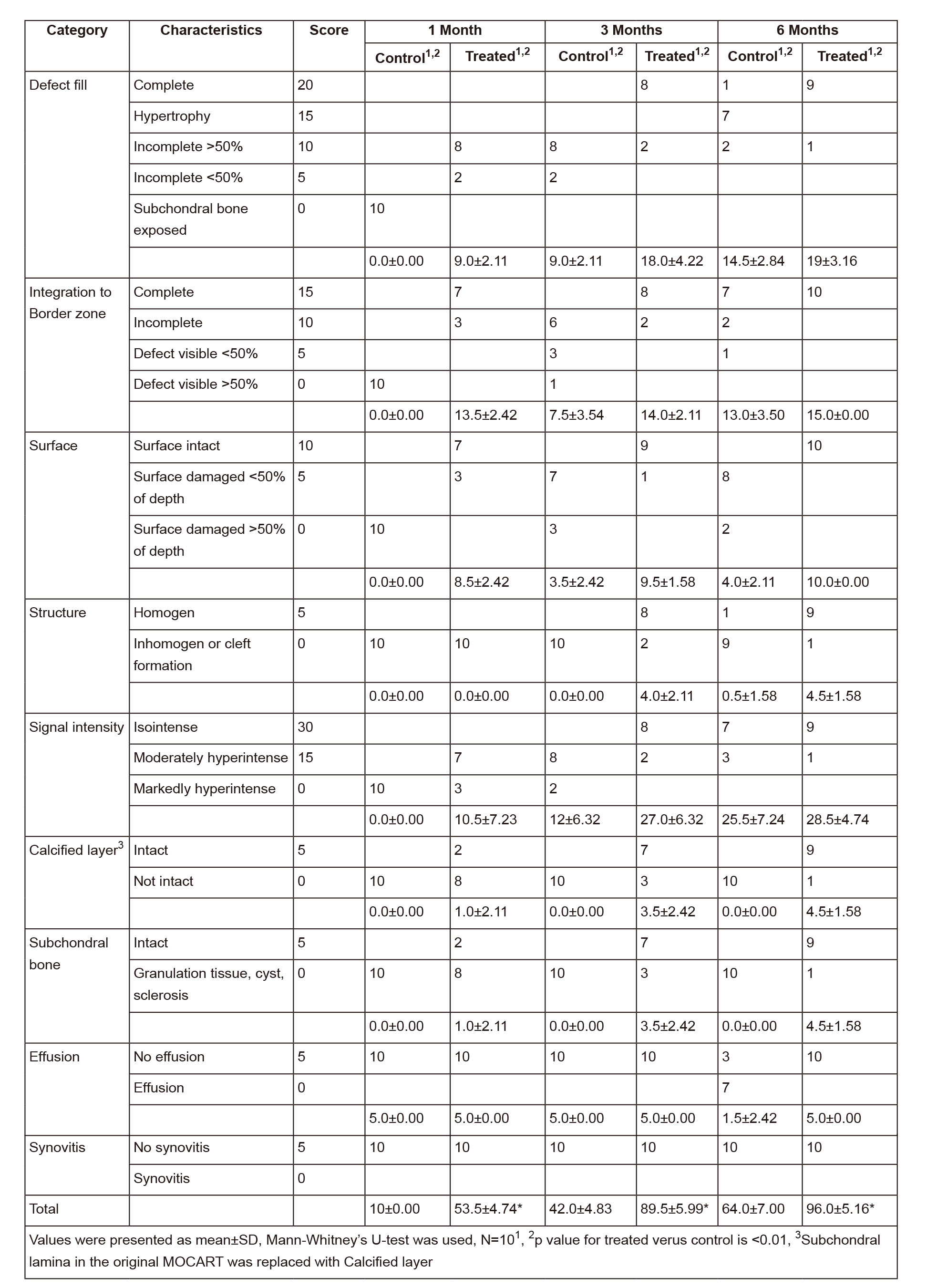
One-step clinical therapies of articular cartilage defects remains a challenge. In this study, a strategy was proposed to utilize type II collagen (Col-II) gels with autologous bone marrow-derived cells (BMDCs) embedded to repair full-thickness chondral defects. Its feasibility and efficacy were further assessed in a minipig model. An 8-mm full-thickness chondral defect was created on the femoral trochlea of two knees in Guizhou minipigs. One knee received Col-II gels with BMDCs implantation versus the untreated one as control. After 1, 3, and 6 months operation, the animals were sacrificed, and the repair outcomes of chondral defects were evaluated using Magnetic Resonance Imaging (MRI), macro- and microscopic observation, and histological analysis. The treatment group showed significantly better repair outcomes of chondral defects than that in the control group at each time point (P < 0.01). Furthermore, the image showed that the repaired tissue in the treatment group was more similar to the surrounding healthy cartilage tissue. Based on the hyaline-like tissue regenerated ability, this one-step strategy provides a promising therapeutic potential for clinical application.
Articular cartilage, found within diarthrodial joints, provides a smooth, lubricated surface that can reduce the coefficient of friction when bones glide past each other. Since articular cartilage functions in harsh weight-bearing environments are subject to high mechanical stress, it lacks vasculature or neurons. As a result, articular cartilage has extremely limited potential for self-repair (1, 2). In clinical practice, the incidence of chondral injury has been found increasing in all age groups year by year, and there were less than 20% patients with knee cartilage injury had a repair procedure (3). A previous study had reported that 60% of patients undergoing knee arthroscopic surgeries were detected chondral injuries, and if left untreated, chondral defects may progress to osteoarthritis (4, 5).
Conventional clinical therapies used for chondral defects include bone marrow stimulation techniques and osteochondral grafting. Although these methods are extensively used, they have many limitations. For instance, these methodologies are not effective enough in older patients or those with larger defects. In addition, bone marrow stimulation techniques could induce the formation of fibrocartilage, which possesses less tensile strength and is more likely to break down than hyaline cartilage (6). In terms of osteochondral grafting, which is also used in clinical practice, the process involves autografts and allografts. Autografts may increase donor site morbidity, while allografts elevate the risk of an immune response and disease transmission (7). Because of the above shortcomings of traditional therapeutic methods, there has been increasing interest in cell-based therapies. The most influential cell-based therapy is autologous chondrocyte implantation (ACI) (8, 9). Despite significant improvements achieved in previous clinical trials (10, 11), ACI has many drawbacks such as donor site morbidity, the need for a second surgery, and the loss of chondrocyte phenotype in monolayer expansion (12, 13). To remedy these limitations, tissue engineering has become a focal point for many researchers. Since tissue engineering holds great promise, engineered cartilage has been applied to clinical settings (14). Recently, a one-step modified cell implantation technique was developed using autologous bone marrow-derived cells to replace the chondrocytes traditionally used in ACI therapies (15, 16). Although the results of this study were encouraging, further investigation was needed.
In this study, a novel strategy was proposed for repairing chondral defects using bone marrow-derived cells (BMDCs) embedded in a type II collagen (Col-II) gel. An 8mm-diameter full-thickness cartilage defect in the femoral trochlea of Guizhou minipigs was created, without breaching the calcified cartilage and subchondral bone, and then operated using our novel strategy. At the beginning of surgery, bone marrow BMDCs were extracted from patients and mixed evenly with Col-II sol at 4 °C. After debridement of the surrounding tissue, the mixture was injected into the defect site. Once the defect space was filled, the temperature was increased to induce a phase change to sol-gel which enabled BMDCs to embed and fix into the Col-II gel. The minipigs were sacrificed 1, 3, or 6 months postoperatively, and the repair outcome of chondral defects was evaluated using magnetic resonance imaging (MRI), macro- and microscopic observation, and histological analysis. We hypothesized that the BMDCs embedded in a Col-II gel should be able to differentiate into chondrogenic lineage cells and effectively repair the chondral defects under the unique microenvironment of articular cavity.
The study subjects consisted of thirty Guizhou mini-pigs (age: 10-12 months, weight: 40-50 kg). All the animal experiments were approved by the Ethical Committee of Third Military Medical University. For each pig, an 8-mm diameter full thickness chondral defect (2-mm depth) was first created in one knee and treated by Col-II gel + BMDC implantation. At the same time, a blank control chondral defect was created in the other knee and left untreated. Each ten minipigs were sacrificed at 1 month (33 ± 3 days), 3 months (92 ± 1 days), and 6 months (183 ± 5 days) postoperatively, and then the repair outcome of chondral defects in both treatment and control groups was evaluated by using magnetic resonance imaging (MRI), macro- and microscopic observation, and histological analysis.
The entire process of collagen isolation was performed at 4°C (17, 18). Fresh cartilage was harvested from pig condyles of femur, cut into small pieces, and washed with saline. After being freeze-dried and smashed, cartilage fragments were mixed with 10 vol of 4 M guanidine-HCl, and the mixture was stirred by magnetic stirrer for 24 h to remove proteoglycans. The precipitation was washed by 0.0.5 M Tris-HCl and 0.5. M acetic acid, and digested by 5 vol of pepsin in 0.5 M acetic acid (1 g/L, pH 2.5~3)for 48 h. After centrifugation (14,336 ×g, 20 min), the supernatant was collected, and its pH value was adjusted to 7.5 with 2 M NaOH. Then, NaCl was added to the supernatant to a final concentration of 3 M. Collagen was salted out overnight and collected by centrifugation (14,336 ×g, 20 min). The collagen was aggregated by dialysis against distilled water for 2 days. After centrifugation (14,336 ×g, 20 min), the collagen was collected. Finally, the collagen was lyophilized.
The lyophilized Col-II was dissolved in 0.1.5 M HCl at a concentration of 20 mg/mL (pH = 3) and kept at 4°C. To neutralize the solution, 1.5. M NaOH was added to adjust the pH to 7.4. The final salt ion concentration of the neutralized Col-II sol was 0.8775% (w/v) and was close to that of saline (0.9%), suggesting a suitable crystal osmotic pressure for cell survival.
To test the gelling ability of Col-II, neutralized sol was injected into an 8 mm chondral defect of a pig knee joint in vitro. The defect was covered by saline immersed gauze, and then exposed to the light of a homemade 37°C constant temperature table lamp 25 cm away from the defect for 6 minutes for slow-heating gelatinization, which was turned off when the temperature of the defect area reached 37°C. After exposure to light, the temperature of the defect site increased, and then the sol was transferred into white gel without liquidity, suggesting good gelling ability of the Col-II solution.
BMDCs were isolated with a commercially available kit (Cat. #LGS1106, TBD Science, China) according to the manufacture’s manual. Under general anesthesia, 15 mL bone marrow aspirates were obtained with a 16-gage syringe at crista iliaca of a Guizhou mini-pig, and mixed with 15 mL porcine BMDC isolation solution from the kit. The mixture was then centrifuged at 896 ×g for 15 min. The second layer from the top was carefully aspirated and transferred into PBS, followed by a centrifugation at 504 ×g for 5 min. After removal of the upper layer, cells were resuspended with PBS, counted, and stored at 4°C. Each aliquot containing 10 uL of harvested cell concentration was cultured in vitro separately, and was further induced for chondrogenic, osteogenic and adipogenic differentiation using different reagents and then evaluated using different staining methods according to the previous methodology published(31-34), with the purpose of identifying the stemness of the cells used. Next, an EP vial containing 1 mL previously prepared Col-II sol was transferred from the refrigerator (4°C) to ice. BMDCs (2×106 cells) were added into the EP vial, followed by vortexing and centrifugation to remove any bubbles. Finally, the Col-II sol embedded with BMDCs was transferred into a syringe for further usage.
All operations were performed under general anesthesia and aseptic conditions. The femoral trochlea was approached through a lateral incision. An 8-mm diameter full-thickness chondral defect (2-mm depth) was created with a stainless hollow drill, while the calcified layer and subchondral bone remained intact. Bleeding was not observed during defect preparation. Next, the Col-II sol embedded with BMDCs was injected into the chondral defect, and the surface of the filled sol was trimmed and covered by saline immersed gauze. The gelling of Col-II sol was achieved by increasing the temperature with a table lamp 25cm away from the operation area for 6 minutes. After gelling, the patella was reset, and the stability of the Col-II + BMDC gel was confirmed by passive flexion of the knee joint 5 times. Then, the incision was sutured in layers. All animals were allowed free movement after surgery and were muscle-injected with benzylpenicillin potassium (4×106 U) 2 times per day for 1 week.
Animals were sacrificed 1, 3, and 6 months after surgery (n=10 at each time point). The complete hind limb was removed, and MRI imaging was performed immediately using T1- and T2-weighted, fat-suppressed gradient echo (19, 20). MRI results were evaluated using a modified Magnetic resonance Observation of CArtilage Repair Tissue (MOCART) scoring system (Table 1) by two independent researchers (21).
For the macro- and stereoscopic microscopic evaluation, knee joints were incised and photographed. The findings, such as the amount of defect filling, color, and cartilage surfaces were evaluated using modified International Cartilage Repair Society (ICRS) macroscopic grading (Table 2) by two independent researchers (22). At each time point, ten knee samples were evaluated per group.
For histological analysis, cartilage samples were prepared by sawing slices perpendicular to the articular surface in the middle of the defects and fixed in 4% formaldehyde for 7 days at 4°C. The fixed tissue was decalcified in 4% ethylene diamine tetraacetic acid (EDTA) for 14 days at 4°C and then embedded in paraffin. Perpendicular to the articular surface, adjacent sections (5-μm thick) were cut from fixed tissue. After deparaffinization, Sirius red, safranin O/fast green, toluidine blue staining and heat-induced antigen retrieval for Col-II were performed in adjacent sections. The protocol of IHC referred to a published study (23) and instructions of kit providers. Mouse monoclonal against Col-II (ab3092, Abcam) (1:1000 dilution) was used to detect the Col-II distribution in the repaired tissue. A streptavidin-peroxidase kit (SP-9002) was used to visualize the antibody. For each time point in a single group, 10 sections for each staining were analyzed. Semi-quantitative histomorphological evaluation of the repaired tissue quality was carried out using the modified O’Driscoll score (Table 3) by two independent researchers (24).
To observe the prognosis of implanted cells in vivo, we designed the cell imaging experiment. Chromosome fluorescence in situ hybridization (FISH) was used to track the implanted cells. Y-chromosome labeled BMDCs from a male pig were used for the repair of the chondral defect of a female pig. One month postoperatively, FISH of Y-chromosome was performed to detect the labeled cells. Chromosome FISH probe synthesis was done by Exon Biotechnology (Guangzhou, China). Sections were preprocessed using methyl alcohol solution, and then dropt onto microslides; The slides were washed using SSC, preheated pepsin, SSC twice successively for 5-min each, and then placed in 70%, 80%, and 100% ethylalcohol for 3-min each; After airing, the slides were added specific probe for reaction for 6 hours; the slides were further washed using SSC solution and NP-40 solution for 5-min each, and then placed in 70%, 80%, and 100% ethylalcohol for 3-min each; After airing, the slides were counterstained using DAPI for 5-min and then been observed. For immunofluorescence (IF) of Col-II, mouse monoclonal antibody against Col-II (ab3092, Abcam) (1:1000 dilution)and goat anti-mouse antibodies (bs-0296G-FITC, Bioss, China) were used as primary and secondary antibody, respectively. The IF procedure was conducted according to the general IF instruction from Abcam and a published study (25).
Data were expressed as the mean ± standard deviation. SPSS version 17.0 (Chicago, IL, USA) was used for statistical analysis. Statistical differences between scores for control and treatment groups at each time point (1, 3, and 6 months postoperatively) were analyzed using Mann-Whitney’s U test. P < 0.0.1 was considered statistically significant.
After surgery, all pigs remained healthy and had normal function in both operated limbs throughout the whole procedure.
MRI showed no joint effusion or synovial hyperplasia in any tested knee. One month after surgery, MRI scans of the control group displayed a high signal intensity in the calcified layer and subchondral bone (Figure 1A), while MRI scans of the treatment group showed filling of the defect area benefiting from the implanted gel-cell complex and lower signal intensity than the surrounding cartilage (Figure 1D). Three months postoperativon, MRI scans or images of the control group detected inhomogeneous repair tissue with rough surfaces and thin thicknesses (Figure 1B). In contrast, the repaired tissue in the treatment group exhibited smooth surfaces and thicknesses similar to those of the adjacent cartilage (Figure 1E). Six months postoperation, the control group showed fibrillation and hypertrophy in the repaired tissue (Figure 1C), while the treatment group showed smooth surfaces and normal thickness (Figure 1F). The modified MOCART scores (Table 1) of the control and treatment groups were 10 ± 0 and 53.5 ± 4.7.4 at 1 month (n=10, p < 0.01), 42 ± 4.8.3 and 895. ± 5.99 at 3 months (n=10, p < 0.01), and 64 ± 6.99 and 96 ± 5.16 at 6 months (n=10, p < 0.01), respectively. These results clearly revealed the efficacy and significance of the one-step repair strategy.
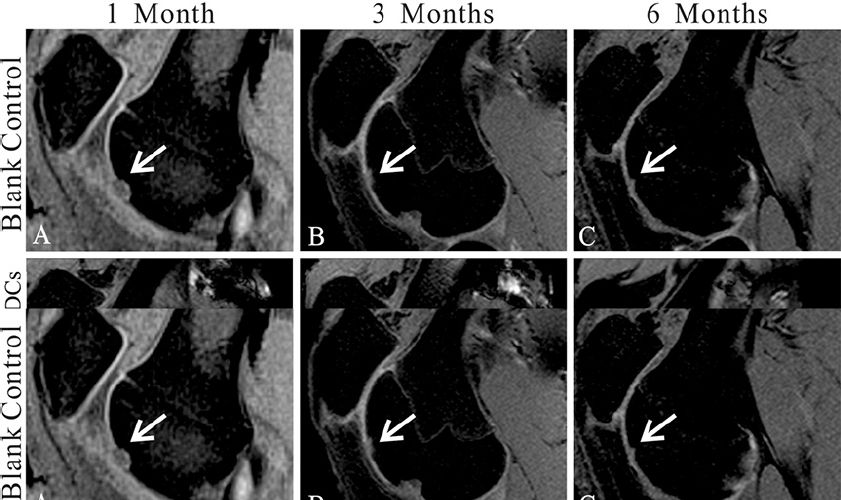 Figure 1
Figure 1Magnetic resonance imaging (MRI) evaluation of the cartilage repair. (A-C) MR images show the tissue repair of the control group 1(A), 3(B), and 6(C) months postoperatively. (D-F) MR images show the tissue repair in the type II collagen (Col-II) + bone marrow-derived cell (BMDC) group 1(D), 3(E), and 6(F) months postoperatively. Sample sagittal MR images are of the tibiofemoral joint acquired using T2-weighted, fat-suppressed gradient echo. Arrows highlight the defects.
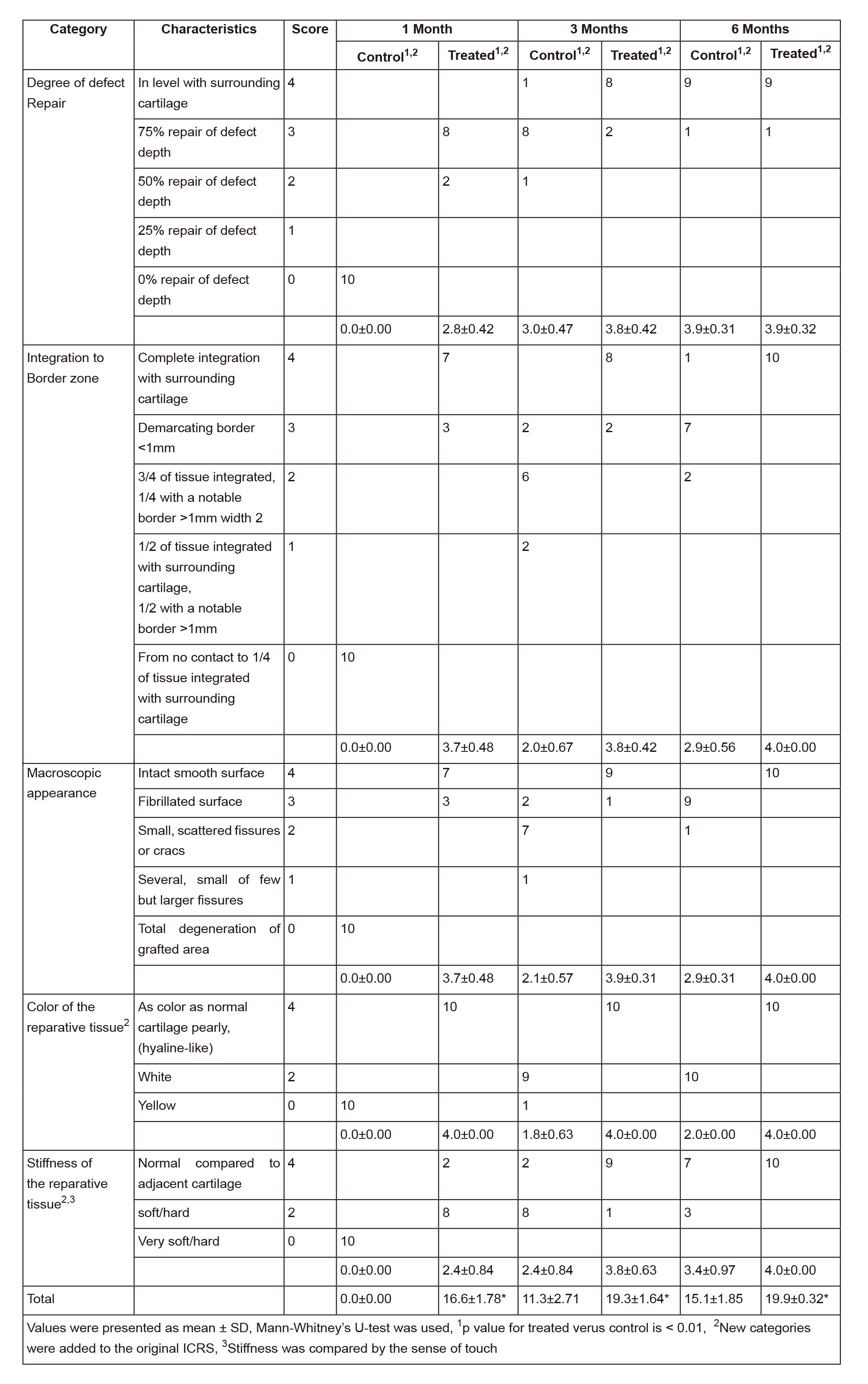
One month postoperation, no healing sign was observed in the control group, the subchondral bone was visible, and the color of the defect surface was red (Figure 2A). In contrast, the defects in the treatment group had nearly complete filling (Figure 2D). Three and six months postoperation, complete filling was seen in both the control and treatment groups (Figure 2 B,C & E, F). However, stereoscopic microscopy observations further showed that the repaired tissues in the control group had rough surfaces, discernible edges, irregular calcified layers, and disordered subchondral bones (Figure 3A). Better repaired tissues were observed in the treatment group, which had smooth surfaces, clear integration with surrounding cartilage, continuous calcified layers, and homogeneous subchondral bones (Figure 3B). Moreover, the color of repaired tissue in the treatment group was similar to that of the normal cartilage. Modified ICRS scores (Table 2) for the control and treatment groups were 0 ± 0 and 16.6 ± 1.78 at 1 month (n=10, p < 0.01), 11.3 ± 2.71 and 19.3 ± 1.64 at 3 months (n=10, p < 0.01), and 15.1 ± 1.85 and 19.9 ± 0.32 at 6 months (n=10, p < 0.01), respectively.
 Figure 2
Figure 2Macroscopic observation of cartilage repair. (A-C) Photographs showed the defects in femoral trochlea of the control group 1(A), 3(B), and 6(C) months postoperatively. (D-F) Photographs showed the defects in femoral trochlea of Col-II + BMDC group 1(D), 3(E), and 6(F) months postoperatively. Black boxes highlight the defects.
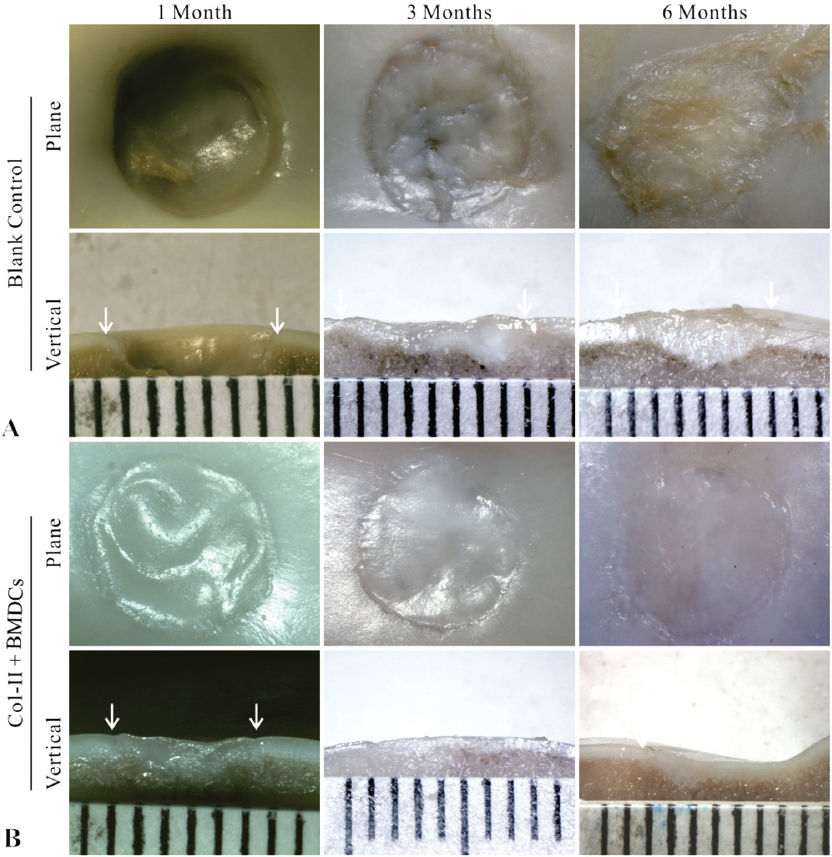 Figure 3
Figure 3Microscopic observation of the cartilage repair. (A) Microscopic images showed the chondral defects in the control group 1,3, and 6 months postoperatively in both horizontal and vertical planes. (B) Microscopic images showed the chondral defects in the Col-II + BMDCs group 1,3, and 6 months postoperatively in both horizontal and vertical planes. Arrows indicated the position of defects.
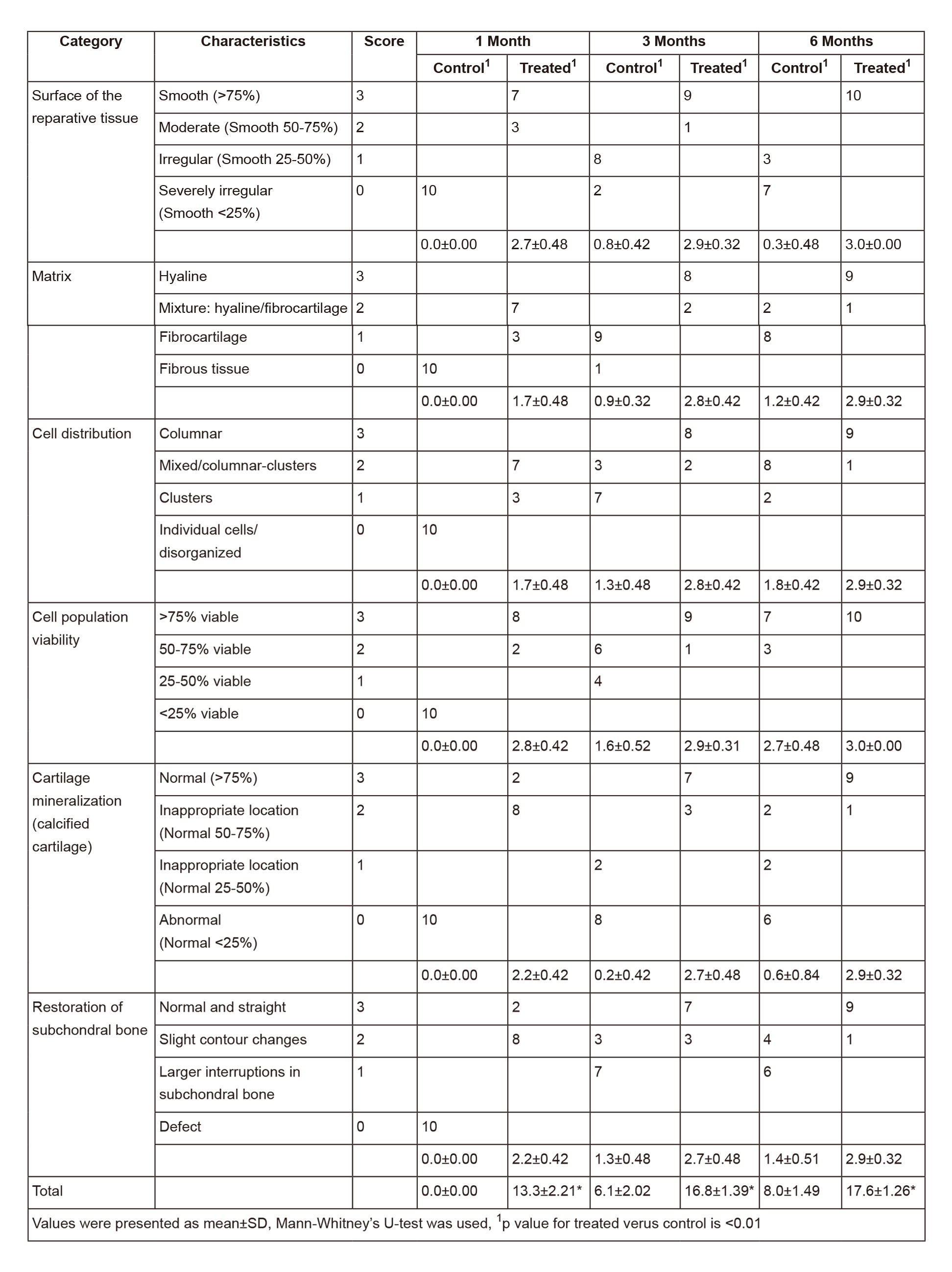
In the control group, the defects remained concave still, and even deepened 1 month after the operation. Fast green staining indicated that the calcified layer disappeared, and the subchondral bone was only covered by a thin layer of fibrous tissue (Figure 4A, blank control). At 3 and 6 months, the defects showed poor healing with abnormal surfaces and thicknesses. Masson’s trichome staining showed that the cells were in a cluster distribution (Figure 4B, blank control). Sirius red staining and Col-II immunohistochemistry (IHC) indicated that the matrix was fibrous cartilage (Figure 5A, B, blank control). Safranin O and toluidine blue staining showed that the repaired tissue was not as well-distributed as the surrounding tissue (Figure 4A, C, blank Control). Fast green staining indicated an irregular calcified layer and a disordered subchondral bone (Figure 4A, blank control). In contrast, the defect areas in the treatment group displayed a consecutively improved healing trend with decent integration, thickness, and surface regularity from 1 month to 6 months postoperatively. Masson’s trichome staining showed that the cells were uniformly distributed (Figure 4B, Col-II + BMDCs). Sirius red staining and Col-II IHC indicated that the matrix was mainly Col-II (Figure 5A, B, Col-II + BMDCs). Safranin O and Toluidine Blue staining intensity of the repaired tissue was similar to the surrounding tissue (Figure 4A, C, Col-II + BMDCs). Fast green staining indicated a continuous calcified layer and a homogeneous subchondral bone (Figure 4A, Col-II + BMDCs). The modified O’Driscoll scores (Table 3) of the control and treatment groups were 0 ± 0 and 13.3 ± 2.2.1 at 1month (n=10, p < 0.01), 6.1. ± 2.02 and 16.8 ± 1.4 at 3 months (n=10, p < 0.01), and 8 ± 1.49 and 17.6 ± 1.26 at 6 months (n=10, p < 0.01), respectively.
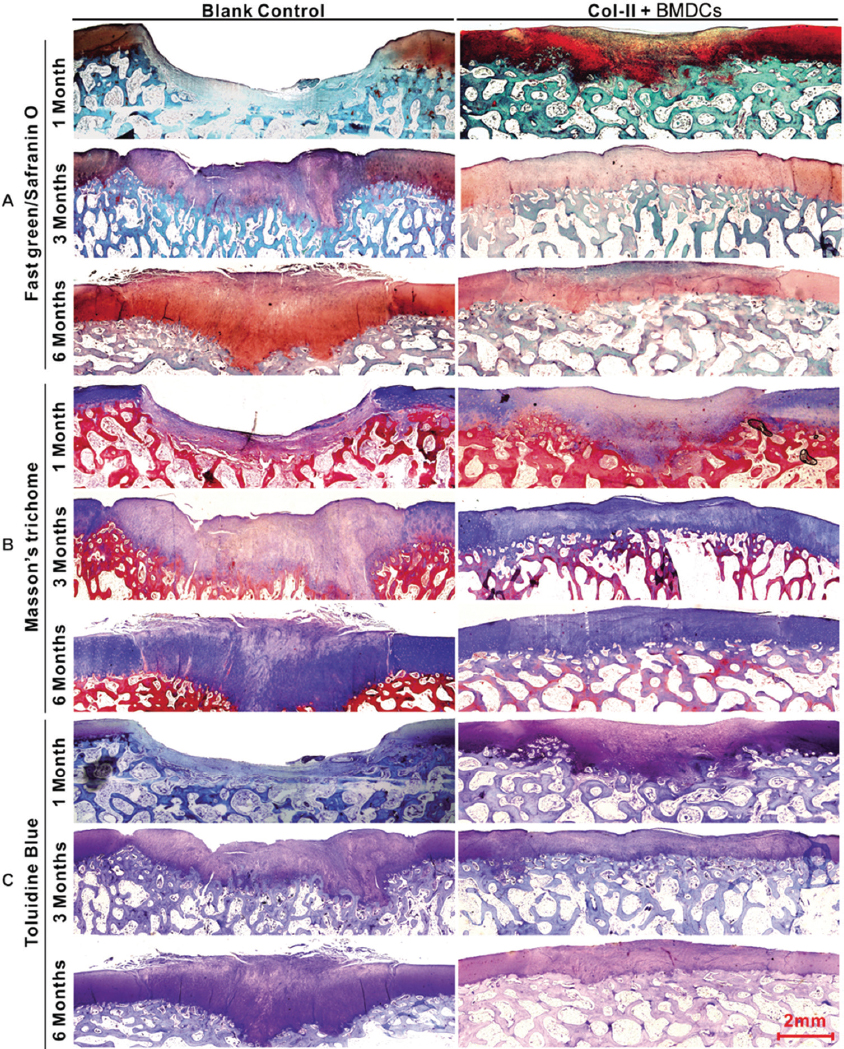 Figure 4
Figure 4Histological appearance of the chondral defects 1,3, and 6 months postoperatively in both the control and the Col-II plus BMDC groups. (A) Fast green/Safranin O staining. (B) Masson’s trichome staining. (C) Toluidine blue staining.
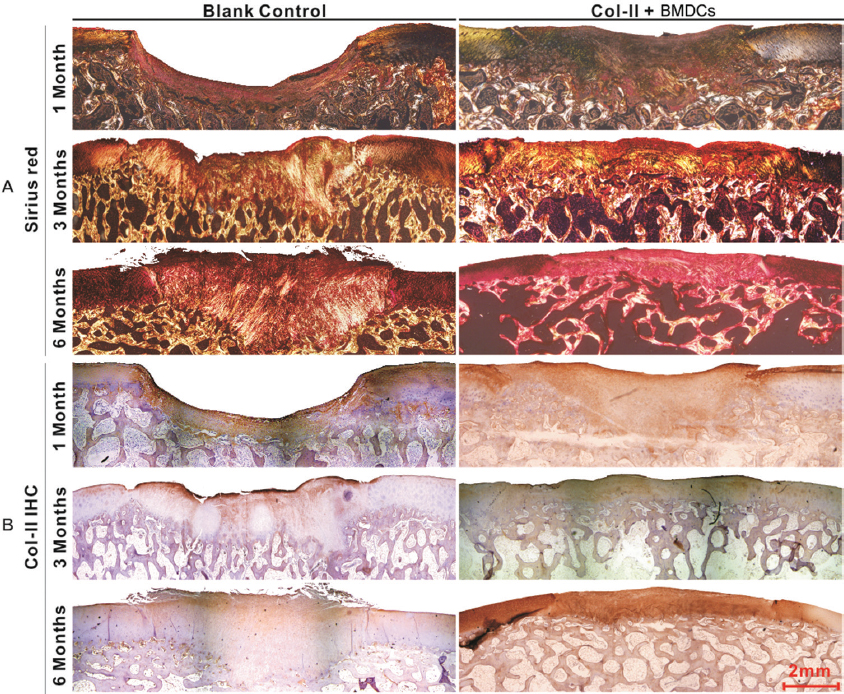 Figure 5
Figure 5Microimages of collagen staining of the chondral defects 1,3, and 6 months postoperatively in both the control and the Col-II + BMDC groups. (A) Sirius red staining. (B) Immunohistochemistry of type II collagen.
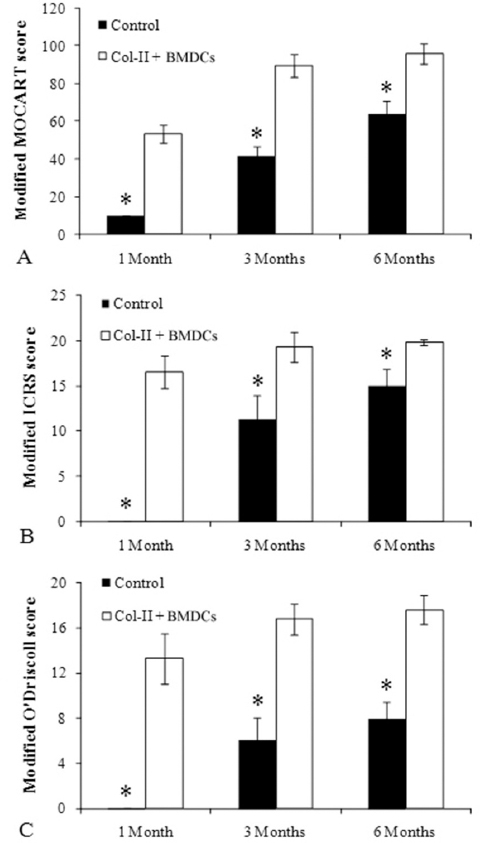 Figure 6
Figure 6Scoring of MRI and macroscopic and histological evaluations of the chondral defect repair. (A) Modified MOCART score. (n=10); (B) Modified ICRS score. (n=10); (C) Modified O’Driscoll score. (n=10); Mann-Whitney’s U-test, *p < 0.0.1.
FISH results showed that the sections of repaired tissue from the selected female pig contained many Y-chromosome-labeled BMDCs 1 month after Col-II + BMDC implantation (Figure 7 A-C). This finding indicated that the implanted BMDCs were still in the defect and participated in cartilage repair. IF Images of Col-II showed abundant Col-II in the repaired matrix and that the cells within the matrix had similar morphology to chondrocytes (Figure 7D). Possible cell division was seen in some of the cells (Figure 7E), and a boundary integration between the repair tissue and the subchondral bone was also observed (Figure 7F). IHC images of Col-II also revealed the cell distribution (Figure 7G). Figure 7H showed a boundary of integration between the repaired tissue and the surrounding cartilage, and the cells distributed more densely in the repaired tissue than in native tissue. Figure 7I indicated a boundary of integration between the repaired tissue and the subchondral bone. The staining results proved that the cells were able to differentiate to chondrocytes, osteoblasts and adipocytes after 2-week induction in vitro (Figure 8A-F), ascertaining their potential stemness.
 Figure 7
Figure 7Microimages of cells in the central area within the repaired tissue in the Col-II plus BMDC group 1 month postoperatively. (A-C) Y chromosome fluorescence in situ hybridization of implanted BMDCs. (A) cell nucleus, (B) Y chromosome, (C) merged image of (A) and (B). (D) Immunofluorescence of type II collagen. (E) Higher-magnification image of box 1 in (D). White arrows indicated the cells during proliferation. (F) Higher-magnification image of box 2 in (D). Arrows indicated the boundary between the repaired tissue and the calcified layer. (G) Immunohistochemistry of type II collagen. (H) Higher-magnification image of box 3 in (G). Black arrows indicated the boundary between repaired tissue and native tissue. (I) Higher-magnification image of box 4 in (G). Black arrows indicated the boundary between repaired tissue and the calcified layer.
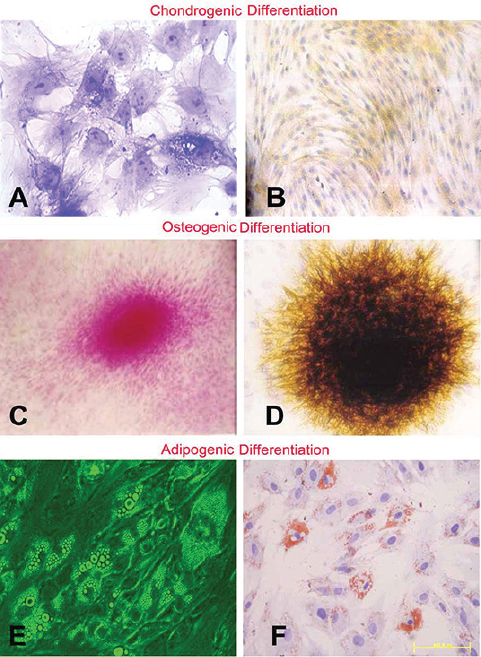 Figure 8
Figure 8Toluidine Blue staining (A), the dark purple color area were chondrocytes. Col-II immunohistochemistry (B), yellow area means Col-II generated by the chondrocytes. Nucleus Fast Red (C), red nucleus were osteoblasts. Von Kossa staining (D), dark yellow and black part was the calcium deposition generated by the osteoblasts. General microimage (E) and Oil Red-O staining (F), red stained area are the adipcytes characterization.
Articular cartilage repair remains a challenge in orthopedic surgery. Although there are many treatment options available, none of them can truly achieve regeneration of hyaline cartilage. To date, the most widely used cell-based therapy ACI, is still not considered as the most effective remedy since randomized clinical trials failed to reveal its solid evidence of benefits when compared to conventional treatments (27, 28). In this study, we proposed a new strategy utilizing Col-II + BMDCs to repair full-thickness cartilage defects. Compared to conventional ACI, this method is a one-step technique that avoids in vitro expansion of seed cells and therefore can be considered time-saving and cost-effective.
We chose Guizhou minipigs as our animal model because the knee joints of pigs are similar to those of humans in terms of size (29) and cartilage-specific properties (30). In the decision of seed cells in cartilage repair, we took advantage of BMDCs rather than chondrocytes, since the former cells have capacity of chondrogenic differentiation (31). Although bone-marrow mesenchymal stem cell (MSCs) has a better repair efficacy and therefore has been widely used in animal experiments (32, 33) and clinical applications (34, 35) for cartilage repair and regeneration, it requires a more than 10-hour cell culture and purification, which is infeasible in this one-step strategy. Moreover, the approach for BMDC isolation is convenient and well-established (36), and avoids the drawbacks of chondrocyte isolation including donor-site morbidity and limited cell numbers harvested. The BMDCs embedded in in situ gels in this study were found to possibly differentiate into chondrocytes and subsequently produce cartilage matrix.
Indeed, bone marrow MSCs have been recognized as a favorable cell source for cartilage repair in recent years (37). Staining results showed that the cells had potential abilities of differentiation, proved the harvested cells were mainly MSCs. This approach using high-density BMDCs which contains MSCs for cartilage repair required only single surgery, and therefore reduced the costs, minimized the donor-site morbidity, and even worked as effectively as chondrocytes for human cartilage repair (38). Previous clinical studies had shown that bone marrow concentrate (mainly BMDCs) reached a satisfactory efficacy for this repair process (15, 16). However, the most suitable scaffold material to pair with cells has not been determined yet. Previous large animal experiments had been carried out using MSCs covered with collagen type I/III membranes (39), embedded in type I collagen gels (40), or suspended in hyaluronic acid (41, 42), all of which had shown promising results. Bone marrow MSCs + type I/III membranes have been further applied in clinical trial, and have been shown to be an effective procedure for the treatment of large full thickness chondral defects (35).
Surprisingly, Col-II, the major component of native cartilage matrix, has been less frequently used as a membrane/scaffold in BMDC-based therapy for the repair of chondral defects. Given the nature of Col-II as similar to cartilage matrix, we believe that Col-II is a better choice for guiding the chondrogenic behavior of BMDCs during the process of chondral defect repair. Although the immunogenicity of Col-II had been reported (43), our study did not find any immunogenic response to the Col-II gel within 6 months in minipigs. In addition, Col-II has been shown to direct MSCs to differentiate toward a chondrogenic lineage (44) and is more suitable than other types of collagen in maintaining chondrocytic phenotype (45). Buma et al compared type I collagen and Col-II for the repair of cartilage defects in rabbits, and suggested that Col-I was more suitable for the repair of deep layer of bone while Col-II was better for the repair of upper layers of cartilage (46). Lee et al used an autologous chondrocyte-seeded Col-II scaffold to repair chondral defects of dogs, and found that the formation of hyaline cartilage was improved compared to the group with autologous chondrocytes only. Another advantage of the Col-II gel was that it was able to adapt to fit into defects of varying sizes and shapes, and then integrated well with the host tissue. Therefore, Col-II gel was additionally used in this study to fill in the chondral defects. Compared with conventional ACI, this strategy had no need for the coverage of cells with a patch/membrane, since BMDCs fixation in the defects was achieved by the thermo-sensitive sol-gel phase change of Col-II. Funayama et al also reported satisfactory efficacy of cartilage defect repair by using injectable Col-II gel embedded with chondrocytes in a rabbit model (47). All above studies and the current one indicated definite benefits using Col-II to repair the cartilage defects.
It is known to us that the size of chondral defect is a determinant of tissue repair and regeneration (48). The larger the defect size is, the more difficult the repair will be. To avoid self-repair, the diameter of chondral defect was set as 8 mm initially, and the subchondral bone was retained unbroken to isolate the bone marrow underneath. However, the chondral defects in the control group began to show limited potential for self-repair after 3 months. As seen in both macroscopic and histological observation, the chondral defects in the control group worsened after 1 month, but partially healed 3 and 6 months postoperatively. It was speculated that the pigs initiated weight-bearing movements immediately after the creation of chondral defect. Without the protection of load-bearing cartilage, subchondral bone underneath the defects was breached within 1 month, and then, the cells from the bone marrow migrated to the defect site and enhanced healing. To assess the repair of chondral defects, a combination of MRI, macroscopic, and histological observation was employed. Macroscopic observation showed a change in the synovial membrane and the surface of repaired tissue but was unable to provide information about the interior. Histological sections showed the internal condition, but cannot contribute to the observation of whole repaired tissue. MRI showed the morphology of both the surface and the inside structure, but lacked information about the tissue components. Therefore, the combination of these three methods enabled an evaluation that was more comprehensive and convincing. The scores of MRI, macroscopic, and histological analysis all showed that the repair outcomes of chondral defects in the treatment group was significantly better than those in the control group (Figure 6).
All of the above integrated results indicated that the proposed Col-II + BMDC complexes in this study had definite and satisfactory efficacy in repairing cartilage defects in this large animal model, which was more representative than mouse or rabbit models when simulating the specific cartilage environment in human beings. In the present study, we first established the extraction procedure for Col-II and confirmed the gelling ability in vitro, and then further investigated the effectiveness and safety of our methodology using this large animal model. Our data confirmed the feasibility and efficacy of this one-step repair strategy and implied its potential in further clinical application. However, there were still some limitations. First, the pigs were allowed free movement after surgery, so that perforation of the subchondral bone plate could occur, which would impede the rehabilitative response. Second, although the morphological characteristics of implanted mixture kept stable during the repair process, it was uncertain whether the implantation provided mechanical function in the defect as no test of mechanics was carried out. Third, since subjects treated using only type II collagen scaffold in our preliminary study showed similar repair pathway (bone marrow underneath) with those left untreated one month after the operation, and because only the efficacy and feasibility of this strategy were the current focus, we decided to exclude an active control group treated with an established procedure (e.g. ACI) or a sham control group (e.g. gel alone) to save pigs, which brought about more than one distinction between the two groups and therefore increased the risk of more potential confounders, resulting in an inferior level of evidence of this study. Fourth, prior to clinical application, the immunogenicity of heterograft should be further considered and tested since the acquisition of human cartilage for extracting Col-II was not as feasible as human bone which has been commercially applied in orthopaedic surgeries for several years. Overall, it may conclude that bone marrow derived cells embedded in Col-II gel can be a promising option in the treatment of large full-thickness chondral defects in the representative large animal model.
This work was supported by the National Natural Science Foundation of China (grant 81271981 to F. Wang, grant 31130021 to L. Yang) and the National Key Research and Development Project of China (grant 2017YFC1104103 to F. Wang).
Abbreviations: MSCs: senchymal stem cells; Col-II: type II collagen; MRI: Magnetic Resonance Imaging; ACI: autologous chondrocyte implantation; MOCART: Magnetic resonance Observation of CArtilage Repair Tissue; ICRS: International Cartilage Repair Society; EDTA: Ethylene diamine tetraacetic acid; FISH: Fluorescence in situ hybridization; IHC: Immunohistochemistry
