The anti-tumor effect of resveratrol has been observed in many cancers. Here, we examined the anti-tumor activity of resveratrol in human nasopharyngeal carcinoma (NPC) cells. Resveratrol, in a dose-dependent manner, inhibited proliferation related proteins (Ki67, PCNA), and cell proliferation, and reduced apoptosis related proteins (cleaved caspase-3, cleaved caspase-9) and apoptosis in nasopharyngeal carcinoma cells. Resveratrol treatment inhibited the increased-expression of Survivin in NPC cells, while the overexpressed Survivin counteracted the effect of resveratrol on cell proliferation and apoptosis in NPC cells, thus establishing Resveratrol-induced reduction in increased-survivin in NPC cells as the underlying mechanism. These findings show that resveratrol can be used to modify the cell growth and death in NPC cells.
Nasopharyngeal carcinoma (NPC) is a type of head and neck cancer with a distinct racial and geographic distribution across the world (1). Most cases of NPC are often found in Southern China and Southeast Asia (2). A great number of studies suggest the vital role of multiple factors, including genetic susceptibility, Epstein-Barr virus (EBV) infection, environmental and dietary factors, in the carcinogenesis of NPC (3). NPC have high tendency of invasion and lymph node metastasis, contributing to the poor prognosis of NPC patients (1). Although the combination of chemotherapy and radiotherapy can improve the 5-year survival rate of NPC patients, the overall prognosis still remains poor with increased rate of relapse or metastasis (4). Therefore, the underlying molecular mechanism of NPC carcinogenesis needs immediate revelation, which may expose novel molecular targets towards its treatment.
Resveratrol (trans-3, 4’, 5-trihydroxystilbene) is a natural polyphenolic phytoalexin which has been found in the skin of many fruits, including grapes, berries and peanuts (5). Accumulating evidence indicate that resveratrol is able to regulate various molecular targets and signaling pathways involved in cell proliferation, inflammation and cell cycle (6, 7). Moreover, several studies also appreciate the anti-tumor effects of resveratrol, for it can trigger apoptosis in a great number of cancer cell lines. For example, Kumar et al. showed that resveratrol can induce superoxide species-independent apoptosis in murine prostate cells and might act as a therapeutic agent against prostate cancer (8). Chen et al. also reported that resveratrol exhibited multiple tumor-suppressing activities in bone cancer by affecting a series of critical events, like inducing apoptosis, in cancer cells (9). Therefore, we hypothesize that resveratrol may act as an effective therapeutic agent in the treatment of NPC.
An imbalance between cell proliferation and apoptosis may result in tumor formation, and apoptosis plays a crucial role in the regulation of tissue homeostasis (10). Survivin is a protein that can effectively inhibit apoptosis and regulate cell cycle (11). A recent study by J.H.Cai et al. elucidated an inverse correlation between the mean survival time of NPC patients and Survivin protein or mRNA expression (12). Thus, Survivin may play an important role in onset and development of NPC.
In our current study, we report the inhibitory effect of resveratrol on Survivin expression in NPC cells, promoting apoptosis and suppressing proliferation of the treated cells. Our findings suggested that resveratrol may serve as an effective therapeutic agent against NPC.
Human immortalized nasopharyngeal epithelial cell line (NP69) and NPC cell lines (CNE1, CNE2, HONE1, C666-1) were purchased from American Type Culture Collection (ATCC). Cells were cultured in RPMI-1640 complete culture medium (Gibco, USA) supplemented with 10% FBS (HyClone, GE Healthcare Life Science, Logan, USA) in a humidified atmosphere of 5% CO2 at 37 °C.
Resveratrol was obtained from Sigma (St. Louis, MO, USA), and a stock solution of 100 mmol/L was prepared by dissolving it in 100% DMSO and stored at -20˚C. NPC cells received different concentrations (25, 50,100 μM) of Resveratrol during experimentation.
Cell Counting Kit-8 (CCK-8) assay was performed to assess cell proliferation. Cells were seeded at a density of 5 ×103 cells per well in 96-well plates and incubated with different concentrations of resveratrol treatment, from Day 0 to Day 5. At indicated time points, 10 μL of CCK-8 solution (Beyotime) was added to the cultures and incubated at 37°C for 2 h. The absorbance was measured at 450 nm using a microplate spectrophotometer (Molecular Devices, Sunnyvale, USA). Triplicate wells were used in each group.
Both naive and Resveratrol treated NPC cells were lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China), according to the manufacturer’s protocol. Protein concentrations were determined by BCA method. Same amount of proteins was separated by 10% SDS-PAGE gel and then transferred into PVDF membranes (Millipore). After blocking with 5% BSA, the membrane was treated with primary antibodies (Santa Cruz Biotechnology: anti-Ki67, anti-PCNA, anti-cleaved-caspase-3, anti-cleaved-caspase-9, anti-Survivin and anti-GAPDH) and incubated overnight at 4˚C. Following day, post wash, membrane was incubated with HRP-conjugated secondary antibodies for 1 h at room temperature, and signals were detected by ECL.
Cell apoptosis analysis was performed using an Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (Multisciences, Shanghai, China). In brief, 5x105 cells, washed with PBS and resuspended in binding buffer, were incubated with 5 μL of Annexin V-FIFC and 10 μL of PI for 15 min in the dark. Post incubation, 400 μL of binding buffer was added and cell apoptosis was analyzed in a flow cytometer (BD Biosciences).
Cells were seeded onto 96-well plate to reach 60% confluence for transfection. To induce Survivin overexpression, following manufacturer’s protocol, NPC cells were transfected with pcDNA3.1.-GFP-Survivin; a full-length of Survivin sequence obtained from GenePharma subcloned into pcDNA3.1.-GFP vector, using Lipofectamine 2000 (Invitrogen, USA).
All data are represented as the mean ± standard deviation (SD). Statistical analysis was performed by Student’s t-test or one-way ANOVA using SPSS 19.0. software. Values of P < 0.0.5 were considered statistically significant.
We first assessed the effect of resveratrol on the proliferation of NPC cells. CNE-1 and HONE1 NPC cells, treated with different concentrations of resveratrol, were subject to CCK-8 assay. Our data showed that resveratrol inhibited the proliferation of both CNE-1 and HONE1 cells in a dose-dependent manner (Figure. 1A-1B, *P < 0.0.5, **P < 0.0.1). We also detected the expression of apoptosis-related proteins through western blot assay. Relative expression of Ki67 and PCNA, in both CNE-1 and HONE1 cells, was significantly down-regulated by resveratrol in a dose-dependent manner (Figure 1C-1D, *P < 0.0.5, **P < 0.0.1). These results suggest resveratrol as a potential inhibitor of proliferation of NPC cells; higher the concentration of resveratrol, stronger the suppression effect on proliferation.
 Figure 1.
Figure 1.Resveratrol inhibits proliferation of NPC cells. NPC cells (CNE1, HONE1) were treated with different concentrations of resveratrol. (A-B) The cell proliferation was measured through CCK-8 assay. (C-D) Relative expression of Ki67 and PCNA was detected through western blot. The bars showed means ± SD of three independent experiments. *P < 0.0.5, **P < 0.0.1 compared with control group.
Next, we explored the effect of resveratrol on apoptosis of NPC cells. Results from cell apoptosis assay indicated that resveratrol induced apoptosis of both CNE-1 and HONE1 NPC cells at all the exposed concentrations. The effect significantly increased with the increase in resveratrol dose (Figure 2A-2D, *P < 0.0.5, **P < 0.0.1). We then measured relative expression of apoptosis-related proteins, cleaved caspase-3 and cleaved caspase-9, through western blot asssay. Resveratrol treatment significantly increased relative protein expression of both cleaved caspase-3 and cleaved caspase-9, compared to control group (Figure 2E-2F, *P < 0.0.5, **P < 0.0.1). Our data suggested that resveratrol treatment resulted in a dose-dependent induction of apoptosis in NPC cells.
 Figure 2.
Figure 2.Resveratrol induces apoptosis of NPC cells. NPC cells (CNE1, HONE1) were treated with different concentrations of resveratrol. (A-D) Cellular apoptosis was detected by flow cytometric analysis. (E-F) Relative expression of cleaved caspase-3 and cleaved caspase-9 was detected through western blot. The bars showed means ± SD of three independent experiments. *P < 0.0.5, **P < 0.0.1 compared with control group.
Then we explored the relationship between resveratrol and Survivin. Relative expression of Survivin was up-regulated in CNE1, CNE2, HONE1 and C666-1 NPC cell lines, but not in NP69 cells (Figure 3A, *P < 0.0.5, **P < 0.0.1). After treating CNE1, CNE2, HONE1, C666-1 cells with different concentrations of resveratrol, the change in Survivin expression was detected through western blot. Our data showed that resveratrol significantly suppressed the relative expression of Survivin in all the treated NPC cells (CNE1, CNE2, HONE1, C666-1), in a dose-dependent manner (Figure 3B-E, *P < 0.0.5, **P < 0.0.1). These results indicated a direct possible effect of resveratrol on Survivin expression in NPC cells.
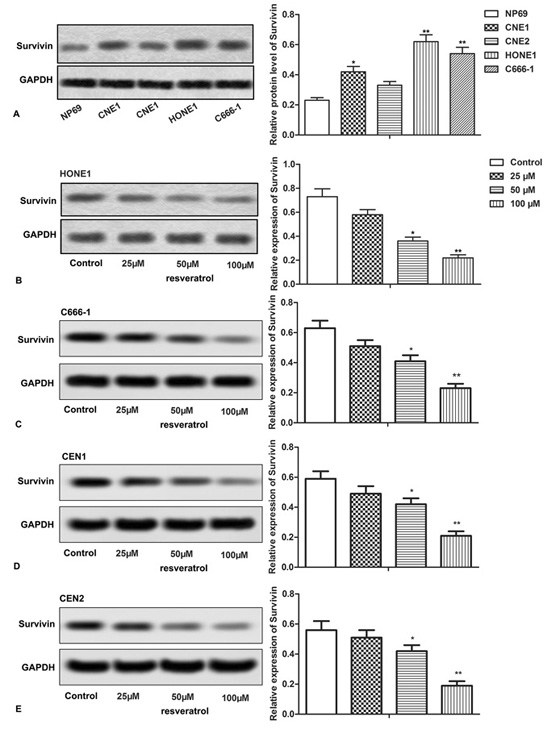 Figure 3.
Figure 3.Resveratrol suppresses the expression of Survivin. (A-E) Relative expression of Survivin was detected through western blot assay. The bars showed means ± SD of three independent experiments. *P < 0.0.5, **P < 0.0.1 compared with NP69 or control group.
To further investigate the interaction between resveratrol and Survivin, CNE-1 and HONE1 cells were transfected with pcDNA3.1.-GFP-Survivin that significantly increased the expression of Survivin (Figure 4A-4D, **P < 0.0.1). Both the survivin overexpressing and naive CNE-1 and HONE1 cells were treated with 100 μM resveratrol, and assessed for cell proliferation and apoptosis. We found that overexpressed Survivin remarkably promoted cell proliferation and inhibited cell apoptosis rate despite the presence of resveratrol (100 μM) (Figure 4E-4H, *P < 0.0.5, **P < 0.0.1, #P < 0.0.5). In addition, resveratrol (100 μM) was unable to counteract the overexpressed-Survivin induced significant increase in the expression of Ki67 and PCNA and decrease in the expression of cleaved caspase-3 and cleaved caspase-9 (Figure 4I-4L, *P < 0.0.5, **P < 0.0.1, #P < 0.0.5). Above results indicated that overexpressed Survivin counteracted the effects of resveratrol on cell proliferation and apoptosis of NPC cells.
 Figure 4.
Figure 4.Overexpressed Survivin counteracts the effects of resveratrol in NPC cells. HONE1 and CEN1 cells were transfected with or without pcDNA3.1.-GFP-Survivin and received 100 μM resveratrol treatment. (A-D) Relative expression of Survivin was detected through western blot analysis. (E,G) The cell proliferation was measured through CCK-8 assay. (F,H) Cell apoptosis was detected by flow cytometric analysis. (I-L) Relative expression of Ki67, PCNA, cleaved caspase-3 and cleaved caspase-9 was detected through western blot assay. The bars showed means ± SD of three independent experiments. *P < 0.0.5, **P < 0.0.1 compared with control group. #P < 0.0.5 compared with resveratrol 100 μM group.
During the past decades, natural antioxidants extracted from plants or fruits have received extensive attention for their anti-tumor effects. Numerous phytochemicals derived from edible plants have been reported to interfere at a specific stage of the carcinogenic process (13). As one of the natural polyphenolic phytoalexins, resveratrol has been found to induce multiple beneficial effects on health, including anti-aging, anti-oxidant and anti-tumor functions (6, 7). In recent years, the anti-tumor effects of resveratrol have been identified in numerous in vitro and in vivo studies with various types of cancers (14-16). In our present study, we demonstrated that resveratrol suppressed cell proliferation while induced cell apoptosis through inhibiting the expression of Survivin in NPC cells, suggesting that resveratrol may serve as an effective agent against NPC.
Previous studies have shown that resveratrol inhibited proliferation of cells in various types of cancers. For instance, a study by Zhou et al. indicated that resveratrol suppressed SKM-1 (an advanced myelodysplastic cell line) proliferation in a dose-dependent manner through inhibiting CCND1 expression (17). Notas et al. reported that resveratrol exerted its anti-proliferative effect on HepG2 cells, a human hepatocyte-derived cancer cell line, by inducing cell cycle arrest at the G1 and G2/M-phases (18). Study by Chai et al. showed that resveratrol inhibits proliferation and migration through SIRT1 mediated posttranslational modification of PI3K/AKT pathway in hepatocellular carcinoma cells (19). In agreement with previous studies, our data showed that resveratrol inhibited cell proliferation of NPC cell lines (CNE1, HONE1), in a dose dependent manner. Cell proliferation is a complex process controlled by a large number of genes including PCNA and Ki67. PCNA is reported to play a crucial role in DNA replication, DNA repair and cell cycle control (20). Ki67 can be detected only during the growth and synthesis phase but not during the resting phase of cell cycle (21). In our study, we observed that resveratrol treatment inhibited the expression of both PCNA and Ki67 in NPC cells. In summary, resveratrol can suppress cell proliferation of NPC cells.
Apoptosis is a normal physiologic process which is important in the maintenance of cellular homeostasis by eliminating malicious cells (22). Numerous studies have shown that resveratrol can induce apoptosis in various types of cancer cells and several mechanisms of resveratrol-mediated apoptosis have also been elucidated. Resveratrol was reported to cause apoptosis of DU 145 prostate cancer cells through activation of MAPK, increase in cellular p53 content, serine-15 phosphorylation of p53 and increased p53 binding to DNA (23). Lin’s study elucidated that resveratrol caused apoptosis in human head and neck squamous cell carcinoma cells (UMSCC-22B cells) by inducing nuclear accumulation of COX-2 (24). Similarly, in our study, we also found that resveratrol increased cell apoptosis rates in NPC cells in a dose-dependent manner. The mechanisms of apoptosis include two main pathways: the extrinsic pathway and the intrinsic pathway which involve two major caspase cascades leading to apoptosis (25, 26). Caspase-9 is an initiator caspase which can activate caspase-3 in the apoptotic signaling cascade, and cleaved caspase-9 is an active form of caspase-9. Caspase-3 is cleaved to form the active caspase-3 enzyme which degrades multiple cellular proteins and is responsible for morphological changes and DNA fragmentation in cells during apoptosis (27, 28). The data from our study showed that resveratrol up-regulated the expression of cleaved caspase-3 and cleaved caspase-9 in NPC cells, suggesting a resveratrol mediated induction of apoptosis in NPC cells. Taken together, we found that resveratrol could act as an effective therapeutic agent against NPC, for it could suppress cell proliferation and promote apoptosis of NPC cells.
The underlying mechanism of the above effect was further assessed by studying Survivin, a member of the “inhibitor of apoptosis protein” (IAP) family that plays a key role in the control of cell division and the inhibition of apoptosis (29). Increased expression of Survivin has been found in majority of human cancers and cancer patients with high Survivin expression often have unfavorable outcomes (30, 31). As reported, overexpression of Survivin increased the cell proliferation in oral squamous cell carcinoma (32) The anti-apoptosis effect of Survivin makes it a useful diagnostic marker for human cancer as well as an attractive target for new anticancer interventions (33). In previous study, resveratrol was reported to inhibit proliferation and induce apoptosis through Survivin down-regulation in hepatocellular carcinoma (34). Another study showed that resveratrol induced melanoma apoptosis by suppression of survivin through STAT3/β-catenin (35). In our experiments, we found that the up-regulated level of Survivin in NPC cells was down-regulated by Resveratrol in a dose-dependent manner. To further elucidate the relationship between Survivin and resveratrol, CNE-1 and HONE1 cells were transfected with pcDNA3.1.-GFP-Survivin to further increase the expression of Survivin. We observed that overexpressed Survivin counteracted the effects of resveratrol on cell proliferation and apoptosis of NPC cells, suggesting that overexpression of Survivin increased the cell growth while resveratrol inhibited cell proliferation and promoted cell apoptosis of NPC cells by suppressing the expression of Survivin. However, the further mechanism was not discussed. We will next study whether resveratrol regulates the STAT3/β-catenin signal.
In conclusion, resveratrol exerted dose-dependent effects on the inhibition of cell proliferation and induction of apoptosis in NPC cells via inhibiting the expression of Survivin. Findings from our study is hoped to help in the identification of novel molecular targets of resveratrol for the treatment of NPC.
We sincerely appreciate the technical support from Chinese PLA Navy General Hospital.
