- Academic Editor
Oral diseases affect over three billion peopleand are among the most commonly observed infections worldwide. Recent studies have shown that controlling the ecology of the oralome is more effective in reducing the risk of caries than the complete removal of both harmful and beneficial microorganisms. This work aimed to develop a strategy for preventing dysbiosis in the oral cavity by applying a postbiotic-based orodispersible film.
Lactiplantibacillus plantarum 226V and Lacticaseibacillus paracasei L26 were cultured in De Man–Rogosa–Sharpe (MRS) broth for 48 hours, followed by centrifugation and filtration. Then, the resultant postbiotics were then subjected to various dilutions (10% (v/v), 20% (v/v), 40% (v/v), 60% (v/v) and 100% (v/v)) and co-incubated with Streptococcus mutans. Antimicrobial efficacy, minimal inhibitory concentration, the time required to inhibit S. mutans growth, and antibiofilm properties of the postbiotics were assessed. Subsequently, an orodispersible film comprising polymers and plasticizers, namely Xanthan gum, maltodextrin, and glycerol, was developed as a vehicle for postbiotic delivery. Formulation optimization, physical property evaluation, and cytotoxicity against the TR146 human oral cell line (TR146 cell line) were conducted.
Postbiotics demonstrated antimicrobial and antibiofilm activity against S. mutans following 24-hour co-incubation. The minimal inhibitory concentration for combined postbiotics administration was 20% (v/v). Remarkably, 79.6 ± 8.15% inhibition of biofilm formation was achieved using 100% (v/v) of the postbiotic derived from L. plantarum 226V. Incorporating postbiotics did not compromise the dissolution time of orodispersible films, all exceeding 20 minutes. Furthermore, solubility improved following postbiotic addition, facilitating ease of handling. Importantly, postbiotic-impregnated orodispersible films were non-cytotoxic when exposed to the TR146 cell line.
These findings underscore the potential of orodispersible films loaded with postbiotics as a promising potential intervention for oral dysbiosis.
Dental caries is the most prevalent infection worldwide among oral diseases, with more than 3.5 billion people experiencing it at least once in their lifetime [1]. Poor oral health impacts mouth well-being and has significant implications for overall health [2]. Studies have linked inadequate oral hygiene to various systemic conditions, including cardiovascular issues, chronic obstructive pulmonary disease, bone resorption, inflammatory bowel disease, and neurodegenerative diseases [3, 4, 5].
Oral health relies heavily on maintaining a balanced oral microbiota. When this equilibrium is disrupted, dysbiosis occurs, and certain microbiota bacteria undergo overgrowth, leading to biofilm formation [6, 7, 8, 9]. The essential characteristic of biofilm formation is the bacterial ability to adhere to surfaces [10]. Moreover, it is crucial to consider that dysbiosis happens before the first symptoms of the oral disease appear, underscoring the importance of effective preventive measures in controlling this condition [11].
As the biofilm starts to form and the environment changes, there is loss in the diversity of the microbiota, which contributes to dysbiosis. Of note is the ability of these bacteria to adhere, a fundamental property for the bacteria in the biofilm to disrupt the homeostasis of the oral cavity. It is important to understand that the first colonizers of the biofilm are usually gram-positive bacteria, namely Streptococcus mutans, which ultimately serve as a bridge for other bacteria to bind, worsening the dysbiotic state [10].
The main bacteria found in dental biofilms include Streptococcus, Actinomyces, prevotella, Porphyromonas, Tannerella, and Fusobacterium spp. [6]. These bacteria produce a matrix rich in glucans and exopolysaccharides (EPS), facilitating their adherence to one another and surrounding tissues [9]. Additionally, biofilms contain endotoxins, namely lipopolysaccharides (LPS), which elicit an inflammatory response in the host. As a protective structure, biofilms prevent chemical agents from reaching the microorganisms, thereby increasing antibiotic resistance [9, 12].
The conventional approach for treating cavities involves mechanically removing the lesion and dental plaque. However, this method removes both beneficial and harmful microorganisms, disrupting microbial balance and creating opportunities for the adherence of dental pathogens to the oral surface by facilitating the obtention of nutrients due to the lack of competition [13]. In contrast, recent research has explored postbiotics as a potential solution for preventing oral dysbiosis. Postbiotics are inactive microorganisms, their components, and metabolites that promote health when administered [14, 15, 16]. Common postbiotic metabolites include short-chain fatty acids (SCFAs) and organic acids, namely acetic, lactic, butanoic, and propionic acids, as well as antimicrobial molecules, such as bacteriocins [16, 17, 18, 19], amino acids [20], EPS, cell wall peptides and lysates, varied enzymes [19, 21], flavonoids, and phenolic compounds [22, 23]. Recent studies have shown that postbiotics can influence commensal microorganisms in the oral cavity, helping to restore balance [14, 24]. The administration of postbiotics in the oral cavity can be associated with an anti-biofilm capacity against S. mutans, a desirable characteristic for controlling dysbiosis. This effect can be correlated with the presence of teichoic acids produced by Lactobacillus spp. [17].
Furthermore, postbiotics present the ability to reduce the levels of oral pathobionts, with SCFAs potentially inhibiting bacterial growth by disrupting their membrane [12, 18, 24]. Postbiotics derived from lactic acid bacteria (LAB) often contain bacteriocins, which exhibit inhibitory activity against pathogens [16]. In this work, the postbiotics were obtained from Lactiplantibacillus plantarum and Lacticaseibacillus paracasei, two lactic acid bacteria that produce bacteriocins and have been considered adequate to use in the oral cavity [1].
One significant advantage of postbiotic administration is the absence of live microorganisms, eliminating the risk of transmitting resistance genes or causing infection in vulnerable groups, such as the elderly, children, and pregnant women [14, 16, 18, 24]. Moreover, postbiotics offer a longer shelf life than probiotics and require less storage and transportation facilities [14, 16, 24, 25].
The effectiveness of postbiotics in promoting oral health depends on the method of administration, which determines their contact time within the oral cavity and how effectively they release their beneficial properties. Orodispersible films (ODF) are a promising delivery method due to their ability to quickly and easily deliver active ingredients. ODFs should be non-toxic, biocompatible, and have no expected adverse effects after use [26, 27, 28, 29, 30, 31, 32]. Furthermore, the manufacturing of ODF is simple, brief, and cost-effective [32].
Although ODFs show promise for administering postbiotics and meeting necessary release conditions, further research is needed to clarify their impact on oral dysbiosis. Therefore, our study aimed to address this gap by developing an ODF incorporating optimized postbiotics. To achieve this goal, we (i) assessed the in vitro antimicrobial and antibiofilm capabilities of specific postbiotics, including those derived from Lactiplantibacillus plantarum, Lacticaseibacillus paracasei, and a combination of both, to determine their effectiveness in combating oral pathogens, (ii) investigated the biological properties of the postbiotics to gain insights into their mechanisms of action and potential health benefits; (iii) optimized the formulation and manufactured the orodispersible films to ensure their efficacy and stability; and (iv) analyzed the physicochemical characteristics of the films to understand their composition and properties.
With this work, we aimed to advance in innovative strategies for maintaining oral health and offer potential alternatives to traditional antibiotic therapy for managing oral dysbiosis.
Lactiplantibacillus plantarum 226V and Lacticaseibacillus paracasei L.26 were obtained as a DELVO-PRO freeze-dried, concentrated starter cultures from DSM (Moorebank, Australia). To obtain the postbiotic solutions, Lactiplantibacillus plantarum 226V and Lacticaseibacillus paracasei L.26 were grown in De Man-Rogosa-Sharpe (MRS) broth (Biokar Diagnostics, Beauvais, France) and isolated in MRS agar (Biokar Diagnostics, Beauvais, France) as previously described by Sornsenee et al. [17] with some modifications. Briefly, after obtaining isolated colonies of both species, each was inoculated in a 15 mL falcon in MRS broth (Biokar Diagnostics, Beauvais, France) and incubated at 37 °C for 48 h.
After that period, the falcon tubes were centrifuged at 8000 g (15 min at 4 °C) using a Hettich centrifuge (Hettich, Tuttlingen, Germany). The supernatant was then filtered with a 0.22 µm membrane to obtain the probiotics’ cell-free supernatant (CFS), the postbiotic solution. To ensure that the supernatant did not contain any cells, the solution was plated in MRS agar (Biokar Diagnostics, Beauvais, France) using the drop method, and the growth was observed after incubation of 24 h at 37 °C.
To incorporate the CFSs postbiotics in the orodispersible films, the postbiotic solutions were added to the polymeric solution 2, adjusting the amount of water to maintain the final volume constant.
To evaluate the postbiotic’s antimicrobial activity, the growth rate of S. mutans 45091 with a concentration of 109 colony-forming units (CFU)/mL was evaluated under distinct postbiotic conditions, according to Jung et al. [33]: those obtained from L. plantarum, those from L. paracasei, and a mixture of both, in the concentrations of 10% (v/v), 20% (v/v), 40% (v/v), 60% (v/v) and 100% (v/v). Different concentrations were selected based on the concentration-dependent activity of the postbiotics. Postbiotics were tested in the maximum range possible (0–100%) to understand this characteristic.
Initially, S. mutans was grown in BHI broth (Biokar Diagnostics,
Beauvais, France) and isolated in BHI agar (Biokar Diagnostics, Beauvais, France)
plates. After obtaining isolated colonies, S. mutans was regrown in BHI
broth (Biokar Diagnostics, Beauvais, France) until a 109 CFU/mL
concentration was reached. Then, a co-culture of the S. mutans in BHI
broth (Biokar Diagnostics, Beauvais, France) and the CFS postbiotic solution were
mixed in a 15 mL falcon tube in a 1:1 ratio and incubated for 24 h at 37
°C. Following that time, a 20 µL sample was taken
from each condition and plated in BHI agar (Biokar Diagnostics, Beauvais, France)
in triplicate. The results were expressed as positive or negative growth.
Afterwards, with an inoculum of 109 CFU/mL, a 96-well plate with all the
different postbiotic concentrations was inoculated to determine the postbiotic’s
antimicrobial activity against S. mutans. The essay was performed in
triplicate. The plate was then incubated at 37 °C while
measuring the Optical Density (OD) at
To understand the standard growth rate of S. mutans, positive control of the microorganism and negative controls of each medium were used. The values were then averaged to obtain the mean results, which were used to analyze the effects of the different postbiotics on the S. mutans growth rate.
The methodology from Drumond et al. [34] was followed to determine the
minimal inhibitory concentration (MIC). For that, samples of S. mutans
were grown in MRS broth at 37 °C until reaching an OD at
The time-kill assay was conducted according to Rossoni et al. [35], with modifications regarding the incubation period. S. mutans was grown until a 109 CFU/mL concentration was reached. The bacteria were then incubated at 37 °C with the postbiotic solutions in their minimal inhibitory concentration. At different time points (0, 1, 2, and 4 h), a sample of 1 mL was taken from the mixture. After serial dilutions, these were plated (20 µL), in duplicate, in BHI agar and incubated at 37 °C for 24 h. The plates were then counted to determine the CFU/mL. A positive control (S. mutans) was also added to plates, incubated, and counted.
S. mutans was cultured in BHI broth until a 109 CFU/mL concentration was reached. For the biofilm formation assay, 100 µL of S. mutans suspension (above) was added to each well of a 96-well microplate. Various postbiotics were then introduced at 5 different concentrations: 10% (v/v), 20% (v/v), 40% (v/v), 60% (v/v) and 100% (v/v). The microplates were incubated at 37 °C for 72 h to allow the biofilm formation. After the incubation period, the content of each well was carefully removed, and the wells were washed with Ringer solution to ensure that only the adhered biofilm remained. Following the protocol by Costa et al. [36], the biofilms were stained with 0.1% crystal violet, and the plates were left to dry at room temperature for 24 h. Finally, the wells were resuspended in glacial acetic acid (30%), and the OD was measured at 630 nm.
The mature biofilm inhibition test was performed based on the methodology described by Sornsenee et al. [17] with some modifications. S. mutans was grown in BHI broth until a 109 CFU/mL concentration was reached. Then, 100 µL were placed in a 96-well microplate and incubated for 5 days at 37 °C to allow biofilm to reach its mature state. The CFS postbiotic solutions were then added to the formed biofilm in different concentrations and left to react for 72 h at 37 °C. The remaining steps were performed as described above.
Orodispersible films were produced following the solving-casting method,
according to Shah et al., 2022 [37], with some modifications. In a
beaker, 2.0 g of Xantham gum (Sigma-Aldrich, Darmstadt, Germany) was added to 140
mL of deionized water and left in a magnetic stirrer for at least 4 h (Solution
1). In another beaker, 2.0 mL of Glycerol (VWR chemicals, Solon, OH, USA) was added to 58 mL
of deionized water and stirred for 2 h. Following that time, the rest of the
excipients were added: 0.2 g of Citric acid (Merck, Darmstadt, Germany) followed
by 3.0 g of Maltodextrin (Sigma-Aldrich, Darmstadt, Germany) and stirred for 2 h
(Solution 2) at 50 °C. Finally, Solution 2 was poured slowly over Solution 1
with constant stirring until complete homogenization. The final solution was left
to rest until the complete disappearance of the bubbles formed during magnetic
agitation, then spread over a plastic container and left to dry for 48 h at room
temperature. Afterwards, the samples were cut using a box cutter into 1
The surface appearance was observed after the ODFs were dried, before and after cutting, to understand if the surface was homogenous, without bubbles, and transparent. The observations were performed for all the samples, with and without the postbiotic solution, and the results were compared.
The disintegration time was performed according to Shah et al., 2022
[37], with a few modifications. The orodispersible films were cut into squares
measuring 1
Each measurement was performed in triplicate, and the mean value was calculated. The results were compared to the orodispersible films without the impregnation of postbiotics, which served as a control.
The pH was then measured, according to Salawi [32]. After the complete disintegration/dissolution of the ODFs in water, the pH was measured using a Crison basic 20 pH probe (Crison, Barcelona, Spain). Each measurement was performed in triplicate, and the average value was calculated. The results obtained with the ODFs impregnated with CFS postbiotics were compared to those obtained with the ODFs without the impregnation of postbiotics to understand the pH variation.
According to Batista et al. [38], the thickness of the ODFs was
measured using a My20 micrometer (Adamel lhomargy, Saint-Baldoph, France).
Squares of 1
According to Choi et al. [39], the mass was measured using an analytical scale (Sartorius, Göttingen, Germany) to understand the variation of the ODF’s weight with the addition of the three conditions of the postbiotic solution. Each measurement was performed in triplicate, and the values were averaged to obtain the mean value. The ODFs without the addition of the postbiotics were used as a control.
The percentages of hydration, moisture loss, and solubility were measured
according to Al-Naamani et al. [40] with modifications. The
orodispersible films, previously cut into 1
All measurements were performed in triplicate on an analytical scale (Sartorius, Göttingen, Germany), and the values were averaged to obtain the mean values.
The percentages of hydration, moisture loss, and solubility were calculated using the following equations:
The contact angle of the ODFs was determined through the sessile drop technique using a tensiometer (Attension Theta, Biolin Scientific, Sweden). For that, 5 µL of deionized water was dispensed on the film samples, and the angle formed between the baseline and the lines tangent to the water droplet. The values were recorded for 1 min, and the average value was calculated to perform the analysis. The ODFs without CFS postbiotic solution were used as a control.
TR 146 human buccal carcinoma cell line was used as an in vitro model of the human epithelial mucosa. The cell line was purchased from Sigma-Aldrich (# 10032305) and validated by STR profiling and tested negative for mycoplasma (Mycostrip 50, InvivoGen, San Diego, CA, USA). After defrosting, it was maintained in HAMS F12 medium (Bio West) with 10% of Fetal Bovine Serum (FBS, Biowest), and 1% of Penicillin-Streptomycin-Fungizone solution (Penstrep, Lonza). The TR146 cell line was then maintained in T75 flasks at 37 °C in a 5% CO2 humidified atmosphere during the experimental time.
TR146 cells were plated onto a 24-well microtiter plate at 105 cells/mL of
density to perform the cytotoxicity assay and allowed to attach overnight. The
medium was replaced, and two pieces of each film with a 0.5 cm diameter were
added to the wells in triplicate per condition. Five conditions were tested: (1)
control cells (cells were cultured only with medium, without any film); (2)
control of the film (cells were cultured in the presence of film without
postbiotics); (3) CFS L. plantarum (cells were culture in the presence
of film with CFS postbiotics obtained from L. plantarum); (4) CSF
L. paracasei (cells were culture in the presence of film with CFS
postbiotics obtained from L. paracasei), and (5) CSF both (cells were
culture in the presence of film with a mix of CFS postbiotics obtained from both
probiotics). After 24 hours of incubation, the metabolic activity of viable cells
was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) test (Sigma-Aldrich). For this purpose, the culture media was removed and
replaced with 450 µL of HAMS F12 and 50 µL of MTT
solution per well. These were incubated for 4 h at 37 °C in a CO2
incubator. The culture media was discarded, and 500 µL of DMSO per
well was added to dissolve the formazan crystals. Subsequently, the plates were
agitated for 10 minutes at room temperature. The absorbance was measured at
All analyses were done with GraphPad Prism version 10.1.1 software (GraphPad Software, Inc., San Diego, CA, USA). The antimicrobial and antibiofilm activity were analyzed using a two-way ANOVA followed by Dunnett’s multiple comparison test to assess significant differences. For the analysis of the ODF’s physical properties and cytotoxicity, the one-way analysis of variance (ANOVA) method was used for multiple comparisons, followed by Tukey’s post hoc test after testing for the normal distribution of all data.
All experiments were performed in triplicate. The results are expressed as
means, and the corresponding standard deviations were calculated. Values were
considered statistically different at a p-value
The minimal inhibitory concentration (MIC) assay was conducted with all postbiotic solutions to determine the concentration (v/v) required to inhibit the growth of S. mutans. This is a crucial assessment since the dysbiotic environment improves the growth of S. mutans, and its metabolic activity creates an anaerobic environment that allows other pathogens, such as Porphyromonas gingivalis and Treponema denticola, to grow. The resulting acid production culminates in an enhanced demineralization process of the dental enamel, ultimately forming a cavity [1].
The minimal inhibitory concentration was defined as the lowest postbiotic concentration, resulting in no visible growth of S. mutans (Fig. 1A). For CFS postbiotics from L. plantarum, the (MIC) was 40%. At the same time, for those from L. paracasei, it was 60%. Interestingly, CFS postbiotics from both probiotics exhibited the lowest concentration needed for complete bacteria inhibition, with only 20% required to inhibit S. mutans’ growth. This suggests an advantage in obtaining CFS postbiotics from two probiotic species due to their synergetic behavior.
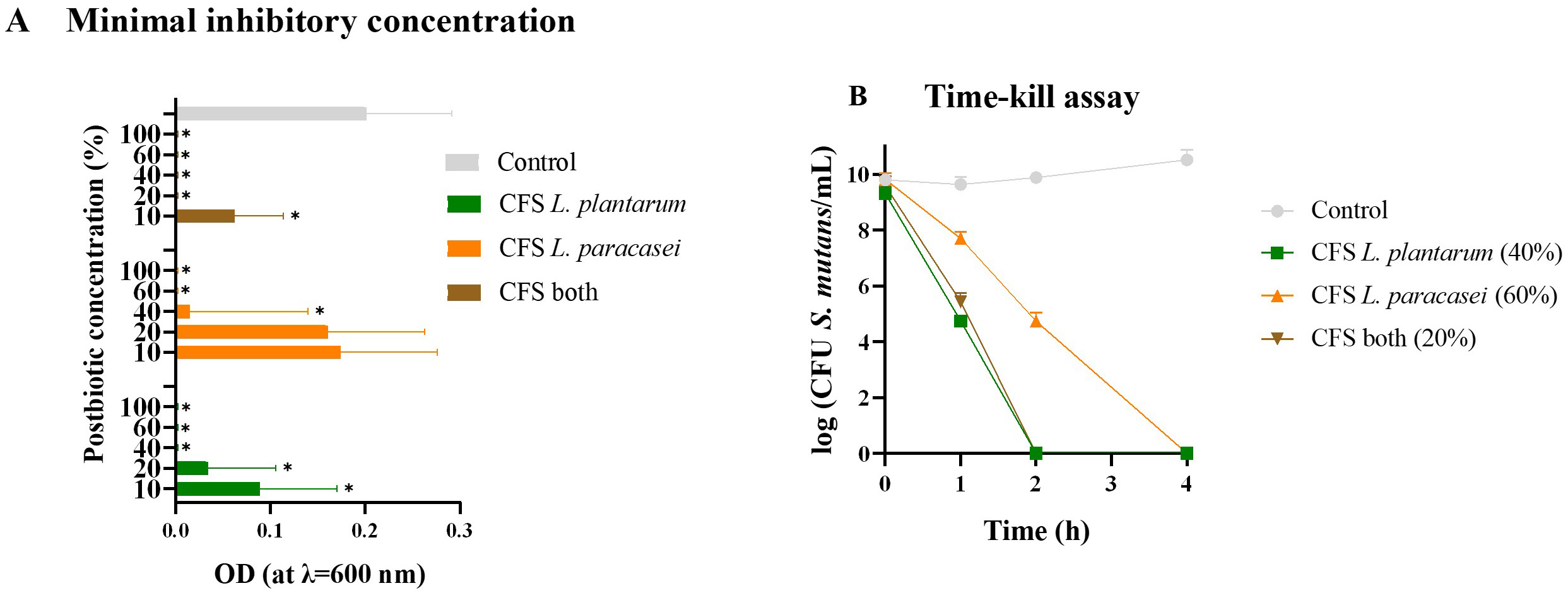 Fig. 1.
Fig. 1.
Minimal inhibitory concentration and Time-kill assay. (A) Optical density (OD)
variation of S. mutans measured at
Additionally, a time-kill assay was performed to evaluate the growth inhibition or death of S. mutans in co-culture with different postbiotics (Fig. 1B). Notably, postbiotics demonstrating higher antimicrobial activity showed a quicker time to kill S. mutans. Both CFS obtained from L. plantarum and those from both probiotics took 2 h before no growth after plating and counting could be observed. In comparison, CFS postbiotics from L. paracasei required 4 h until no growth was detected.
A positive control was used in both tests to ensure the growth of S. mutans. However, it is important to notice that the CFU/mL of S. mutans was adjusted to 106 in the MIC assay.
These findings are promising for incorporating postbiotics into ODFs, as they exhibit rapid antimicrobial activity without requiring significant time to exert their effects, thus making them suitable for oral cavity administration.
The results obtained in this study are in accordance with those in the literature [12, 17, 34, 35].
Regarding the antimicrobial measurements performed with the postbiotics against S. mutans, it was critical to understand how the co-culture of the different postbiotics in distinct concentrations affected the microorganism’s growth rate. Firstly, a co-culture of various concentrations of S. mutans with the three postbiotics (at 50% concentration) was plated, and growth inhibition was observed (data not shown). Based on these results, the growth rate of S. mutans at a concentration of 109 CFU/mL was evaluated for each postbiotic during 24 h.
In co-culture with CFS postbiotic from L. plantarum, the growth rate of S. mutans varied notably with increasing postibiotic concentration (see Fig. 2A). Interestingly, even at 10% concentration (sub-MIC) of CFS postbiotic, a discernible difference was observed. When a concentration of 40% was reached, the growth was minimal, and no pronounced difference was noted between the higher concentrations (40, 60, and 100%). These findings align with those of the MIC (Fig. 1A). Similarly, in co-cultures with CFS postbiotics from L. paracasei, a decline in S. mutans growth rate was observed with increasing postbiotic concentration (Fig. 2B). However, only at a high concentration (100%) did the postbiotic notably reduce bacterial growth. This indicates that the antimicrobial activity of the L. paracasei postbiotic was less effective compared to that of L. plantarum, corroborating the results of the MIC assay (Fig. 1A).
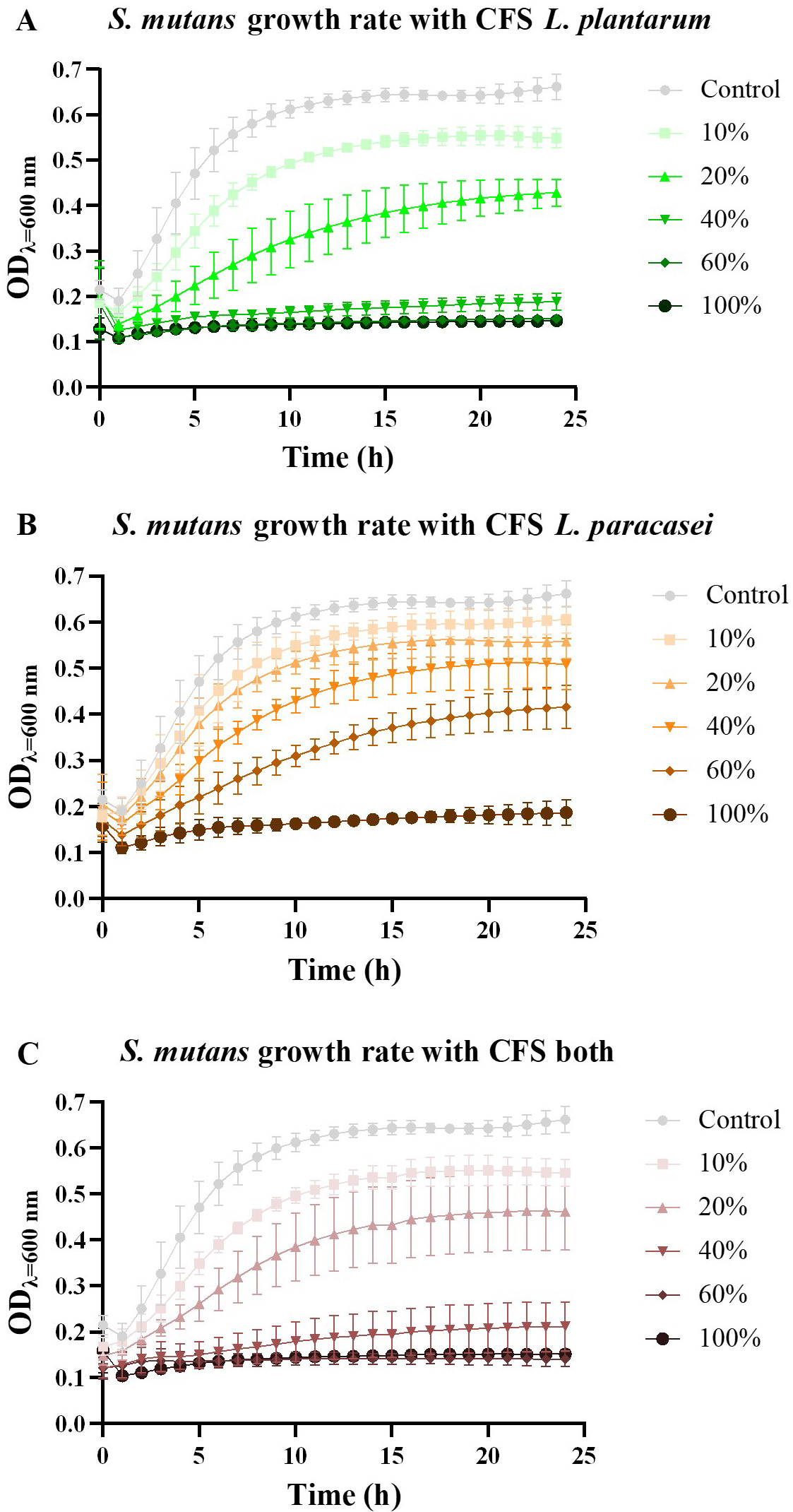 Fig. 2.
Fig. 2.
Growth rate measurement. (A) The Optical Density (OD) variation of S.
mutans measured at
Analysis of co-cultures with postbiotics obtained from both probiotics revealed a significant impact on S. mutans growth rate (Fig. 2C). Notably, the postbiotic mixture from both probiotics exhibited higher antimicrobial activity than individual postbiotics. Once again, these findings are consistent with those of the MIC assay, indicating that a lower concentration of the postbiotic mixture is needed to effectively inhibit S. mutans growth (Fig. 1A). From Fig. 2, it is clear that at 10% (sub-MIC) concentration, inhibition of S. mutans can be observed in all tested CFS’s.
Studying the potential antibiofilm activity is crucial in controlling oral cavity homeostasis, considering S. mutans’ ability to adhere to oral mucosa and dental surfaces, leading to biofilm formation. Two key aspects must be addressed: inhibiting biofilm formation and disrupting mature biofilms. The former is vital for preventive approaches, while the latter is essential for co-adjuvant therapy. Given that disease symptoms manifest later than dysbiosis onset, postbiotics could be applied in an asymptomatic oral cavity already experiencing dysbiosis. Moreover, microorganisms become more resistant to therapies once the biofilm forms. Hence, understanding the mature biofilm disintegration/inhibition capacity of different postbiotic solutions tested in this study is imperative.
The results obtained in our study are in accordance with other findings [12, 17, 33].
The antibiofilm activity correlated directly with postbiotic concentration (Fig. 3A) in inhibiting biofilm formation. The CFS postbiotic from L.
plantarum exhibited the highest inhibition percentage at 100% concentration
(79.6
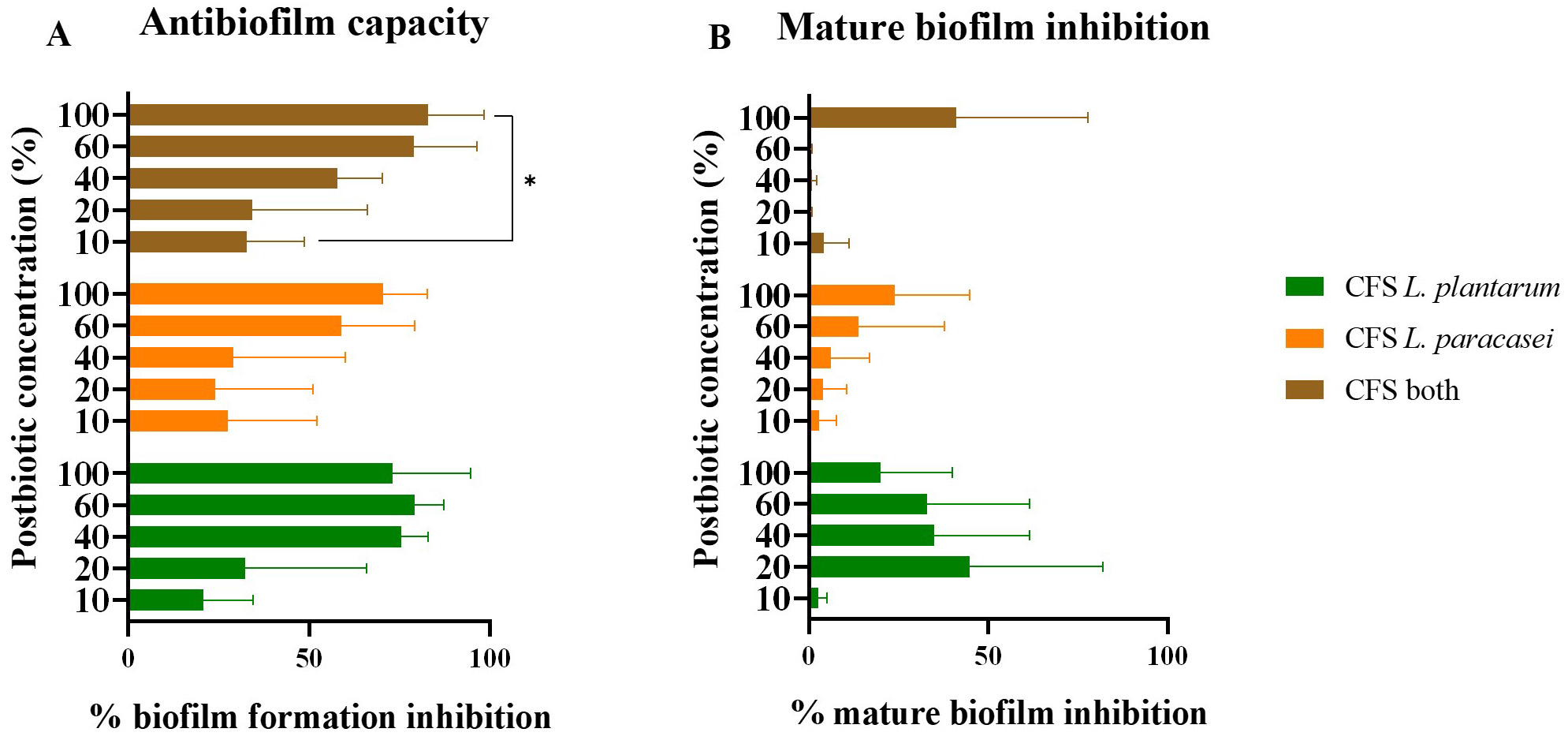 Fig. 3.
Fig. 3.
Antibiofilm capacity. (A) Inhibition percentage of S.
mutans biofilm formation with different CFS postbiotics in distinct
concentrations. (B) Inhibition percentage of S. mutans mature biofilm
formation with different CFS postbiotics in distinct concentrations. *
indicates significant differences between the samples (p ˂ 0.05). The
data is presented as mean
Interestingly, the postbiotic from L. plantarum displayed a notable
inhibition percentage (75.5
The mature biofilm inhibitory properties of the tested postbiotic were expected to be lower compared to biofilm formation inhibition due to the heightened resistance of bacteria in mature biofilms to treatment. As shown in Fig. 3B, none of the tested postbiotics reached 50% inhibition, indicating the significant challenge in removing mature biofilms.
Curiously, mature biofilm inhibition activity appeared unrelated to postbiotic
concentration. The highest inhibition percentage (44.7
However, the potential activity demonstrated by CFS postbiotics from L. plantarum warrants further analysis. The lack of correlation between antibiofilm activity and concentration requires deeper investigation, as these results were unexpected.
The orodispersible film formulation was optimized for elasticity, appearance, and maneuverability. According to Mura et al. [41], an ODF that presents elasticity and flexibility ensures a pleasurable sensation in the oral cavity. The optimized formulation was based on Cugini et al. [42], who stated that film-forming polymers should constitute up to 50% of the total concentration, followed by up to 20% of plasticizers, 10% of sweetening agents, and 10% of saliva stimulants. In this sense, the final optimized concentration of the film-forming solution was 25% (m/v) film-forming polymers (Xantham gum and Maltodextrin), 15% (m/v) plasticizer agent (Maltodextrin and Glycerol), 1% (m/v) saliva stimulant (Citric acid), and 1% (v/v) sweetening agent (Glycerol). After drying, the postbiotic-free ODFs presented adequate handling; they were thin and easy to cut. Regarding color, the ODFs were transparent. However, the same was not observed regarding the ODFs impregnated with the postbiotic solutions. During the formulation of the ODF, a significant number of bubbles were formed in the solutions; however, when left to rest before pouring, the complete disappearance of the bubbles was noted. However, the bubbles persisted in the solution impregnated with the postbiotic, even after the rest period; they were darker and not homogenous. Nonetheless, the ODFs impregnated with CFS postbiotics were more accessible to handle since they were thicker and less gelatinous.
The choice of natural polymers was based on the fact that they are biodegradable and biocompatible, do not present toxicity for the oral cavity, and and they are generally recognized as safe (GRAS) by the FDA [43]. Maltodextrin was chosen based on the appearance it provides the films; however, its mechanical properties are often limited [37]. To overcome this problem, xantham gum was used concomitantly as a film-forming polymer.
A significant value to determine regarding ODFs is the disintegration time, i.e., the time it takes to dissolve entirely after contact with the oral cavity. There are still no guidelines regarding the dissolution time. However, the time should be long enough to ensure that the postbiotics can be delivered to the oral cavity and exert their activity. Still, according to Lordello et al. [28], the disintegration time is directly related to the polymer concentration in the ODF.
The ODF without the impregnation of CFS postbiotics served as a control and
presented a dissolution time of 24
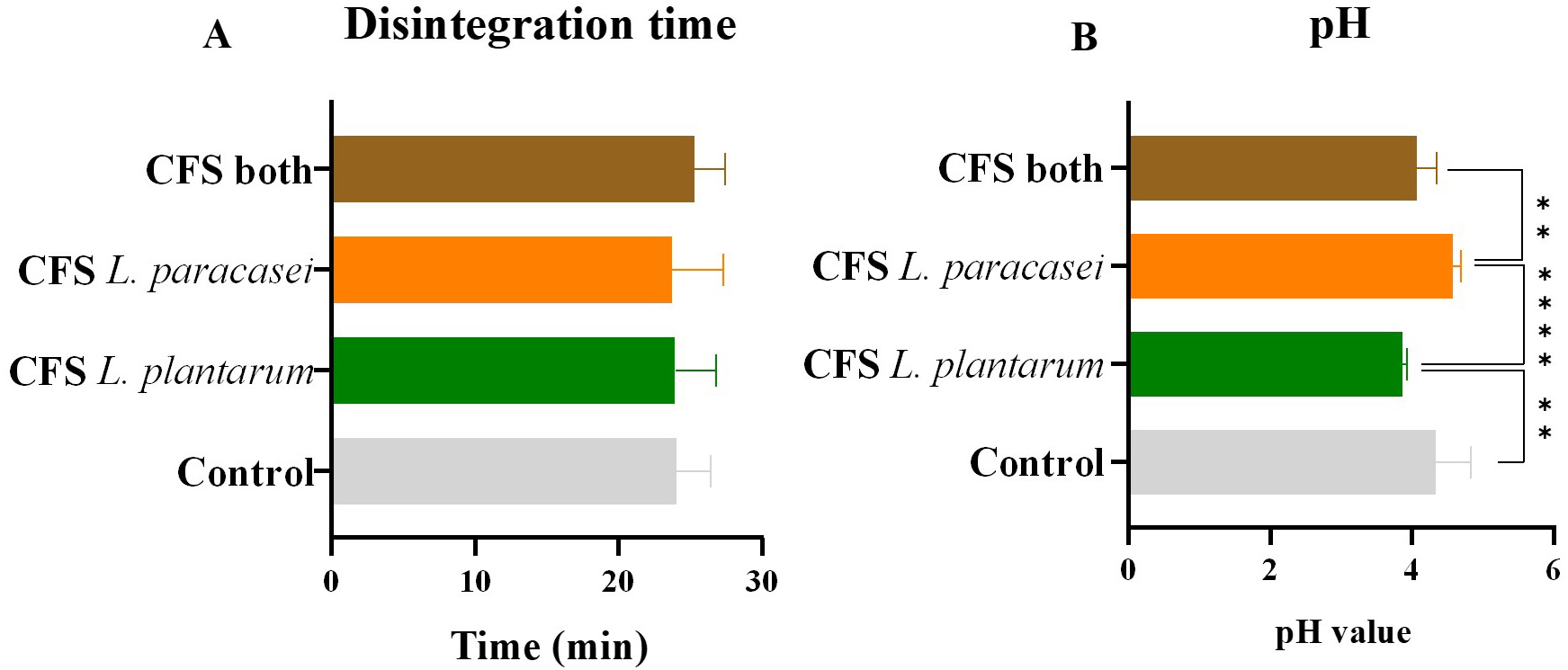 Fig. 4.
Fig. 4.
Disintegration time and pH values. (A) Time, in minutes, until
complete dissolution of ODFs and (B) pH values of the different oral dispersible films (ODFs) impregnated
with postbiotic solutions. The data is presented as mean
However, it is essential to remember that in vitro behavior differs drastically from in vivo performance. If the ODFs are applied to the oral cavity, the time it takes to dissolve them is expected to decrease since they will be affected by deglutition, speech, and the normal movement of the tongue.
The pH was measured after the complete dissolution of the ODFs; data is shown
below in Fig. 4B. The ODF without the impregnation of the postbiotics served as a
control and showed a pH value of 4.33
This demonstrates that, probably during growth, L. plantarum produced
acidic metabolites, such as lactic acid, which offers more antimicrobial activity
against pathogens. It is also likely that L. plantarum produces more
acidic metabolites during growth, which can be noted when comparing the
antimicrobial activity with the postbiotic obtained from L. paracasei.
The ODF impregnated with CFS postbiotics obtained from both probiotics had a pH
value of 4.06
The films’ pH was desired to be closer to neutral (around 7) [44]. However, lower pH values were expected since the chosen probiotics are LAB that produce acid during growth [28]. Cytotoxicity assessments showed that none of the tested ODFs presented increased cytotoxicity when in contact with oral cavity cells. This demonstrates that the low pH does not present a safety limitation. However, its application could be limited since it might exacerbate the already acidic environment in an unhealthy oral cavity. Additionally, some acids produced during Lactobacillus spp. growth are responsible for disrupting the bacterial membrane, which could translate into an absent antimicrobial activity after the neutralization of the ODFs.
The low pH of the control was due to the addition of citric acid, which acts as a saliva stimulant. These low pH values might contribute to the films’ antimicrobial activity and the maintenance of a desired salivary flow, which can contribute to removing harmful bacteria in the oral cavity. However, multiple factors could influence this activity, and further analysis is needed to determine if the antimicrobial effect persists after neutralizing the film-forming solutions.
Another physical property of the ODFs assessed was the variation of their thickness before and after the addition of the different CFS postbiotic solutions (Fig. 5A).
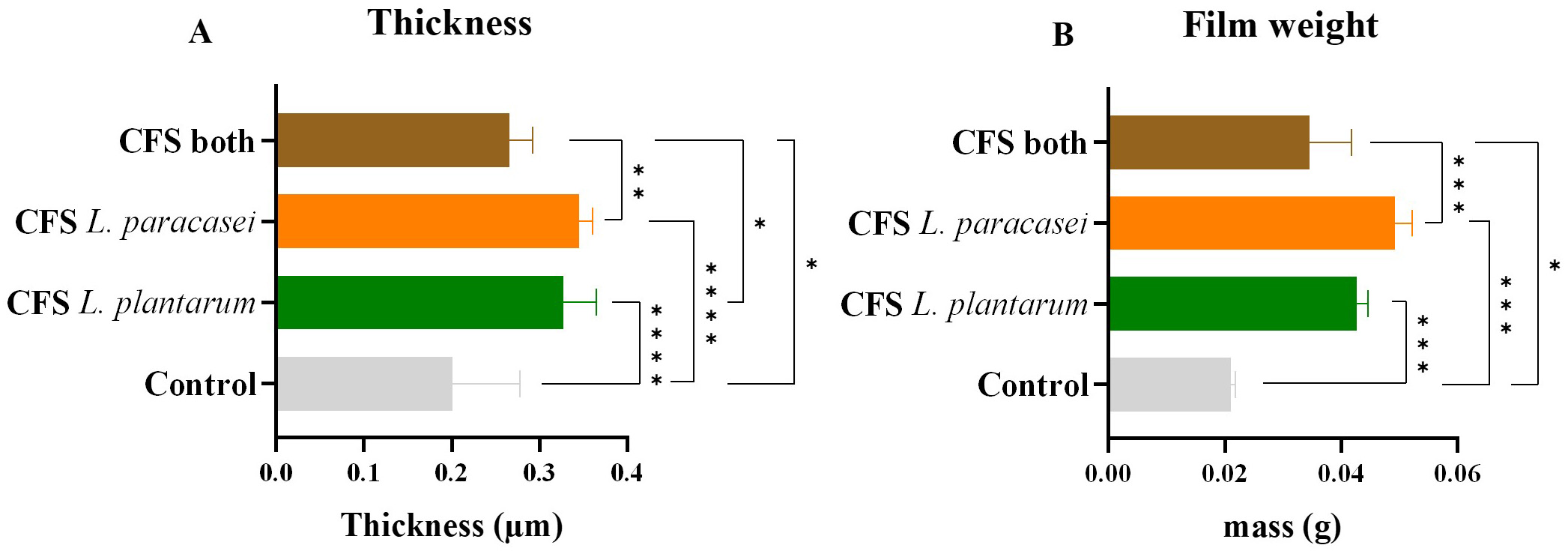 Fig. 5.
Fig. 5.
Thickness and film weight. (A) Thickness values, in
µm, of the different samples of postbiotic-impregnated ODFs. (B)
Film weight values, in grams, of the different samples of postbiotic-impregnated
ODFs. The data is presented as mean
The ODF without impregnating the postbiotic solutions was considered a control,
presenting a thickness of 0.175
Measurements were taken using an analytical scale to understand the variations in film weight before and after incorporating different postbiotic solutions. The ODF without any CFS postbiotic solution served as a control. All ODFs showed significant weight increases with incorporating CFS postbiotics (Fig. 5B).
The control weight was 0.0210
As expected, the weight variations were similar to thickness changes observed with different postbiotic solutions. However, some authors suggest that the increased weight and thickness after CFS postbiotics impregnation could exceed the recommended levels for optimal behavior in the oral cavity [38, 45].
Another critical factor to consider is the hydration percentage or swelling capacity. This value is directly related to the hygroscopic properties of the ODF. It is affected by the film-forming polymers and can influence the physical characteristics of the final product [37, 45]. Films with high glycerol concentrations display lower mechanical stress since it increases their hygroscopic tendency [37].
The results showed a significant drop in hydration percentage when CFS
postbiotics were added to the film-forming solution (Fig. 6A). The control ODF
had a hydration percentage of 1710
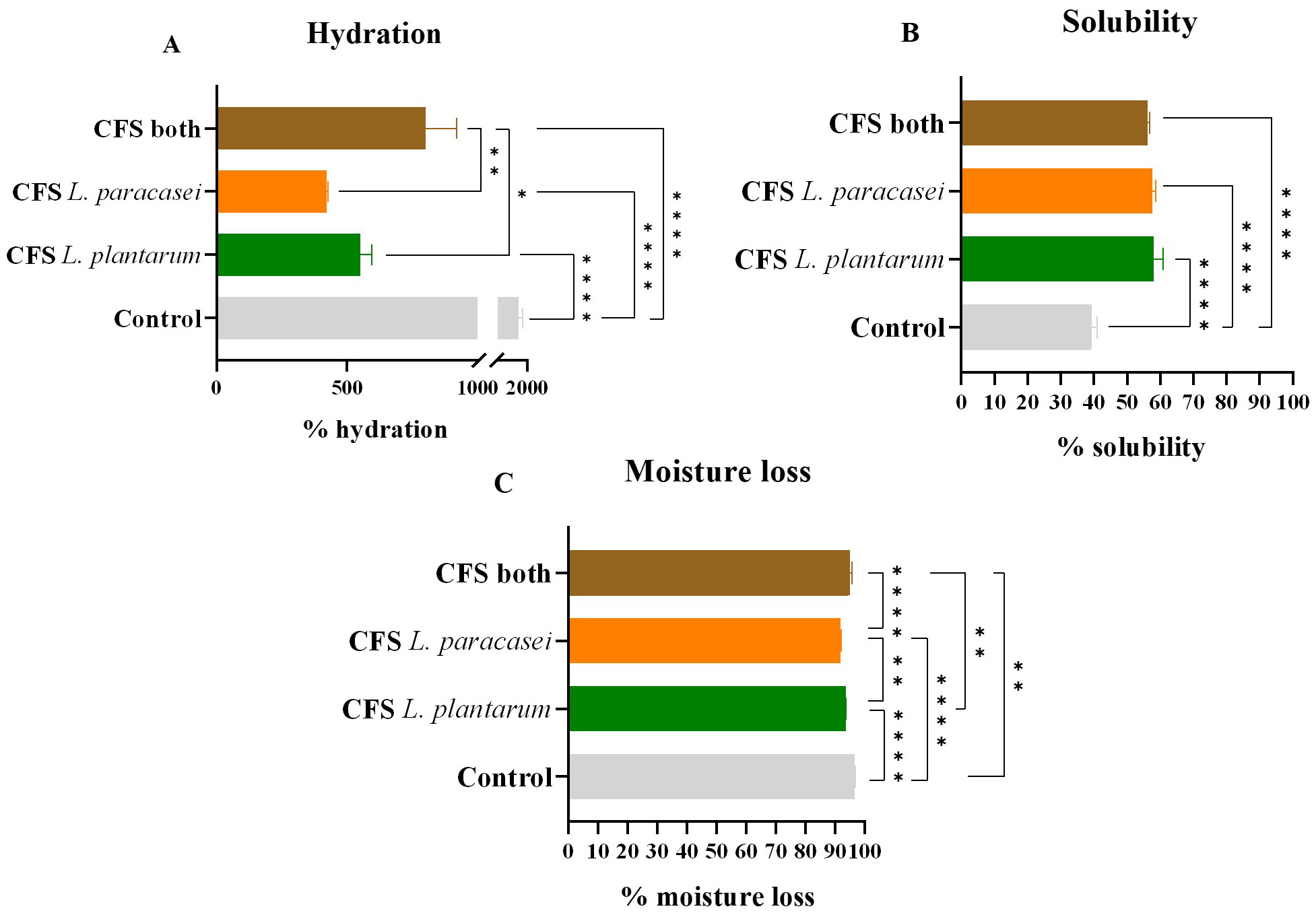 Fig. 6.
Fig. 6.
Hydration, moisture loss, and solubility percentages. (A)
Hydration percentage values of the different samples of postbiotic-impregnated
ODFs. (B) Solubility percentage values of the different samples of
postbiotic-impregnated ODFs. (C) Moisture loss percentage values of the different
samples of postbiotic-impregnated ODFs. The data is presented as mean
Alongside hydration, moisture loss and solubility percentages were also
measured. Higher solubility was found in samples with lower hydration, which is
desirable for better postbiotic dissolution in the oral cavity. Increasing
solubility is desired since it allows for better dissolution of the postbiotics
in the oral cavity. The control ODF had a solubility of 39
Moisture loss variability between samples was less substantial but still
statistically significant. The control ODF had a moisture loss percentage of 96
The water contact angle, which indicates the hydrophilicity of the ODFs, was the
final physical property analyzed (Fig. 7). This angle, formed between the ODF
base and the tangent to the water droplet’s exterior plane, can impact
dissolution time and hydration percentages. Lower contact angles signify higher
hydrophilicity. As anticipated, the contact angle decreased after adding CFS
postbiotics, correlating with increased solubility. The control ODF, without CFS
postbiotics, had a contact angle of 54.6
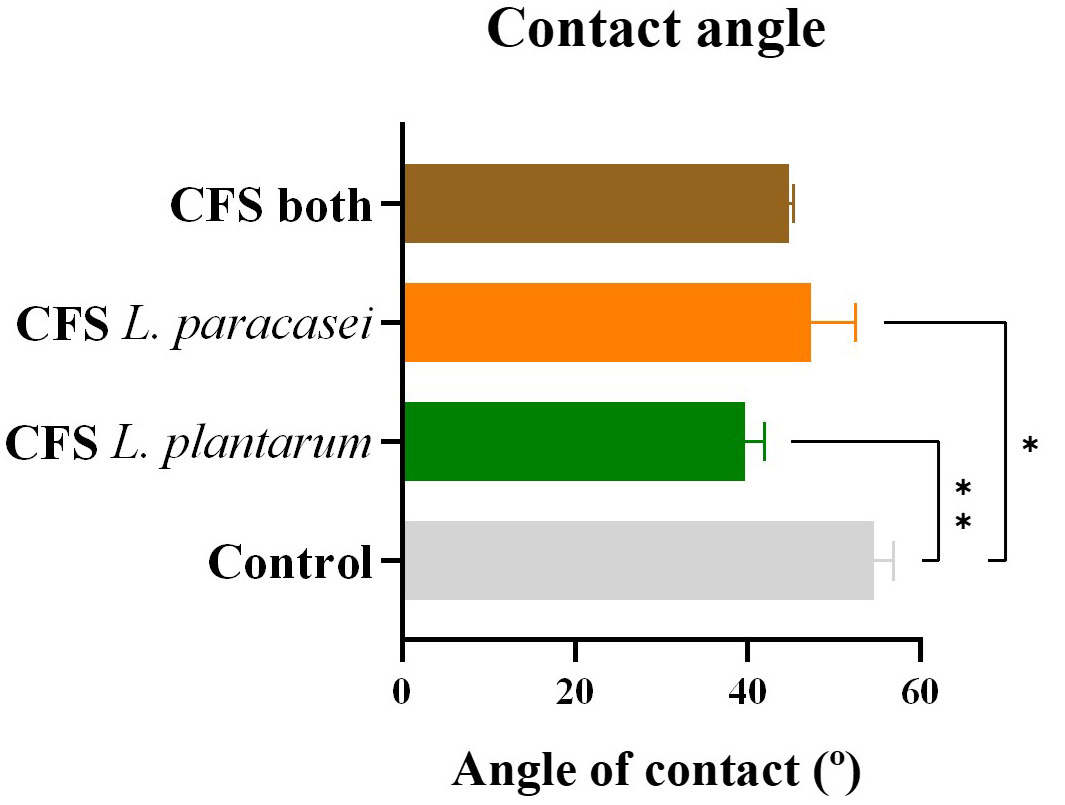 Fig. 7.
Fig. 7.
Contact angle. Angle of water contact of the different samples
of postbiotic-impregnated ODFs. The data is presented as mean
ODFs present a promising avenue for maintaining oral cavity homeostasis, with
postbiotics impregnated in the film-forming solution exhibiting antimicrobial and
potential antibiofilm activity upon oral administration. To ensure safety (cell
viability maintenance) when in contact with the different ODFs tested, a
cytotoxicity assay was conducted, with two defined controls: a cell control
without adding ODFs with or without postbiotics and a control of ODFs without
impregnating CFS postbiotics. The loss of cell viability between controls was not
statistically significant, decreasing from 100
Across the three postbiotics tested, cell viability decreased in all samples,
with the highest loss attributed to the CFS postbiotic from L. plantarum
(56.2
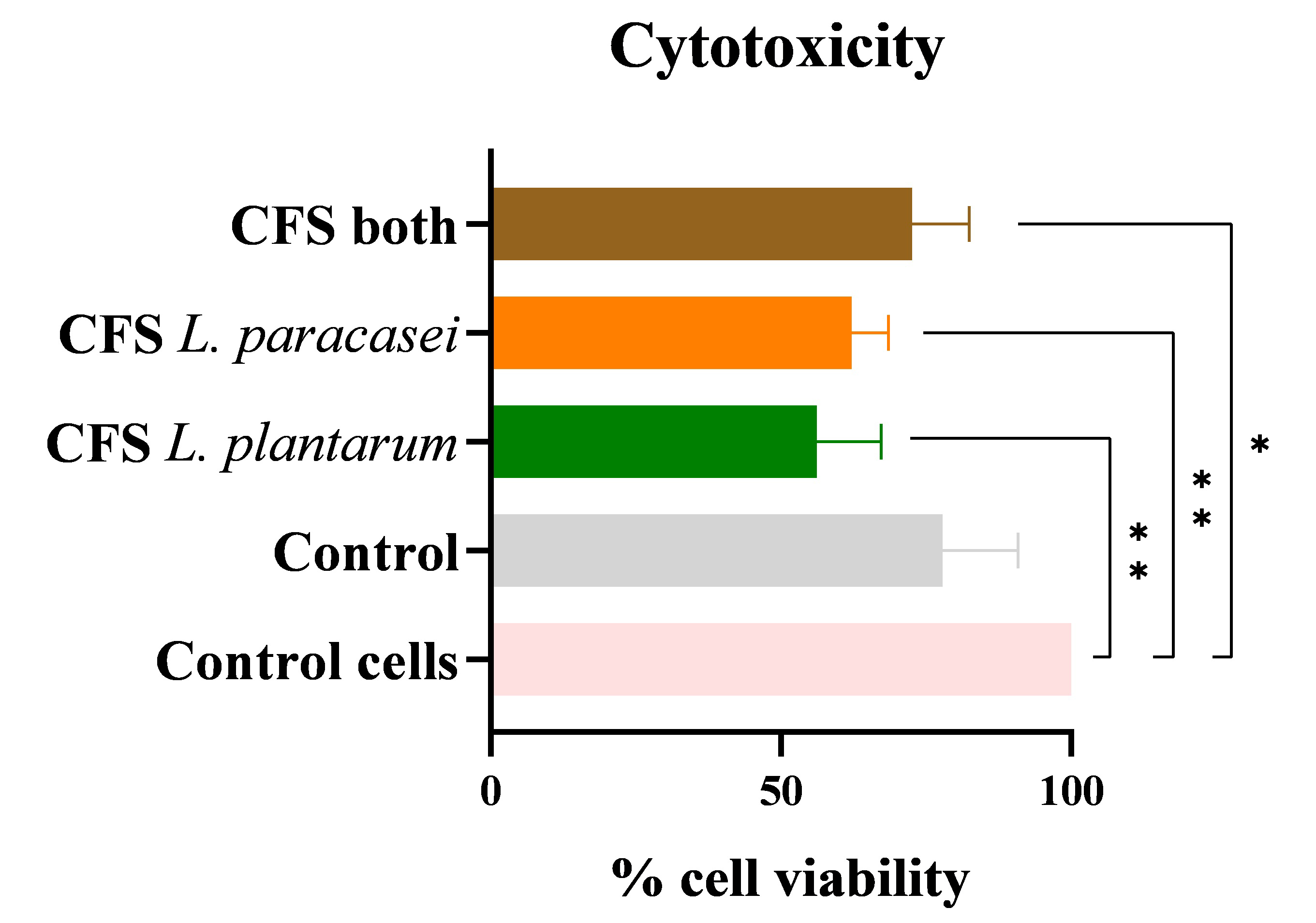 Fig. 8.
Fig. 8.
Cytotoxicity. Percentage of cell viability of TR146 cell line
in contact with ODFs impregnated with the different CFS postbiotics, representing
cytotoxicity. The data is presented as mean
These findings underscore the potential of ODFs impregnated with postbiotics as a preventive treatment option for controlling dysbiosis in the oral cavity, mainly when S. mutans is the major pathogen involved.
These results are in accordance with those obtained in the literature [17, 45].
Oral diseases stem from an imbalance in the oral cavity microbiome known as oral dysbiosis. To address this, preventive measures should prioritize restoring the natural balance of the microbiota rather than eliminating it.
In our study, we developed an ODF incorporating postbiotics derived from L. paracasei and L. plantarum. Evaluating the antimicrobial properties of these postbiotics revealed significant activity against S. mutans, varying effectiveness based on their concentration. Postbiotics from both sources demonstrated superior antimicrobial effects when compared to those from L. paracasei alone, suggesting potential synergies. Additionally, these postbiotics displayed antibiofilm properties, particularly when applied during biofilm formation. Furthermore, these ODFs showed no cytotoxicity to human oral cells.
However, it is essential to recognize that there are limitations that should be overcome before considering this approach. The lack of antimicrobial activity against mature biofilms is concerning since this condition is prevalent in a dysbiotic environment. Additionally, the low pH attained can be considered a limitation since it can aggravate the dysbiosis in an already unhealthy oral cavity, worsening the disease or the dysregulated condition. Lastly, more detailed studies of ODF’s physical characteristics should be performed, considering that the lack of understanding of the mechanisms by which it delivers the active compounds in the oral cavity is a lenient limitation. Additionally, it should be understood how the postbiotics behave in the long term to determine their viability accurately.
All things considered, further investigations are warranted to understand their physical characteristics, storage requirements, and shelf life post-impregnation with postbiotics. Optimization of ODF formulation and delivery methods are also essential.
Notably, in vitro findings may not fully translate to in vivo scenarios due to environmental factors unique to the oral cavity, underscoring the need for clinical trials to assess ODF efficacy. For this, preclinical work should be performed with a three-dimensional model of the oral cavity that takes into account the salivary flow and the mastication effects in the ODF. It is also vital to carry out clinical tests in the future to determine the actual extent of the benefits of using postbiotic-impregnated ODFs.
Lastly, standardized guidelines for postbiotics acquisition and formulation are necessary to ensure safe and effective application in improving oral health.
The data utilized and/or examined in the present study can be obtained from the corresponding author upon reasonable request.
MBR and FKT designed the research study. MBR and CSO performed the cell culture assays. MBR analyzed the data and wrote the manuscript. CSO and FKT provided supervision and directions to the manuscript. CSO and FKT contributed with writing-review, editing and validation. All authors contributed to editorial changes in the manuscript and approved the final version of manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
According to the Portuguese Catholic University’s Ethical Commission for Health (CES-UCP), this study does not involve human or animal subjects, therefore, ethical committee approval is not necessary.
The authors would like to thank the CBQF staff for their assistance with this project.
This work was supported by National Funds from Fundação para a Ciência e a Tecnologia (FCT) through the project [UIDB/50016/2020].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
