate cancer (CRPC) [3]. Since traditional therapies including chemotherapy and radiotherapy are not highly effective and have obvious side effects of cytotoxicity for CRPC, novel therapeutic strategies are urgently needed. Oncolytic adenovirus as a new viral therapy agent for CRPC has gained increasing attention due to several advantages such as high selectivity, low cytotixicity and oncolysis characteristics.
Adenovirus (Ad) has been the workhorse of virotherapy since 1950s. Unfortunately, due to the rapid development of chemotherapy and safety concern of virotherapy, the enthusiasm for virotherapy suddenly faded away later [4]. Only recently, virotherapy has become a hot topic with the development of Ad and retroviral vectors for the delivery of a variety of transgenes targeting different types of cancers [4]. Therefore, in this review we summarize current status on the design and application of oncolytic Ad vectors for PCa therapy.
To develop Ad vector as a novel approach of cancer therapy, it is important to understand the genome structure of Ad in order to design recombinant Ad vectors. Ad is a non-enveloped virus with double-stranded DNA genome. Total 103 Ads can be divided into seven ‘species’ named A to G based on their genotypes [5]. Among them, A and C species are the most prevalent, and Ad serotype 5 (Ad5) is mainly used in the studies on Ad [6].
There are five early transcription units (E1A, E1B, E2, E3 and E4), two delayed transcription units (IX and Iva2) and one late transcription unit (L1-L5) in Ad 5 coding region [7]. E1A conserved region 2 (E1A-CR2) interacts with retinoblastoma (Rb) in host cells to promote S-phase entry and viral DNA replication [7]. E1B contains E1B-19K and E1B-55K. E1B-19K is a functional Bcl-2 homologue and plays a dual role in apoptosis and autophagy [8]. E1B-55K could promote virus survival in tumor cells, but is not necessary for oncolytic effects of adenoviruses [9]. E2 region encodes polymerase, DNA binding protein (DBP) and preterminal protein (pTP), which are important for viral transformation and replication. E3 region encodes adenovirus death protein (ADP), which promotes the cytolysis of host cells and the spread of the virus to the surrounding cells [10]. E4 region plays an important role in transition and late viral gene expression, and is vital for viral replication and virion assembly (Fig. 1).
 Fig. 1.
Fig. 1.Illustration of the structure and function of Ad5 genome. LITR and RITR indicate left and right inverted terminal repeats, respectively. Abbreviation: E1A-CR2, E1A conserved region 2; Rb, retinoblastoma; ADP, adenovirus death protein; DBP, DNA-binding protein; pTP, precursor terminal protein; Pol, polymerase.
Ad5 is categorized into two vectors based on oncolytic character: conditionally replicating adenoviral (CRAd) vector which only propagates and lyse cancer cells, and could not replicate in normal cells; and replication-defective adenoviral (RDAd) vector with the deletion of E1, which could not replicate in cells but can carry therapeutic genes [11].
To achieve optimal anti-tumor efficacy of Ad vector, we need develop Ad vector
with improved tropism and transduction. Therefore, we need identify cellular
receptors that mediate the tropism and transduction of Ad. Cell attachment of
oncolytic adenovirus is initiated by the attachment of the fiber protein to CAR
receptor [12]. Next, the interaction between Arg-Gly-Asp (RGD) motifs in Ad
penton-base protein and
Both CAR and
The upregulation of
Coagulation factor X (FX) is an adapter for coagulation factors involved in liver tropism following systemic delivery [23]. Ad5 recognizes FX via hexon hypervariable region, and FX then interacts with heparan sulfate proteoglycans (HSPGs) on the surface of liver cells [24]. Ablating the binding of FX to Ad5 can diminish virus localization to the liver. Warfarin pretreatment significantly reduced liver sequestration and hepatic toxicity of Ad vectors [25].
On the other hand, Ad shows high affinity to scavenger receptor-A (SR-A) and scavenger receptor expressed on endothelial cell-I (SREC-I). Kupffer cells (KCs) and liver sinusoidal endothelial cells (LSECs) recognize Ad and remove them from the circulation to inhibit efficient hepatocyte transduction (Fig. 2). The efficiency of hepatocyte transduction by Ad could be increased by blocking SR-A and SREC-I [26]. Two peptides PP1 and PP2 have been designed to block SR-A and SREC-I, respectively, and they significantly improved Ad-mediated hepatocyte transduction efficiency [27].
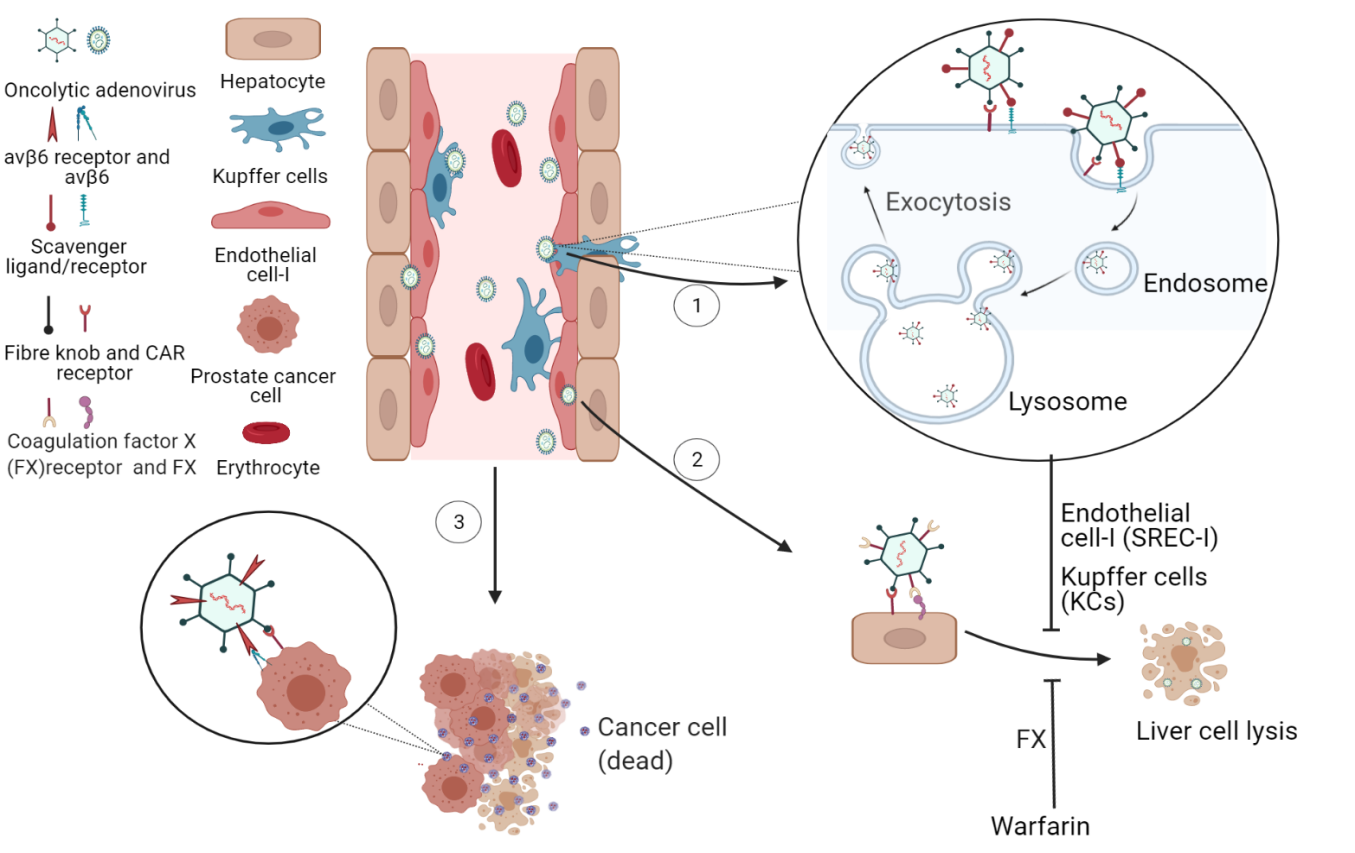 Fig. 2.
Fig. 2.The ligands and receptors involved in Ad tropism and
transduction. (1) Ad is picked by SR-A and SREC-I on KC and LSEC, and then is
internalized through pinocytosis and then passed to lysosome. Most Ads are
released from hepatocyte by first-pass effect which reduces the tropism and
transduction and protects the hepatocytes from lysis. (2) Ad binds to FX and then
FX binds to HSPGs on liver cells. Warfarin significantly reduces liver
sequestration and hepatic toxicity. (3) Ad binds to CAR on PCa cells and then is
internalized via
To improve the potency of oncolytic adenovirus, incorporating exogenous genes or
deleting genes of adenovirus backbone is the first approach. For example,
p53 gene, a well-known pro-apoptosis gene, was introduced into Ad to
generate Ad-p53 to induce cell death pathways in tumor tissues [28]. Oncolytic
mutant Ad
The incorporation of prostatespecific promoter/enhancer leads to virus
replication and the induction of the expression of exogenous genes only in
prostate cancer cells [8]. Prostate specific antigen (PSA) promoter has been
utilized in Ad mediated gene therapy against PSA-positive PCa. For example,
Ad/PSAP-GV16-
Prostate specific membrane antigen (PSMA) is primarily expressed in PCa cells and highly expressed during PCa metastasis [31, 32, 33]. PSMA promoter based Ad vector Ad-PSMA (E-P)-CD drove the expression of cytosine deaminase and efficiently kill PSMA-producing CL-1 xenograft tumor with combined use of prodrug 5fluorocytosine [34].
PB promoter as a prostate-specific promoter was also utilized. Ad-ARR2PB-Bax vector contained PB promoter and two androgen response elements (ARR). PB promoter drove the expression of pro-apoptotic Bax and induced apoptosis in LNCaP xenograft tumor. Therefore, PB promoter based Ad vector has potential to target AR positive PCa [35].
Prostate cancer gene 3 (PCA3/DD3) is a PCaspecific marker identified recently. Hao et al. [36] designed OncoAd.mK5.DD3 vector to drive the expression of mK5 (the mutational kringle5 of human plasminogen) by DD3 promoter specially in PCa cells and the results showed that OncoAd.mK5.DD3 was able to inhibit PCa efficiently. Taking advantages of the sensitivity and specificity of DD3 as PCa marker, a test kit Progensa™ (Gen-Probe Inc. San Diego, CA, USA) was developed for the detection of PCa cells in urine [37].
Ad5 recognizes coxsackievirus and adenovirus receptor (CAR) on host cells via fiber protein. Therefore, the modification of fiber could change virus tropism [38]. The incorporation of RGD motif in fiber protein into AdRGD-PGp53 led to enhanced transduction in PCa cells and upregulation of p53 expression, with effective anti-tumor activity both in vitro and in vivo [39]. On the other hand, Ad.5/3-CTV oncolytic virus was engineered with the change of Ad.5 fiber knob to Ad.3 fiber knob, and it facilitated virus infection in a CAR independent manner, showing higher efficiency in human PC cells with low CAR expression [40].
Since anti-tumor effect of Ad is partially mediated by virus induced immune response, oncolytic immunotherapy gains more attention recently. An oncolytic Ad (Ad5-yCD/mutTKSR39rep-mIL12) was designed to express pro-inflammatory cytokine IL-12 and suicide gene, the high anti-tumor efficacy provided the support for further development of this approach in clinical trials [41].
GM-CSF (Granulocyte-macrophage Colony Stimulating Factor) is an
immune-modulatory cytokine that induces the activation of monocytes and
macrophages, and promotes T-cell mediated anti-tumor response [42].
Ad5
The combination of Ad with CD40L-based costimulatory molecule induced both humoral and cellular immunity against many types of cancer. Ad-PL-PPT-E1A was constructed with the fusion of PSA and CD40L and it exhibited enhanced anti-tumor activity, which could be a promising approach for gene therapy of advanced PCa [45].
Combination therapy with immune checkpoint blockade efficiently kills tumor [46]. PD-L1 inhibits T cell function against solid tumors, which may decrease anti-tumor effect of chimeric antigen receptor-modified T cells (CAR T-cells) [47]. Therefore, Ad vector engineered to express PD-L1 blocking antibody has become a strategy to enhance anti-tumor efficacy of CAR T-cells [48].
To avoid the destruction of virus particles by the immune system and enhance systemic delivery of virus particles, the use of autologous cells as carriers has been explored recently [49]. Mesenchymal stem cells (MSC) mediated delivery of Ad vector overcame the barrier to systemic delivery of Ad vector, and improved intratumoral dissemination of Ad vector [50].
Oncolytic Ads are often combined with other therapies. The combination of
virotherapy and chemotherapy has shown synergistic response in PCa cells to
effectively kill tumor cells and reduce side effects [51]. The combination of
Ad
Acute single high dose rate (HDR) radiation of PCa cells 24 h before the infection with Ad vector containing PSA enhancer and PB promoter led to significantly enhanced virus replication and cell lysis [54]. In addition, the uptake of radioiodine by injecting Ad carrying the hNIS gene linked to PSMA (Ad. PSMApro-hNIS) had been estimated. The anti-tumor efficacy of radioiodine was significantly improved in C81 cell xenograft model [55]. The results based on 125I nuclide labelled 125I-RSOAds-hTERT/PSA could provide new options for the treatment of PCa [56].
Interleukin (IL)-24 exerted inhibitory effects on various cancer cells by enhancing immune regulation and inhibiting tumor growth, angiogenesis and metastasis [57]. Recently, we reported that the combination of Ad vector ZD55-IL-24 with chemotherapy or radiotherapy significantly inhibited the growth of androgen-independent PCa cells and activated the apoptosis of these cancer cells xenografts in vivo [58, 59]. These data suggest that the combination of chemotherapy or ionizing radiation and oncolytic Ad vector expressing IL-24 leads to synergistic anti-tumor effect on PCa.
Elucidating the molecular mechanism of virus-host interactions of oncolytic
adenovirus is essential to the development of better therapy for PCa. The
identification of FX,
On the other hand, we should pay attention to the variety in the phenotypes and surface receptors in PCa cells. Although primary cancer cell culture is a golden standard of in vitro model, it could not mimic in vivo situation of PCa perfectly. A patient-derived xenograft (PDX) model was developed based on direct engraftment of patient tumor into immunocompromised mice, and it retained most characteristics of primary tumor [61]. The design of novel PCa in vivo model will hope realize personalized therapy for PCa patients in near future.
LM and JW designed the study. CW, FW, ZX collected and analyzed the literatures. RW and JC analyzed the literatures and made the figures. All authors participated in writing the draft and approved the final manuscript.
Not applicable.
Not applicable.
This study was supported by grants from the Project of Invigorating Health Care Through Science, Technology and Education (No. CXTDA2017034), and Jiangsu Provincial Medical Youth Talent (No. QNRC2016794).
The authors declare no conflict of interest.
