- Academic Editor
Intrinsic factor (IF) is a glycoprotein crucial for cobalamin (vitamin B12) absorption in the human body. This study aimed to evaluate the binding affinity of nitrosylcobalamin (NO-Cbl), a cobalamin analog, to recombinant human IF derived from plants, using hydroxocobalamin (OH-Cbl) as a comparative standard.
Surface plasmon resonance (SPR) was employed to assess the kinetic parameters of NO-Cbl and OH-Cbl interactions with plant- derived IF across various concentrations.
SPR analysis demonstrated that NO-Cbl and OH-Cbl exhibited high binding affinities to IF, with equilibrium dissociation constant (KD) values in the picomolar range. OH-Cbl showed a slightly stronger binding affinity (KD = 4.79 × 10-11 M) than NO-Cbl (KD = 8.58 × 10-11 M). Despite NO-Cbl and OH-Cbl both being bound to IF, differences in binding affinity and stability were observed, particularly at higher concentrations.
Variations in IF binding between NO-Cbl and OH-Cbl may be attributed to the saturation of binding sites or recognition issues specific to plant-derived IF. This study underscores the potential of NO-Cbl as a targeted therapeutic agent capable of leveraging natural cobalamin uptake pathways. These results also highlight the suitability of using recombinant plant-derived IF as a model for predicting the biological activity of cobalamin analogs despite the nuanced differences from native human IF.
Cobalamin (vitamin B12) is an essential cofactor in various metabolic processes, including DNA synthesis, fatty acid metabolism, and myelin production [1]. Its absorption in the human body is mediated by intrinsic factor (IF), a glycoprotein secreted by the parietal cells of the stomach [2]. IF binds to cobalamin in the small intestine, facilitating its transport across the intestinal epithelium and its subsequent delivery to tissues via other cobalamin-binding proteins, such as transcobalamin II [3].
The study of cobalamin analogs has garnered significant interest due to the potential therapeutic applications of these analogs, particularly in diagnosis and cancer treatment of cancer [4, 5, 6]. Nitrosylcobalamin (NO-Cbl) is a modified form of cobalamin in which a nitric oxide (NO) group is attached to the cobalt center of the corrin ring [7]. This modification is designed to exploit the natural cobalamin uptake mechanisms while introducing the ability to deliver nitric oxide selectively to target cells, such as cancer cells, that overexpress cobalamin (CD320) receptors [3, 8, 9, 10]. The ability of NO-Cbl to bind IF effectively is a critical determinant of its potential to be recognized by other cobalamin-binding proteins, thereby ensuring its bioavailability and therapeutic efficacy.
Intrinsic factor serves as an ideal model for studying the binding kinetics of cobalamin analogs due to its highly specific interaction with cobalamin. By evaluating the binding affinity and kinetic behavior of NO-Cbl in comparison to hydroxocobalamin (OH-Cbl), a naturally occurring and biologically recognized form of cobalamin [11], researchers can gain insights into the potential biological activity and therapeutic applicability of NO-Cbl. Such studies are particularly relevant given the growing interest in developing cobalamin-based drug delivery systems and targeted therapies [12, 13, 14, 15, 16].
Previous research has demonstrated that modifications to the corrin ring of cobalamin can significantly impact its binding affinity and recognition by cobalamin-binding proteins [10]. SInterestingly, chemical modifications can abrogate the biological activity of cobalamin analogs by disrupting their interaction with transport proteins such as transcobalamin II (TCII) [17]. Similarly, the use of surface plasmon resonance highlighted the importance of structural integrity for effective binding to IF, with even subtle changes leading to altered binding kinetics [18].
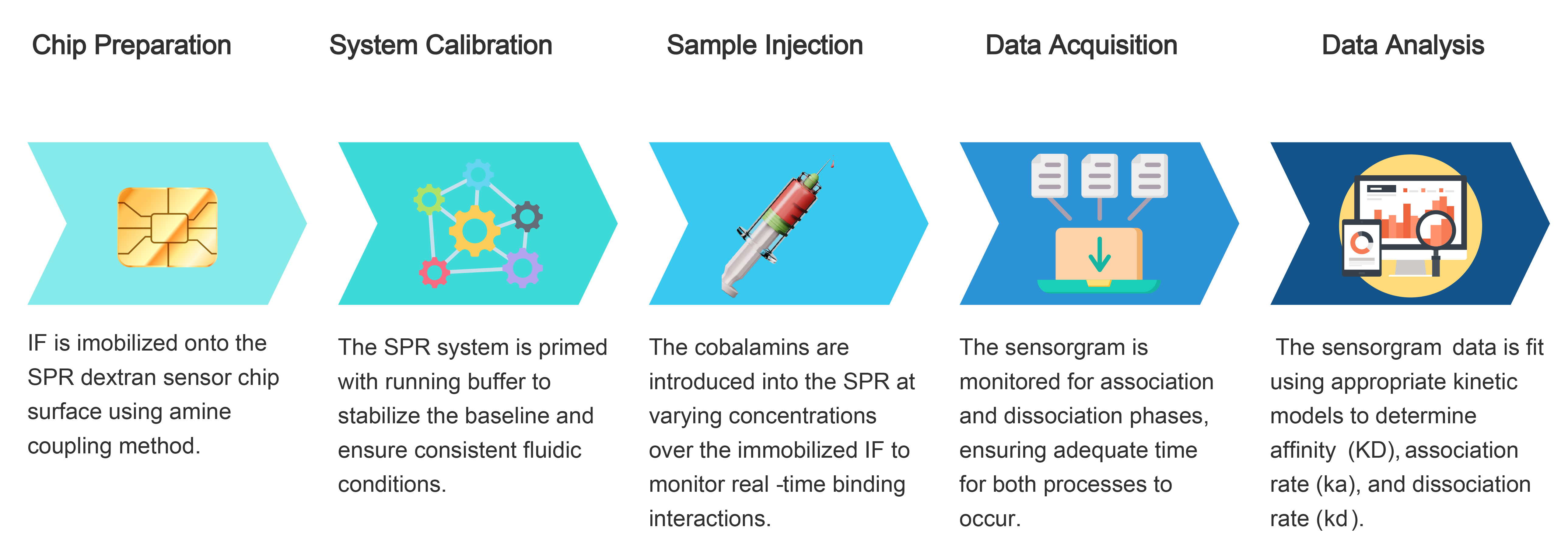
Surface plasmon resonance (SPR) is an experimental technique used to study biomolecular interactions between two analytes in real time. SPR is useful in drug discovery to help identify receptor-ligand interactions as well as biomarkers for disease [19, 20, 21, 22, 23]. The general experimental flow involves immobilizing analyte A on a sensor chip surface, flowing analyte B over the surface, and detecting changes in refractive index as binding occurs. This non-invasive approach allows for characterization of molecular interactions under near-physiological conditions, making it possible to study complex biological systems such as cobalamin and its binding partners.
In this study, we explore the binding kinetics of NO-Cbl and OH-Cbl to recombinant human IF derived from plants (Arabidopsis thaliana) [24]. The use of plant-derived IF offers several advantages, including the potential for large-scale, pathogen-free production. Crucially, plants do not produce cobalamins as they are exclusively synthesized by certain bacteria and archaea [25]. Consequently, recombinant intrinsic factor (rIF) produced in plants is completely free of cobalamin. In contrast, human-derived intrinsic factor (hIF) may retain trace amounts of bound cobalamin or haptocorrins (cobalamin binding proteins) potentially leading to reduced binding efficiency in a SPR model [26].
This study builds upon previous research on NO-Cbl and OH-Cbl by specifically focusing on their binding affinities and kinetics with IF, an aspect critical to understanding their therapeutic relevance and biological function. By investigating these interactions, we aim to bridge the gap between the established therapeutic potential of NO-Cbl and its biochemical basis, distinguishing this work from prior studies that primarily explored its anticancer properties and pharmacodynamics. This study not only provides valuable insights into the biological relevance of NO-Cbl but also contributes to the broader understanding of cobalamin binding dynamics and the development of novel cobalamin-based therapeutics.
Fresh nitric oxide gas (CP grade 95%) was introduced into a solution of methylene chloride at a pressure of 100 psig within a continuously stirred tank reactor. The use of methylene chloride as the solvent was critical because it lacks electron-donating properties which could otherwise interfere with the binding process. This synthesis approach, as originally described by Bauer [7], allowed for the production of a biologically active and oxygen-stable form of NO-Cbl, which has been widely studied for its therapeutic potential in targeting tumor cells [17, 27].
The chemical identity and purity of the NO-Cbl reaction method was subsequently confirmed using the stability-indicating high pressure liquid chromatography (HPLC) method described by Dunphy et al. [27]. The compound’s storage stability (–80 °C) for nitric oxide content was monitored by ultraviolet-visible spectroscopy (UV/Vis).
Surface plasmon resonance (SPR) experiments were performed using a Biacore T200 system (Cytiva, Marlborough, MA, USA). CM5 sensor chips (carboxymethyl dextran surface) and reagents for amine coupling, including N-ethyl-N′-dimethylaminopropylcarbodiimide, N-hydroxysuccinimide, and 1 M ethanolamine HCl (pH 8.5). Recombinant human intrinsic factor protein from transgenic plants (rhIF; cobalamin binding capacity 40 nmol, corresponding to 2 mg active rhIF; Cobento Biotech, Aarhus, Denmark) was immobilized onto the CM5 dextran chip using amine chemistry, resulting in a response unit (RU) of 4800. Prior to analyte injection, the sensor chip surface was equilibrated with the control buffer (running buffer) to establish a stable baseline. The control buffer was also used for all blank injections and as a diluent for analytes. All experiments were conducted at 37 °C to maintain consistency in binding kinetic measurements. After each binding analysis, desorption was conducted using 0.5% w/v sodium dodecyl sulfate followed by 1M glycine at pH 2.5. The sensor surfaces were preconditioned at a flow rate of 100 µL/min as directed by the Biacore 2000 owner’s manual. Kinetic parameters were determined using the Langmuir binding model. The SPR workflow is shown in Fig. 1.
The kinetic parameters Rmax, RI, Req,
and kobs for both NO-Cbl and OH-Cbl were compared between four
concentrations (1
The binding affinities of OH-Cbl and NO-Cbl to rhIF were evaluated using SPR analysis. The key kinetic parameters for both cobalamins, along with their responses at increasing concentrations, are summarized in Table 1 below.
| OH-Cbl (M) | Rmax (RU) | RI (RU) | Req (RU) | kobs (1.s) |
| 1 × 10–11 | 6.59 × 103 | 8.76 | 1.14 × 103 | 3.07 × 10–4 |
| 1 × 10–10 | 535 | –8.73 | 361 | 7.85 × 10–4 |
| 1 × 10–9 | 125 | 2.72 | 120 | 5.56 × 10–3 |
| 1 × 10–8 | 101 | 11.1 | 101 | 0.0533 |
| ka (1/Ms) | kd (1/s) | KA (1/M) | KD (M) | Chi2 |
| 5.31 × 106 | 2.54 × 10–4 | 2.09 × 1010 | 4.79 × 10–11 | 9.89 |
| NO-Cbl (M) | Rmax (RU) | RI (RU) | Req (RU) | kobs (1.s) |
| 5 × 10–12 | 2.39 × 104 | 19.1 | 1.32 × 103 | 2.41 × 10–4 |
| 1 × 10–11 | 755 | –1.87 | 78.8 | 2.55 × 10–4 |
| 1 × 10–10 | 1.4 × 103 | –0.0961 | 756 | 4.94 × 10–4 |
| 1 × 10–9 | 84.2 | –5.73 | 77.5 | 2.88 × 10–3 |
| 1 × 10–8 | 96.3 | 5.99 | 95.4 | 0.0268 |
| ka (1/Ms) | kd (1/s) | KA (1/M) | KD (M) | Chi2 |
| 2.66 × 106 | 2.28 × 10–4 | 1.17 × 1010 | 8.58 × 10–11 | 8.8 |
Definitions: Rmax, maximal response (saturation); RI, refractive index; Req, response at steady state (equilibrium); kobs, observed rate constant; KA, affinity constant; ka: Association rate constant; KD, Dissociation constant; kd: Dissociation rate constant; RU, resonance units.
The SPR sensorgrams (Figs. 2,3) demonstrate the association and dissociation phases critical for kinetic calculations of the cobalamin-rhIF binding interactions. The initial upward curve represents the association phase, during which the analyte binds to the immobilized ligand on the sensor surface. This binding is followed by the dissociation phase, characterized by a decline in response units (RU) as the analyte detaches from the ligand once analyte solution flow ceases. Refer to Supplementary Material Section for further details as to key kinetic parameters, Langmuir fit description, and schematic illustration of a sensorgram.
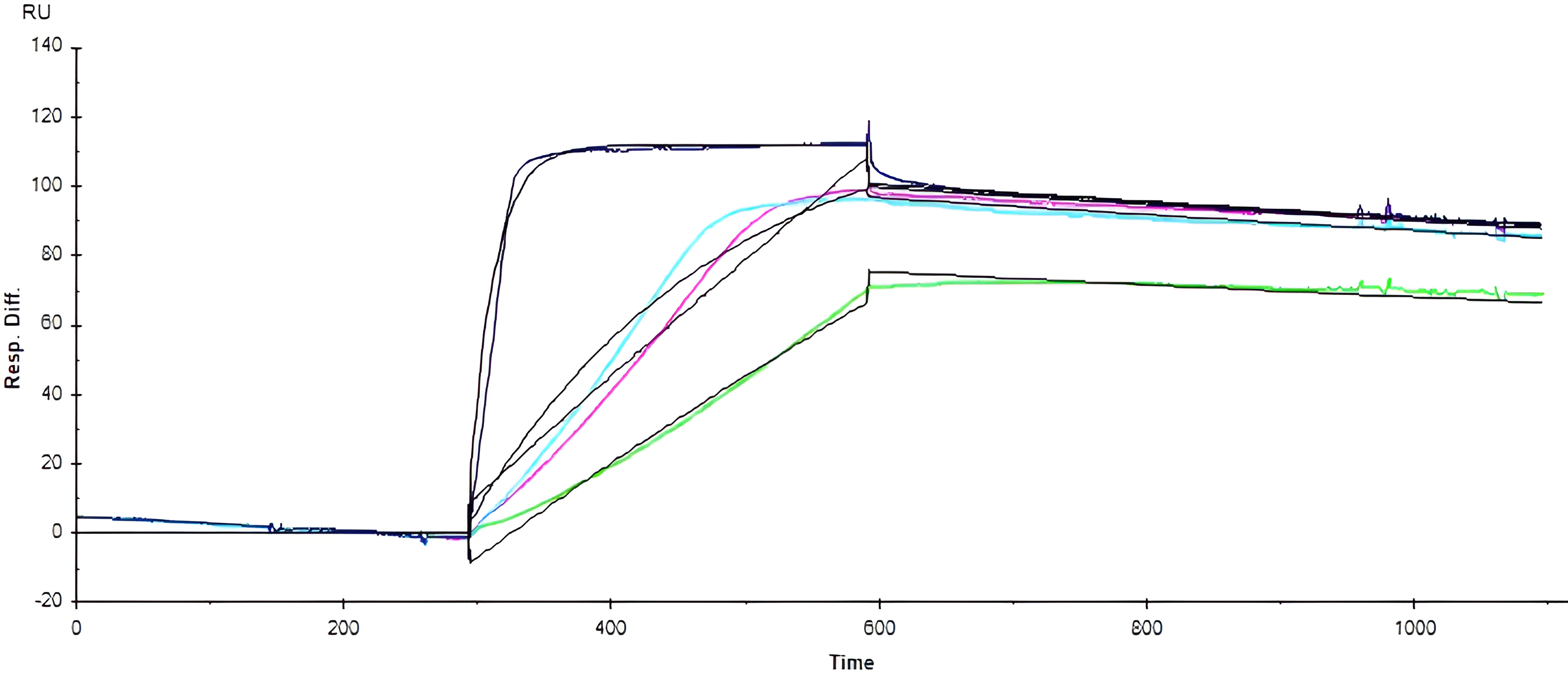 Fig. 2.
Fig. 2.
Sensorgrams of hydroxocobalamin binding to intrinsic factor.
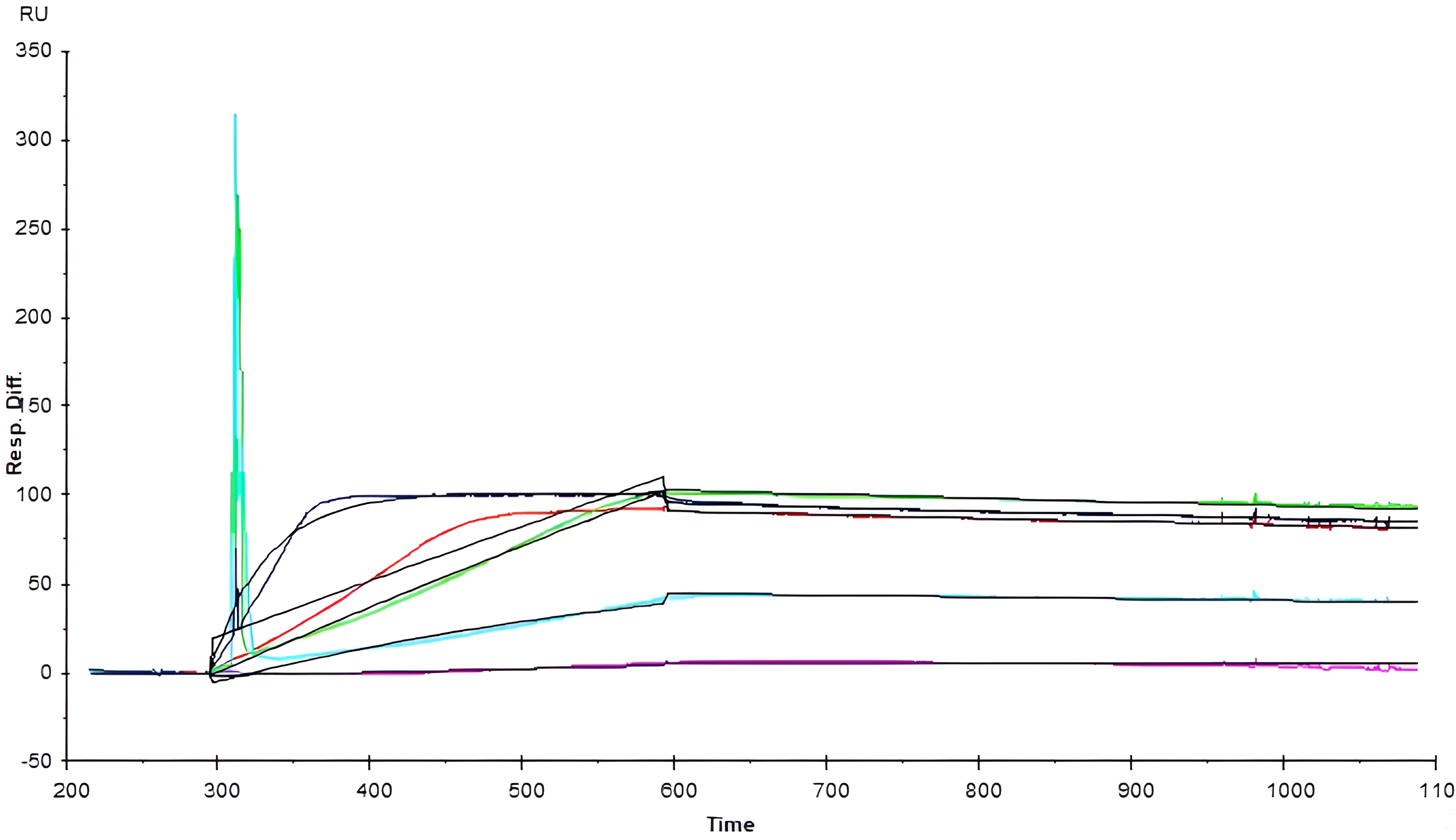 Fig. 3.
Fig. 3.
Sensorgrams of nitrosylcobalamin binding to intrinsic factor.
The sensorgrams for OH-Cbl (Fig. 2) demonstrate the interaction between OH-Cbl
and rhIF at increasing analyte concentrations. The concentrations tested ranged
from 1
The NO-Cbl sensorgrams (Fig. 3) display the interaction between NO-Cbl and rhIF
across five concentrations, ranging from 5
The Spearman rank correlation was performed to evaluate the monotonic
relationship between the parameters Rmax, RI,
Req, and kobs (1.s) across the four concentrations (1
Overall, OH-Cbl demonstrated a slightly higher binding affinity to rhIF than did
NO-Cbl, as evidenced by the lower KD value (4.79
The kinetic data for OH-Cbl and NO-Cbl revealed both similarities and differences in their interactions with rhIF. Notably, both cobalamins demonstrated concentration-dependent binding, with increases in kobs as the concentration increased, indicating that their interactions with rhIF become less stable at higher concentrations. Both OH-Cbl and NO-Cbl exhibited a decrease in Req with increasing concentration, suggesting that as more binding sites on rhIF were occupied, the overall stability of the binding interaction decreased for both compounds. The similar response observed in kobs and Req, highlighted that rhIF interacted with both NO-Cbl and OH-Cbl in a similar manner.
SPR has emerged as a research tool for studying molecular interactions in real time, obviating the need for fluorescent dyes or radioactive markers which could alter binding interactions [28]. SPR is used in drug discovery to identify and validate new drug candidates [29, 30, 31, 32] and to help predict absorption, distribution, metabolism, and excretion (ADME) profiles [33, 34]. Specifically, SPR has been instrumental in characterizing the binding of small molecules to target proteins, in optimizing monoclonal antibodies, and in screening fragment libraries to identify lead compounds [19, 22, 31, 35, 36]. In the context of cobalamin biochemistry, SPR offers an ideal platform for assessing the binding properties of drug candidates with cobalamin binding proteins as a tool to assess biological relevance [37, 38, 39, 40].
Our SPR analysis confirmed that both OH-Cbl and NO-Cbl exhibited high binding affinities to rhIF, with KD values in the picomolar range. These results were consistent with those from a study by Deme et al. [41], which utilized SPR to quantify the interactions between cobalamins and lysosomal transport proteins. In our study, OH-Cbl demonstrated a slightly stronger affinity to rhIF than did NO-Cbl. However, NO-Cbl demonstrated a noticeable degree of binding affinity suggesting its therapeutic potential through the cobalamin uptake pathway. This strong binding affinity becomes crucial for NO-Cbl’s intended use to deliver NO to target cells, particularly tumor cells that overexpress cobalamin (CD320) receptors.
OH-Cbl presented a nearly linear response curve, suggesting a stable and robust interaction with rhIF across all concentrations tested. In contrast, NO-Cbl exhibited a concentration-dependent binding response, with lower Rmax and higher KD values, indicating a slightly weaker interaction with rhIF, especially at higher concentrations. This difference in binding stability was further supported by the faster dissociation rate (higher kobs) demonstrated by NO-Cbl. This concentration-dependent response could be due to competitive binding or to saturation of binding sites, a common phenomenon observed in SPR research when ligand concentrations are elevated [42]. Structural differences between OH-Cbl and NO-Cbl, such as modifications to the corrin ring or to functional groups, most likely influenced binding kinetics. Despite these differences, both cobalamins were effectively recognized by rhIF.
Non-specific binding and fluctuating RI values observed during the study raise concerns about the accuracy of kinetic data. These fluctuations are not uncommon in SPR experiments, especially at higher ligand concentrations, where saturation effects or interactions with the sensor surface can contribute to variability. Non-specific interactions between cobalamins and the sensor surface, or between other molecules in the system, may affect binding parameters such as Req and kobs. Saturation of the sensor surface at higher concentrations could further lower Rmax values, particularly for NO-Cbl, complicating data interpretation.
The use of IF derived from plants introduces potential variability in binding behavior due to differences in glycosylation patterns compared to native human IF [24]. These glycosylation differences could influence protein structure and interaction with cobalamins, particularly at higher concentrations where saturation or recognition issues may arise.
To address these limitations, future studies should refine experimental designs to incorporate negative controls, such as the cobalamin precursor uroporphyrinogen, which has limited binding to IF. In addition, cyanocobalamin, adenosylcobalamin, and methylcobalamin should be included to allow for a broader comparison between cobalamin forms. An analysis of proteins that naturally interact with cobalamin such as cubilin, megalin, transcobalamin II and lysosomal cobalamin transporters, such as lysosomal cobalamin transport escort protein (LMBD1) and ATP binding cassette subfamily D member 4 (ABCD4), would add to the biological relevance of the study. Also, to ensure mass transport does not influence binding kinetics, various surface densities on the CM5 chip should be evaluated. Optimization of buffer conditions, immobilization of binding proteins, flow rates, and surface regeneration could improve the reliability of kinetic data. Additionally, further investigations are necessary to understand whether glycosylation differences in plant-derived IF influences cobalamin binding interactions compared to other sources of IF.
The SPR data confirm that NO-Cbl, while slightly less stable than OH-Cbl, still exhibits a strong interaction with rhIF, supporting its potential as a targeted therapeutic agent. The data provides a foundation for the future development of cobalamin-based therapeutics for treatment or amelioration of diseases that are cobalamin-dependent. The results of this study reinforce the importance of studying structural analogs of cobalamin in therapeutic applications.
ChatGPT 4o was used as an editing tool to create a more concise structure and to analyze the data. The authors programmed, reviewed and edited the content and verified the data using other analytical software such as Excel Data Analysis Tool Pak.
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
JAB contributed to the design of the research study, conducted the experiments, and analyzed the data. AMS and JAB wrote and edited the manuscript. AMS also performed the statistical analysis. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The authors extend our thanks to Daniel J. Lindner, MD, Ph.D. and Satya P. Yadav, Ph.D. (Cleveland Clinic) for their help in performing the SPR analysis. A special thanks to Cobento Biotech for their gift of the rhIF. A special thanks to Eric D. Roush, Ph.D. (Biacore Application Scientist, Cytiva Life Sciences) and Smarajit Bandyopadhyay, Ph.D. (Molecular Biotechnology Core, Cleveland Clinic) for their review of the sensorgrams. We would like to express our sincere gratitude to the reviewers for their valuable comments and constructive suggestions. Their insightful feedback has significantly improved the quality and clarity of our study, and we deeply appreciate their time and effort in reviewing our work.
This research received no external funding.
Joseph A. Bauer is affiliated with Nitric Oxide Services, LLC. However, the judgments in data interpretation and manuscript writing were not influenced by this affiliation. Both authors declare no conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/FBE26810.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.