Cerebral ischemia reperfusion (I/R) injury is associated with a high incidence of neurological morbidity and mortality worldwide. Higenamine has anti-inflammatory, anti-oxidative and anti-apoptotic capacities and has been successfully used in myocardial and intestinal ischemia reperfusion. We hypothesized that higenamine might serve the same effects in cerebral I/R. In a rat model of cerebral I/R, higenamine improved functional state of nerves, significantly inhibited the I/R-induced increase in the serum level of tumor necrosis factor α (TNF-alpha) and interleukins (ILs) such as IL-1, IL-6 and IL-18, and CD14+ cells, while decreasing the axonal nerve degeneration. Together, the data demonstrate that higenamine has therapeutic effect in cerebral I/R injury.
Cerebral ischemia is triggered by insufficient blood supply to the brain, resulting in irreversible brain damage with high morbidity and mortality (1). Reperfusion induced oxidative damage would aggravate cerebral injury by activating free radical reactions in the brain tissue. The most vulnerable region to cerebral ischemia-reperfusion (I/R) injury is the hippocampal CA1 area, which is responsible for learning and memory (2). Further, cerebral I/R could dysfunctionalize cerebral microcirculation, resulting in increased permeability of cerebral blood-brain barrier (BBB) and elevated extravasation of serum proteins in the perivascular space. Thus, early and appropriate treatment is crucial to lowering brain damage.
Higenamine (1-((4-hydroxyphenyl)methyl)-1,2,3,4-tetrahydroisoquinoline-6,7-diol), a plant based alkaloid, is mainly extracted from different plants, e.g., Nandina domestica, Annona squamosa, Nelumbo nucifera, Aconitum, Gnetum parvifolium and Tinospora crispa. According to published reports, higenamine could activate α-AR signaling to suppress platelet aggregation (3) and motivate β-AR signaling to control muscle contractions (4). Recently, researchers discovered that higenamine could defend brain cells against anoxic damage via enhancing HO-1 levels (5), suggesting that ischemic injuries could be potentially treated using higenamine.
Limited ability of nerves to regenerate is the key reason for invalid restoration after focal cerebral ischemia. Therefore, improving the morphological and functional recovery of nerves can be the prime target in the treatment of cerebral I/R injury. Previous studies have suggested that some traditional Chinese medicines possess neuroprotective effects, demonstrated by inhibiting the death of nerve cells and loss of axons in various nerve diseases. For example, the polysaccharides of Lycium barbarum have been demonstrated to play an important role in neuroprotection (6). Besides that, MPTP/MPP+ induced dopaminergic neurodegeneration and motor disturbance could be alleviated by harpagoside by up-regulating glial cell line-derived neurotophic factor (7). Furthermore, Hiroshi and colleagues observed that both spontaneous and nerve-evoked acetylcholine could be up-regulated by higenamine (8). Thus, higenamine may be a potential drug in treating cerebral I/R injury.
The main pathological feature of cerebral I/R injury is neuroinflammation characterized by various complex cellular activities and processes such as production of inflammatory factors that includes tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-18 and tumor necrosis factor (TNF)-α (9). Furthermore, higenamine can also reduce inflammatory reaction by inactivating NF-κB, and thus can be beneficial in conditions like endotoxemia (10). In addition, in a murine intestinal I/R injury model, higenamine could bring down the intestinal injury score by increasing the expression of HO-1, while also reducing the levels of inflammatory cytokines, in vivo (11). These studies indicate the potential of higenamine in the treatment of cerebral I/R injury.
In the current study, we investigated whether higenamine could blunt cerebral I/R injury and the underlying molecular mechanisms in vivo. Cerebral I/R rats were treated with higenamine at different concentrations. Results suggested that cerebral I/R injury could be significantly suppressed by higenamine through inducing axonal regeneration and inhibiting inflammatory reaction.
150 SPF male rats (weighting 250±20 g) were obtained from the Center of Laboratory Animals, Zhejiang University and housed at 22±2°C under a 12-hour light/dark cycle for 1 week. The experimental procedures were performed strictly following the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
The cerebral I/R injury model was established through middle cerebral artery occlusion (MCAO) as originally described (12). In brief, post-anesthesia, the left middle cerebral artery (MCA) of sprague dawley (SD) rats were occluded by filament at the level of alar foramina. Reperfusion was accomplished 2 hour later, by withdrawing the filament. During the procedure, the internal temperature of rats was maintained at 37°C using a heating pad. Post procedure, surgical wounds were closed using sterile techniques.
Higenamine (Figure 1A) was purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). 150 SD rats were randomly divided into 5 groups (n=30); Control group: healthy rats, sham group: rats which received surgery without artery ligation, model group: I/R model rats, I/R + higenamine (low) group: I/R rats which received higenamine (10 mg/kg body weight) by intraperitoneal injection once a day, and I/R+ higenamine (high) group: I/R rats received higenamine (50 mg/kg body weight) by intraperitoneal injection once a day. Every 6 rats in each group were sacrificed at day 3, 7, 14 and 28 for subsequent analysis.
 Figure 1
Figure 1Higenamine alleviated brain ischemic injury caused by I/R. Rats were randomly divided into 5 groups. Control group: healthy rats; Sham group: rats received surgery without artery ligation; model group: I/R model rats; I/R+ higenamine (low) group: IR rats treated with higenamine (10 mg/kg); I/R+ higenamine (high) group: IR rats treated with higenamine (50 mg/kg). A. The structure of higenamine. B. BBB was detected using EB extravasation. C. mNSS was used to evaluate nerve injuries. D. HE staining of brain tissues was applied to assess the morphological changes of hippocampus CA1 area. Experiments were repeated at least 3 times, and error bars represent ± SD. (**P < 0.01, ***P < 0.001 versus control and sham groups; #P < 0.05 versus I/R model group).
BBB permeability was measured by detecting EB extravasation, as described elsewhere (13), at 3, 7, 14, and 28 days post-surgery. Briefly, EB solution was injected intravenously into I/R rats. Post sacrifice through heart perfusion, the collected brain samples were incubated in 50% trichloroacetic acid, followed by homogenization and centrifugation. The extracted supernatant was diluted with ethanol and EB concentration was measured using spectrophotometer (Bio-Rad680, USA; 620 nm excitation, 680 nm emission).
Neurological deficit was detected using modified Neurological Severity Score (mNSS) at 3, 7, 14, and 28 days after reperfusion following MCAO, as reported elsewhere (14).
Using HE staining, the morphological structure of CA1 hippocampal region was assessed in all experimental groups of SD rats, at 2 weeks post-MCAO, as reported previously (15). Images were captured under the Olympus CX23 light-microscope (Olympus, Tokyo, Japan).
Venous blood samples were extracted from rats at 2 weeks post-MCAO. The concentrations of IL-6, TNF-α, IL-1β and IL-18 were measured using the respective ELISA Assay Kit (Meixuan Co. Ltd, Shanghai, China), according to manufacturer’s instructions.
After the rats in each group were euthanized, at 2 weeks post-MCAO, brain tissues were carefully recovered to assess the status of axons within. The samples were subject to immunohistochemical staining for neurofilament 200 (NF-200), as described before (16). Images were captured under optical microscope (Olympus, Tokyo, Japan).
Total proteins were extracted from brain tissue using BCA kit (Boster, Wuhan, China), at 14 days after MCAO. It was further assessed for the levels of NF-200, growth associated protein 43 (GAP-43), repulsive guidance molecular A (RGMa), CD14, toll like receptor 4 (TLR4), TAK1 (TGF-β activated kinase 1) (Abcam, Cambridge, UK) and nucleoproteins (NF-κB p65, MIP-2, COX-2) (Abcam, Cambridge, UK) using western-blot technique. The experimental protocol strictly followed manufacturer’s instructions.
At 14 days after MCAO, the presence of CD14+ cells in peripheral blood was assessed by flow cytometry. The method was performed strictly according to manufacturer’s instructions using FACS (BD Bioscience, Shanghai, China).
Statistical analysis was performed using SPSS 17.0 (Statistical Package for the Social Sciences, IL, USA). All results were presented as mean ± SD. The statistical significance of the studies was analyzed using one-way ANOVA. The difference was considered statistically significant at P < 0.05.
The EB extravasation assay demonstrated that both high and low concentration of higenamine could suppress the increased BBB permeability at 14 and 28 days post-MCAO (Figure 1B). mNSS score analysis showed that the score was closest to 5 points at 14 days and 28 days in I/R + higenamine groups, compared with other groups (Figure 1C). Thus, 14th day was chosen as the optimal time point for the subsequent experiments. HE staining suggested that the neuronal degeneration was reduced in IR + higenamine groups, compared to I/R model group, especially when dosed at higher concentration (50 mg/kg) of higenamine (Figure 1D). These results indicate that higenamine could attenuate brain ischemic injury caused by I/R.
The impact of higenamine on axonal regeneration was assessed by detecting the expression level of related proteins. Immunofluorescence staining revealed a significant decrease in the level of FN-200 in I/R model group, compared to control and sham group (Figure 2A-B). However, treating with higenamine, especially at the concentration of 50 mg/kg, significantly increased the FN-200 expression in I/R rats, compared to untreated group (Figure 2C). Western blot analysis revealed a similar result in GAP-43 expression, the expression of which was increased in higenamine treated I/R rats. Further, increased levels of RGMa in I/R rats was also inhibited by higenamine (Figure 2C). These results suggest that higenamine may induce axonal regeneration.
 Figure 2
Figure 2Higenamine induced axonal regeneration. Rats were randomly divided into 5 groups. Control group: healthy rats; Sham group: rats received surgery without artery ligation; model group: I/R model rats; I/R+ higenamine (low) group: IR rats treated with higenamine (10 mg/kg); I/R+ higenamine (high) group: IR rats treated with higenamine (50 mg/kg). A. The level of NF-200 in brain tissues was measured using immunohistochemical staining. B. Histogram represents the statistical analysis of NF-200 expression according to the result of immunohistochemical staining. C. Western-blot was applied to detect the expression levels of NF-200, GAP-43 and RGMa in brain tissues. Experiments were repeated at least 3 times, and error bars represent ± SD. (**P < 0.01, ***P < 0.001 versus control and sham groups; #P < 0.05, ##P < 0.01 versus I/R model group).
ELISA was employed to determine the anti-inflammatory effect of higenamine in I/R rats. As shown in Figure 3, the I/R induced enhanced expression of TNF-α, IL-1β, IL-6 and IL-18 were remarkably repressed by higenamine, especially at the concentration of 50 mg/kg. The results demonstrated that I/R iinduced inflammatory response could be effectively suppressed by higenamine.
 Figure 3
Figure 3Higenamine reduced inflammatory response in I/R rats. Rats were randomly divided into 5 groups. Control group: healthy rats; Sham group: rats received surgery without artery ligation; model group: I/R model rats; I/R+ higenamine (low) group: IR rats treated with higenamine (10 mg/kg); I/R+ higenamine (high) group: IR rats treated with higenamine (50 mg/kg). The expressions of TNF-α, IL-1β, IL-6 and IL-18 in serum were measured by ELISA assay. Experiments were repeated at least 3 times, and error bars represent ± SD. (**P < 0.01 versus control and sham group; #P < 0.05, ##P < 0.01 versus I/R model group).
Various inflammatory signaling pathways were activated in the process of I/R injury. The effect of higenamine on these pathways was assessed by measuring the associated proteins in peripheral blood using flow cytometry and western-blot. The results revealed that the I/R induced increase in the number of CD14+ cells was remarkably suppressed by higenamine (Figure 4A). Further, the increased levels of CD14, TLR4, TAK1, NF-κB, MIP-2, COX-2 in I/R model group, compared to control and sham group, were significantly reduced in I/R + higenamine (high) group (Figure 4B). These findings illustrate that the higenamine can inactivate inflammatory signaling pathways to control inflammatory response.
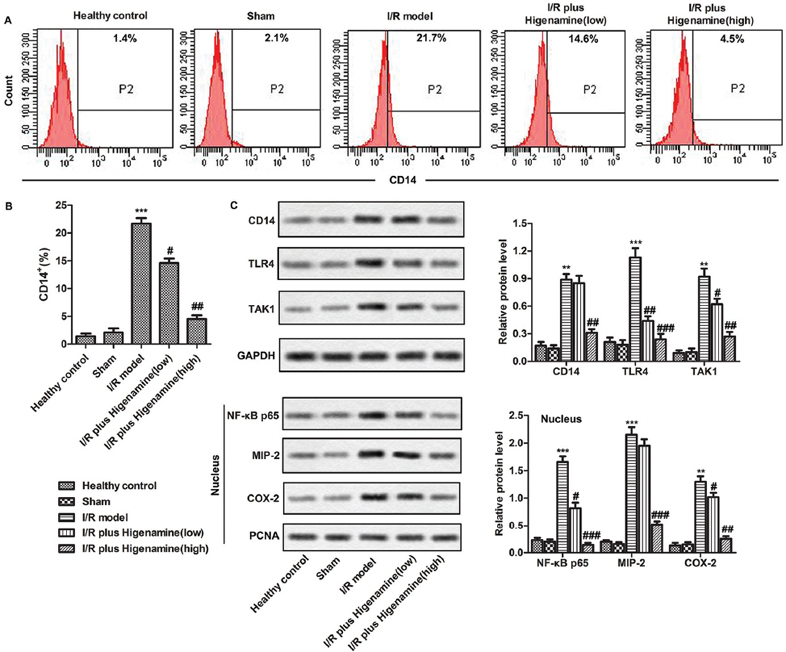 Figure 4
Figure 4Inflammatory signaling pathways were inactivated by higenamine. Rats were randomly divided into 5 groups. Control group: healthy rats; Sham group: rats received surgery without artery ligation; model group: I/R model rats; I/R+ higenamine (low) group: IR rats treated with higenamine (10 mg/kg); I/R+ higenamine (high) group: IR rats treated with higenamine (50 mg/kg). A. The production of CD14+ cells in peripheral blood was assessed by flow cytometry. B. Histogram represents the statistical analysis of the percentage of CD14+ cells in hemocytes. C. The expressions of total proteins (CD14, TLR4, TAK1) and nucleoproteins (NF-κB p65, MIP-2 and COX-2) from brain tissues were evaluated using western-blot. Experiments were repeated at least 3 times, and error bars represent ± SD. (**P < 0.01, ***P < 0.001 versus control and sham group; #P < 0.05, ##P < 0.01, ###P < 0.001 versus I/R model group).
Temporary or permanent decrease in cerebral blood flow would result in cerebral ischemia, which is connected with neuronal injury, apoptosis, inflammation, excitotoxicity, depolarization and peri-infarct (17). Although rapid reperfusion is essential for treating cerebral ischemia, the occurrence of injury after perfusion is often an important reason for the aggravation of brain damage (18). Despite various efforts in understanding the underlying mechanism and drug discovery have been taken, the effective treatment of cerebral I/R has so far been extremely limited. Increasing number of Chinese herbs, such as ampelopsin (19), salvianolic acid B(20), astragaloside IV (21) and triptolide, have been used for treating cerebral I/R injury (22). These studies suggested the prospects and possibilities of Traditional Chinese medicine in treating cerebral I/R injury.
Higenamine is regarded as a traditional heart stimulant and anti-inflammatory in China. According to published reports, higenamine possesses a variety of pharmacological properties, including dilation of blood vessels and bronchi, immunomodulation, anti-inflammation, anti-apoptosis and anti-oxidation (23). Studies over the last few years on higenamine have revealed its crucial role in treating ischemia reperfusion injury. Wu et al. pointed out that myocardial ischemia reperfusion injury and myocyte apoptosis could be attenuated by higenamine via β2-AR/PI3K/AKT signaling pathway (24). Similarly, Lee and colleagues observed that apoptotic cell death could be decreased by higenamine via inducing heme oxygenase-1 in rat myocardial I/R injury (25). Furthermore, higenamine could regulate Nrf2-HO-1-Hmgb1 axis and reduce the damage of intestinal I/R in mice (11). A similar conclusion was drawn in our research, where higenamine exerted protective effects on cerebral I/R injury via decreasing neuronal degeneration in rats.
Cerebral I/R is often accompanied with irreversible neurological damage, which would further aggravate focal cerebral ischemia. NF-200, a neurofilament protein, is the typical bio-marker of mature neurons in central nervous system. Some investigators have indicated that many Chinese herbs, especially alkaloids, played positive role in nerve regeneration. Dendrobium nobile Lindl. alkaloids, has been exhibited to keep the synaptic integrity of cultured neurons via enhancing the neurogenesis related proteins; synaptophysin and postsynaptic density-95 (26). Arecoline, an alkaloid, could attenuate local inflammatory conditions, promoting restoration of a severe peripheral nerve injury (27). Similarly, in our study, higenamine (alkaloid) could defend nerve cells against cerebral I/R injury, with evidences of increased axonal regeneration markers (NF-200, GAP-43 and RGMa), while decreased mNSS scores and neuronal degeneration in higenamine treated cerebral I/R model rats.
BBB is closely associated to the homeostasis of the brain microenvironment via limiting the transport of substances from the blood to the brain (28). BBB breakdown is one of the major mechanisms leading to the progression of brain damage and long-term neuronal defects. Accumulated studies have indicated that various Chinese herbs could blunt BBB damage. For example, tetramethylpyrazine could maintain integrity of BBB via alleviating apoptosis and permeability of brain microvascular endothelial cells (29). In addition, the integrity of the BBB could be protected by tanshinone IIA through suppressing the level of critical endothelial tight junction proteins (30). Similarly, in our study, higenamine tremendously reduced the BBB permeability caused by cerebral I/R injury. These results suggested that the higenamine could repress the BBB permeability and thus may ameliorate cerebral I/R injury.
Inflammation plays a vital role in the pathogenesis of cerebral I/R injury. Autoimmune responses and neuroinflammation could be induced by TLR4 signaling during the process of cerebral ischemia. The downstream of TLR4 signaling include activation of TLR4, CD14 and NFκB, and the release of inflammatory mediators such as COX-2 and TNF-α (31). The anti-inflammatory effect of higenamine was demonstrated by researchers in various diseases models. Si Chen et al. demonstrated that TNF-α could be directly bound by hypaconitine, mesaconitine, higenamine and quercetin, reducing TNF-α-mediated cytotoxicity in L929 cells (32). Moreover, Young and colleagues observed that iNOS expression was remarkably suppressed by higenamine through reducing the activation of NF-κB, which may be beneficial in various inflammatory diseases (33). Similarly, in our study, higenamine significantly repressed the elevated CD14+ cells in I/R model rats. In addition to this, the increased expression of inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-18) was inhibited by higenamine, while also inactivating the inflammatory pathways (TLR4, TAK1, NF-κB, MIP-2 and COX-2). These results strongly demonstrated the anti-inflammatory effects of higenamine.
Overall, in this report, we provide evidence that higenamine could mitigate cerebral I/R injury via inducing axonal regeneration and repressing inflammatory reaction, thus demonstrating the protective ability of higenamine in cerebral I/R injury. This study may enrich the thoughts for the treatment of cerebral I/R injury with higenamine and may provide experimental evidence towards its translation into clinical trials.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations: Blood Brain Barrier (BBB); Evans Blue (EB); Enzyme Linked Immunosorbent (ELISA); Modified Neurological Severity Scores (mNSS); ischemia reperfusion (I/R); ischemia reperfusion (I/R); interleukins (ILs); tumor necrosis factor α (TNF-α)
