1 Symbiosis School of Biological Sciences, Symbiosis International (Deemed University), 412115 Pune, Maharashtra, India
2 RIKEN Center for Biosystems Dynamics Research, 230-0045 Yokohama, Kanagawa, Japan
3 Department of Biosciences and Technology, MIT World Peace University, 411038 Pune, Maharashtra, India
Abstract
Cancer is a common, deadly disease with an unknown etiology. Meanwhile, current therapeutic options possess significant risks. However, probiotic bacteria and their metabolites have been reported to have antiproliferative and apoptotic effects on cancer cells. Therefore, because of their selective specificity and lack of treatment-associated comorbidities, these bacteria and their metabolites could be potential alternatives to conventional chemical and radiation therapies. Given their superior immunomodulatory and anti-cancer effects and lack of side effects, commensal bacteria derived from healthy humans are currently used as next-generation probiotics. This review summarizes current findings on these probiotic properties and anti-cancer activities of healthy human commensal bacteria. Additionally, the review focuses on small metabolites, proteins, and enzymes secreted by human commensal bacteria for their therapeutic applications against cancer. Further, utilizing a protein engineering strategy to reduce the toxicity of L-asparaginase, an enzyme-based anti-leukemia drug used for the last forty years, is also discussed. A possible workflow outline for isolating, identifying, screening, and characterizing human commensal bacterial strains for their therapeutic applications in cancer treatment is also proposed. This review emphasizes the need to explore various human commensal bacteria, not just mainstream lactic acid bacteria, for novel cancer therapeutics that provide multiple health benefits.
Graphical Abstract
Keywords
- anticancer
- bacterial metabolites
- human commensal bacteria
- L-asparaginase
- probiotics
- postbiotics
Cancer is a rapidly growing disease and the second leading cause of death worldwide after cardiac diseases [1, 2]. Every fourth person is at potential risk of developing cancer during life [3]. Chemotherapy and radiation therapy are the mainstream existing cancer therapeutics, but they possess limited efficacy, ambiguity and adverse side effects [4]. Diagnosis of the cancer at an advanced stage further contribute to the failure of the existing therapeutics [5]. Innovative methods for treating cancer include targeted therapy and cancer vaccinations [6]. Natural resources, such as bacteria, plants, fungi, and marine microorganisms, are observed as abundant therapeutics supply against human diseases, including cancer. In this regard, bacterial metabolites having anti-cancer activity have caught research attention due to their natural origin, ease of production and target-specific action. Moreover, approximately 13,000 naturally occurring chemical compounds with various pharmacological properties have been identified till date from distinct bacterial strains [5, 7].
To overcome issues associated with chemo and radiation therapy in cancer treatment, exploration of microbes and their metabolites is crucial because of their advantages to overall health benefits, immunomodulation, cancer prevention and treatment [8, 9]. Furthermore, whether used alone or in combination with the traditional anti-cancer therapies, the microbiome treatment ensures improved efficacy and reduced toxicity [10, 11]. Human commensal bacteria have co-evolved and naturally adapted to the human host and are involved in maintaining host homeostasis and robustness of the immune system [12, 13]. As a result, they can be more beneficial than bacteria from other sources such as food, marine, soil, and plants and could be the most promising candidate for addressing metabolic, immunological and cancer-related health problems [12, 14]. Additionally, these commensal bacteria secrete bioactive compounds such as short chain fatty acids, bacteriocins, indoles, indole derivates, and exopolysaccharides that can have anti-cancer activity besides their anti-inflammatory, anti-pathogenic, and immunomodulatory properties [15, 16]. It is intriguing that although two human individuals share 99.9% genetic similarity, their gut microbiome has 80 to 90% genetic diversity [17]. The bacterial secretions such as, toxins, enzymes [e.g., lipases, proteases and L-asparaginases (ASNase)], efficiently target cancer cells [18]. Because of the specificity towards cancer cells, bacterial secretions have the potential to serve as targeted therapeutic agent. However, the Food and Drug Administration (FDA)-approved L-asparaginase for the treatment of blood cancer is associated with therapeutic toxicity. Therefore, in the present review, we have proposed the bidirectional approaches—(i) protein engineering of existing L-asparaginase and (ii) exploring alternative L-asparaginase sources-to address the toxicity issues. Further, the current findings of human commensal bacteria and their metabolites in cancer prevention and therapy have also been summarized. Furthermore, the detailed workflow for exploring human commensal bacteria and their metabolites as next-gen probiotics and novel biotherapeutics for ant-cancer therapy is also proposed. The following sections discuss the potential of human commensal bacteria and their metabolites for the treatment and management of different cancer types.
Probiotics are live and safe microorganisms that are adequately consumed to
promote health by rebalancing the intestinal habitat [19, 20]. Utilizing
undigested food components in the host, these organisms secrete various
metabolites that balance the intestinal pH, exhibit antibacterial properties,
stimulate and activate immune cells, metabolize cancer-causing substances and
maintain the gut microbial equilibrium to rule out dysbiosis [20, 21, 22]. Currently,
a number of bacteria and their metabolites have been assessed for their
anticancer properties and are being further explored as potential alternatives to
persisting anticancer therapies [23, 24]. Bacterial metabolites are small
molecular weight, biologically active natural products that significantly impact
health and disease, affecting local and systemic environments [25, 26]. Bacteria
mainly produce short-chain fatty acids (SCFAs), indole derivates and polyamines
by utilizing the host diet, and these metabolites contribute to reducing tissue
inflammation and cancer cell proliferation [22, 27]. Bile acids and their
derivatives are host-derived metabolites modified by bacteria that aid in
metabolism and innate immune cell function [16, 28]. Bacteria directly synthesize
adenosine triphosphate (ATP), Vitamin K, various Vitamin B, and capsular
polysaccharides that promote overall antitumor immunity [29, 30]. Bacterial
secretions such as enzymes, toxins, lipases and proteases efficiently target
cancer cells [24, 31]. Gram-negative bacteria (Salmonella,
Escherichia, Pseudomonas, and Proteus) and
gram-positive bacteria (Clostridium, Bifidobacterium, and
Lactobacillus) have been reported to have cytotoxic, antiproliferative
and apoptotic effects on cancer cells [24, 32]. Some bacterial components can be
used as adjuvants for vaccine production [33]. Additionally, bacterial
metabolites have direct and indirect immunomodulatory effects on both immune and
non-immune cells that confer natural and acquired immunity by regulating the
proinflammatory cytokines; interleukin (IL) -1 beta (
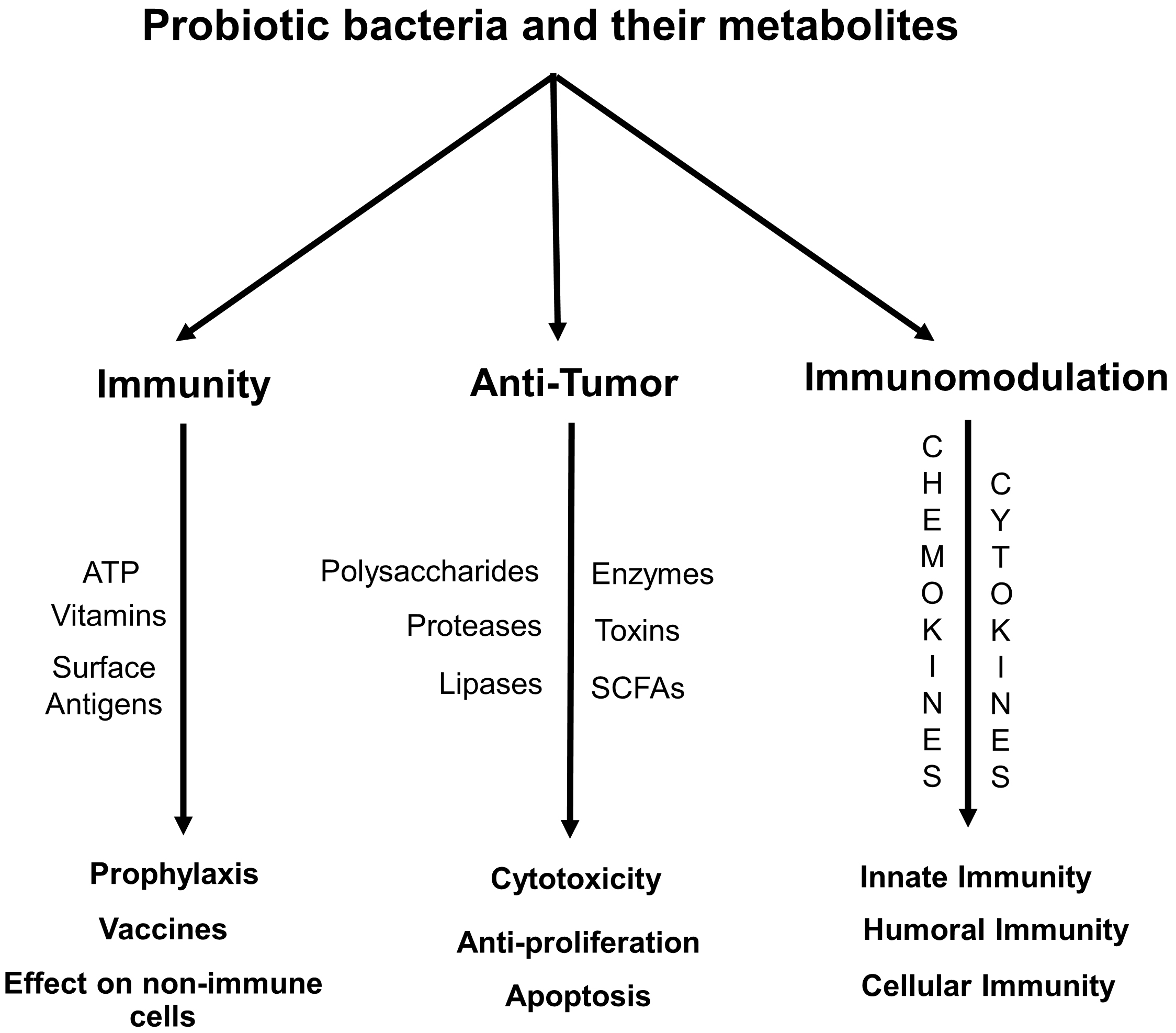
 Fig. 1.
Fig. 1.
Flow chart summarizing the role of probiotic bacteria and their metabolites in providing immunity and protection against cancer. SCFAs, short-chain fatty acids.
It is known that during tumor development, the microbiota composition changes [37] and, in some instances, the alteration may even start before the formation of tumor [38]. Due to invasiveness and biofilm-forming capabilities, some pathogenic bacteria such as Escherichia coli and Bacteroides fragilis, can abundantly manifest during the early time point of transformation. Metagenomic sequencing of fecal bacteria from different stages of colorectal cancer patients have revealed increased abundance of Bacteroides massiliensis, Bacteroides ovatus, Bacteroides vulgatus, and E. coli [37]. Due to the changes in metabolic, adhesive, and nutrient availability caused by oncogenic transformation, locally, the microbiota also gets redistributed. Interestingly, in some cases resistance to chemotherapy has been linked with enrichment of Gammaproteobacteria and Fusobacterium nucleatum [37]. It has also been demonstrated that depletion of commensal bacteria can also reduce the effectiveness of chemotherapy or radiotherapy. On the other hand, presence of Akkermansia muciniphila, B. fragilis, Enterococcus, and some Bifidobacterium strains have been reported to enhance the effectiveness of immunotherapy in cancer patients.
Known for their beneficial role as probiotics, mainstream lactic acid-fermenting bacteria have been consumed in the diet for decades. However, food-derived lactic acid bacteria have been shown to be delicate and unable to tolerate extended exposure to acidic pH following fermentation, oxygen during refrigeration storage, and human gastric pH [39, 40]. Thus, beyond the traditional sources of probiotics such as food and dairy, researchers are searching for human commensal candidates and their metabolites for a wider spectrum of health benefits, either operating alone or in combination with commercial anticancer drugs [41, 42, 43]. These findings would also help us develop new therapeutic approaches and modulate the microbiome for prevention and/or cure of cancer.
The application of next-generation sequencing for metagenomics and identification of microbes, has widened the spectrum of traditional probiotics, which include a sizable number of bacteria with potential therapeutic properties [44]. Moreover, natural bacterial strains residing in healthy human individuals can serve as novel sources of probiotics [8, 19, 45]. Interestingly, gut-derived bacteria have greater adhesion ability and tolerance to high bile salt concentrations and acidic pH, making them plausible candidates for next-generation probiotics [45, 46, 47]. Furthermore, probiotic investigations should target the production procedure, stable storage and delivery mode [48, 49]. Importantly, different strains of the same bacterial species have varied impacts on metabolic regulation, inflammation and cell proliferation [50, 51]. Further, the culture supernatants of Enterococcus faecalis strains isolated from the stool of healthy volunteers showed an antiproliferative effect on human colorectal carcinoma cell lines, but E. faecalis strains isolated from the stool of colorectal cancer patients did not show any effect [51]. Additionally, the strains isolated from healthy volunteers did not affect the human fetal colon epithelial cell line CRL-1790 [51].
Bazireh et al. (2020) [45] reported the potential in vitro
probiotic properties of five lactic acid bacterial strains isolated from human
feces and saliva. The cell-free extract of these strains were found to be
effective against colon carcinoma cell line (Caco-2). A microbial biotherapy
candidate, Lactobacillus plantarum 5BL, a vaginal commensal bacterial
strain was found to induce apoptosis and thereby showed significant
anticancer activity against the cervical (HeLa), colon (HT-29), gastric (AGS),
and breast (MCF-7) cancer cell lines (p
The examples described in this section imposed that human-derived bacteria, other than mainstream lactic acid-fermenting bacteria, can provide alternative novel probiotic candidates and novel metabolites for anticancer therapy. However, individual human microbiota strains differ in their probiotics and anticancer activities, and therefore more exploratory research is needed.
Lactic acid bacteria components and their metabolites are widely studied for their in-vitro anticancer activity [53]. In a gene expression study by quantitative real-time polymerase chain reaction (qRT-PCR), the bacterial supernatants of the probiotic L. acidophilus ATCC 4356 strain showed dose and time-dependent apoptotic effects on colorectal cancer cell lines through the upregulation and downregulation of the SURVIVIN and SMAC genes, respectively [54]. Similarly, the proteinous metabolites isolated from vaginal human commensal Enterococcus strains had significant anticancer effects on cervical, lung, colon, and breast cancer cell lines [55, 56]. Also, culture-free supernatants obtained from vaginal normal-flora, viz., Enterococcus hirae 20c, Enterococcus faecium 12a and L12b showed pronounced cytotoxic effect on cervical and lung cancer cell lines. Furthermore, these strains showed in-vitro probiotic properties such as bile and gastric acid tolerance, potent antimicrobial activity, biofilm formation and adhesion to epithelial cells, etc. [55]. Another vaginal commensal, E. faecalis 16H, exhibited potent in vitro probiotic properties, and the secreted proteinaceous metabolite showed a selective apoptotic effect on multiple cancer cells (gastric, cervical, breast and colon) and no effect on normal cell lines. Furthermore, the functional characterization of the anticancer metabolite from vaginal E. faecalis would be useful for the cancer therapy [56].
Individual human commensal bacteria and their metabolites have target-specific effects; however, their effectiveness as probiotics and anticancer agents may vary [57]. Several human gut-derived Lactobacillus strains exhibit in vitro probiotic properties, and their extracellularly secreted metabolites have effective cytotoxic effects on colorectal cancer cell lines, such as Caco-2 and HRT-18, without affecting normal Vero cells, confirming their therapeutic potential in colorectal cancer [58]. Mixed Bifidobacterium strains, representative of skin and gut human commensals, showed potent anticancer effects on colon adenocarcinoma cell lines and were ineffective against normal rat-derived epithelial cells. Further, in mouse models, it decreased the expression of colon cancer markers, epidermal growth factor receptors and cyclooxygenase-2 and led to disease stabilization, colon integrity and halting of cancer growth and metastasis. The higher anti-proliferative activity observed was considered to be due to the quorum-sensing ability of mixed Bifidobacterium strains [59].
Exploring human commensal bacteria for their additional roles in cancer
prevention and therapy, cancer immunity and microbiota maintenance is a need of
the hour. Different metabolites with anticancer potential that are produced by
human commensal bacteria are summarized in Fig. 2.
6-N-Hydroxyaminopurine (6-HAP) from the human skin commensal
Staphylococcus epidermidis not only selectively reduced melanoma tumor
growth but also protected against induced tumors. 6-HAP restricted the growth of
murine melanoma cell lines and murine T-cell lymphoma cells by blocking adenosine
and thymidine (A = T) base pairing leading to inhibition of DNA synthesis.
Compared with vehicle, intravenous inoculation of 6-HAP in melanoma-infused mice
reduced tumor growth by more than 60%. Interestingly, when the tumor-induced
mice were subjected to ultraviolet (UV) radiation and then tropically injected
with 6-HAP, the incidence and frequency of tumor growth were low [60]. Oral
administration of fecal samples from healthy humans to germ-free (GF) mice
resulted in a notable increase in interferon gamma (IFN
 Fig. 2.
Fig. 2.
Metabolites and molecules with anticancer potential obtained from human commensal bacteria. These metabolites include short chain fatty acids (A), organic acids (B), nucleotide derivatives (C), polysaccharides (D), small peptides (E) and lipopolysaccharides (F). 6-HAP, 6-N-hydroxyaminopurine; 6-TG, 6-thioguanine.
The consortia of 11 unique bacteria composed of seven Bacteroidales (with
IFN
Another metabolite - exopolysaccharides (EPSs), obtained from
Lactobacillus strains, showed anti-proliferative activity against colon
and cervical cancer cell lines by inducing apoptosis and autophagy [65, 66, 67]. EPSs
with higher mannose/glucose ratio showed better antiproliferative effects via
time-dependent apoptosis of HT-29 cells, highlighting the significance of mannose
in cancer drug design [65]. The EPSs from the vaginal L. gasseri
G1 and L. gasseri H15 strains inhibited cell proliferation and
triggered apoptosis by upregulating Bax and Caspase 3 genes in
HeLa cells and also showed an anti-inflammatory effect by increasing IL-10 and
decreasing TNF-
On the contrary, decreased production of short chain fatty acids – butyrate and propionate have been linked with the complication during chemo and radio therapies in cancer patients. Interestingly, a study by Okubo and colleagues [69] showed that higher abundance of Bacteroides was associated with increased fear of breast cancer recurrence. On the other hand, higher abundance of Lachnospiraceae, and Ruminococcus or increased alpha diversity was associated with reduced fear of cancer recurrence [69]. Therefore, studying the bacterial strains and the associated metabolites found amongst the patients showing reduced morbidity and cancer recurrence during treatment might lead to better therapy regimens.
In the light of above evidence, it can be suggested that human commensal
bacteria-derived proteinaceous compounds, exopolysaccharides, and short-chain
fatty acids (SCFAs) have selective cytotoxicity against cancer cells while
sparing healthy ones. These metabolites regulate important cancer markers like
SURVIVIN and SMAC, induces apoptosis, suppress the inflammatory
pathways, and thereby display their potential as anticancer agents.
Interestingly, these metabolites in combination with immune-modulating agents
like IL-2 or TGF-
The human commensal bacteria proven for their beneficial role against different types of cancer are listed in Table 1 (Ref. [45, 52, 54, 55, 56, 58, 59, 60, 62, 63, 65, 66, 67, 68]).
| S. No. | Beneficial bacterial strains | Isolation source | Effective in cancer type | Findings | Reference |
| 1. | Lactobacillus fermentum strains | Gut and mouth | Colon | Showed probiotics properties and cytotoxicity on Caco-2 cell lines. | [45] |
| 2. | Lactobacillus plantarum 5BL | Vagina | Breast, Cervical and Gastric | Induced apoptosis in breast cancer cell line (MCF-7). | [52] |
| No cytotoxic effects on normal cells. Also effective in gastric cancer. | |||||
| 3. | L. plantarum GD2, L. rhamnosus E9, L. brevis LB63 | Gut | Colon | Secreted exopolysaccharides (EPS) with high mannose to glucose ratio induced apoptosis in HT-29 cell lines. | [65] |
| 4. | L. gasseri strains (G10 and H15) | Vagina | Cervical | Bacterial strains and secreted exopolysaccharides showed anti-proliferative, apoptotic and anti-inflammatory effect on HeLa cells. | [66] |
| 5. | L. acidophilus | Gut | Colon | Cell-bound exopolysaccharide (cb-EPS) induced autophagy and arrested the proliferation of HT-29 colon cancer cells. | [67] |
| 6. | L. acidophilus 606 | GI Tract | Cervical, Colon and Leukemia | Heat killed (HK) bacteria and its soluble polysaccharide showed dose and time dependent cytotoxicity against cervical cancer cell lines. Also effective against leukemia and colon cancer. | [68] |
| 7. | Lactobacillus acidophilus (ATCC 4356) | Human microbiota | Colon | Secreted metabolite regulates expression and induced apoptosis in colon cancer cell. | [54] |
| 8. | Enterococcus sp. | Vagina | Cervical, Lung and Hepatic | Secreted metabolites showed cytotoxicity against cervical cancer cell lines. Also effective in hepatic and lung cancer. | [55] |
| 9. | Enterococcus faecalis 16H | Vagina | Breast, cervical, Colon, and Gastric | Secreted metabolites induced apoptosis in breast cancer cells. Also effective against cervical, colon, and gastric cancer cells. | [56] |
| 10. | Lactobacillus acidophilus strains | Gut | Colon | Cell-free supernatants showed cytotoxicity on Caco-2 cells. No effect on Vero cells. | [58] |
| 11. | Bifidobacterium species* | Skin and Gut | Colon | Mixed Bifidobacterium strains showed potent anti-cancer activity against colon adenocarcinoma cell lines. | [59] |
| 12. | Staphylococcus epidermidis* | Skin | Skin | Purified 6-HAP molecule restricted growth of murine melanoma cell and mice T-cell lymphoma cells by inhibiting DNA synthesis. | [60] |
| 13. | Escherichia coli strain Nissle 1917 (EcN)* | Intestine | Breast and Gastric | Enhanced immunity and Suppressed breast tumor growth and malignant transformation in combination with galunisertib (transforming growth factor beta (TGF- |
[62] |
| 14. | Escherichia coli KUB‑36 | Gut | Breast and Gastric | Produces SCFA and showed anti-inflammatory activity and cytotoxicity against breast cancer cell lines. Effective against colon and gastric cancer cells too. | [63] |
‘*’ denotes in-vivo study.
In addition to small metabolites, proteins and enzymes secreted by human commensal bacteria have also been shown to have therapeutic applications in cancer treatment. For instance, L-asparaginase obtained from E. coli K12 strain is FDA-approved drug for the treatment of leukaemia for last 45 years [70]. Though L-asparaginase selectively targets cancer cells, it also shows some side effects on normal human cells [71]. In contrast to normal cells, cancer cells lack the L-asparagine synthetase enzyme and thus cannot synthesize L-asparagine. Hence, the malignant cells rely on circulating plasma L-asparagine to grow and proliferate [31, 71, 72]. Externally supplied L-asparaginase (~3 µM) utilizes circulating plasma L-asparagine and deprive the tumor cells of this amino acid, leading to starvation and, ultimately death by p53-dependent apoptosis [73, 74]. However, the treatment is associated with adverse reactions mainly exhibiting hypersensitivity reactions and organ related toxicities [72]. This is primarily due to the antigenicity and intrinsic L-glutaminase activity of the currently used L-asparaginases [75]. The intrinsic L-glutaminase activity deprives L-glutamine in the blood, which is crucial for normal cell division, tricarboxylic acid (TCA) cycle, asparagine synthesis and cell survival [76, 77]. This can result in organ toxicity (liver, pancreas and brain), diabetes mellitus, and blood abnormalities (hyperammonaemia, leukopenia and thrombosis) due to reduced protein synthesis such as albumin, fibrinogen, insulin and coagulation factors [72, 75, 78]. To circumvent the treatment challenges associated with currently available L-asparaginase, numerous bacterial sources viz, soil, plants, water, and extreme environments have been explored so far [79, 80, 81, 82, 83] and human commensal bacteria were still unexplored for screening and analysis of glutaminase-free L-asparaginase. Recently, we reported three strains (two of E. coli 3F1, 3F2 and one Klebsiella pneumoniae 3S3) that produced glutamine-free-asparaginase and also showed probiotic properties [84].
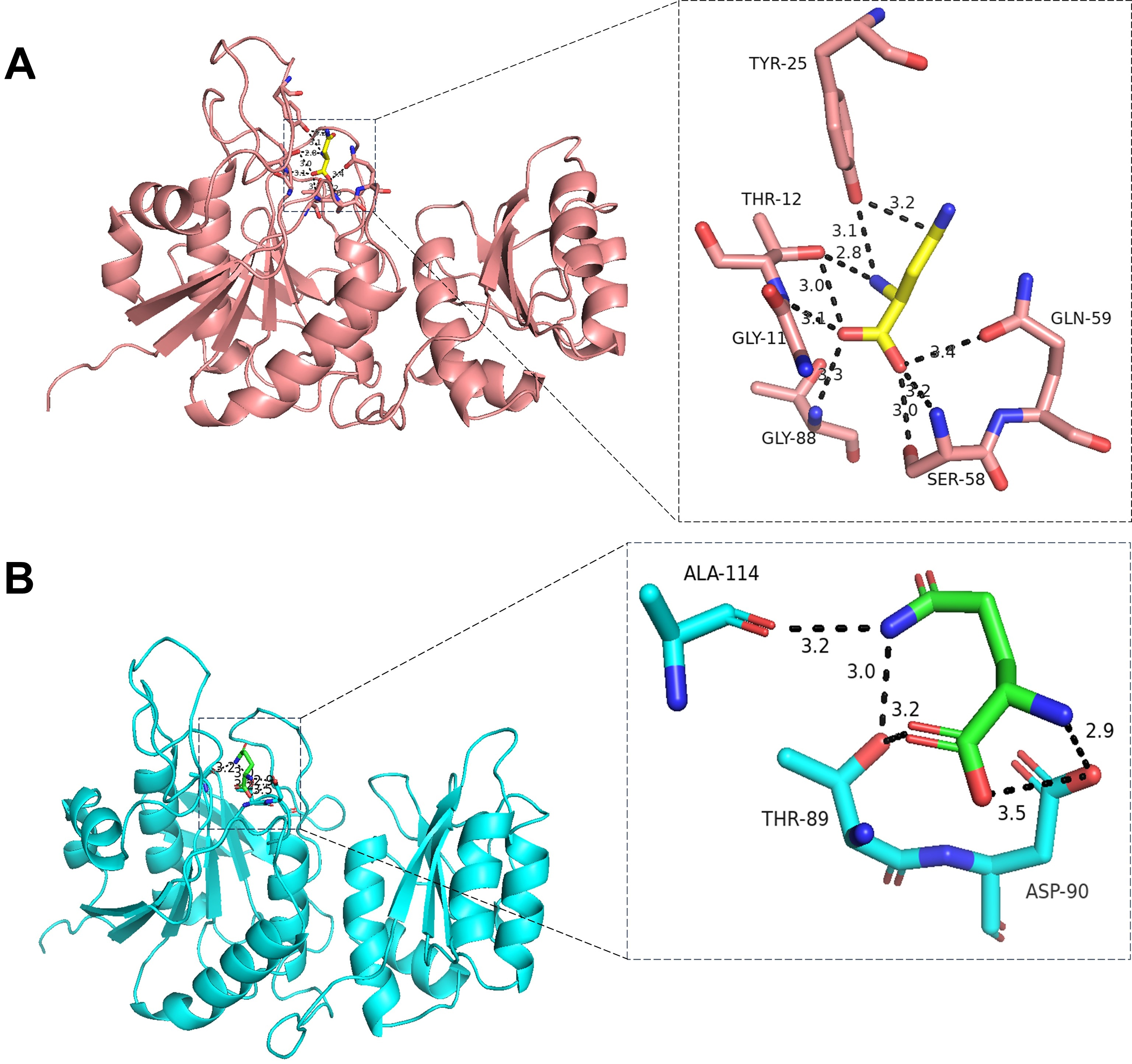
Alternatively, engineering of commercially available L-asparaginase can also be attempted to reduce its affinity towards L-glutamine. To attempt the same, we have inspected the structure of L-asparaginase (PDB Id 6PAC), there are four molecules in the asymmetric unit (Fig. 3). As calculated using computed atlas of surface topography of proteins (CASTp) program, the volume of the catalytic site is ~35.27 Å3. The L-asparagine and L-glutamine binds to the catalytic pocket with energy values ~–4.29 kcal/mol and ~–4.20 kcal/mol, respectively. The L-asparagine forms hydrogen bond with Gly11NB, Thr12OG1, Tyr25OH, Gln59OE1, Ser58NB/OG and Gly88NB (Fig. 3), whereas L-glutamine forms hydrogen bond interaction with Thr89OG1, Asp90OD2, Ala114OB residues (Fig. 3). The later three amino acids at active site, can be explored for engineering commercial L-asparaginase to reduce its affinity towards L-glutamine and thereby reduce the side effects.
 Fig. 3.
Fig. 3.
Molecular docking of L-asparaginase from E. coli K12 (PDB Id 6PAC) with L-asparagine (A) and L-glutamine (B) was performed using AutoDock Vina v 1.1.2. The protein data bank (PDB) with partial charges (Q) and atom types (T) (PDBQT) files for protein and ligand molecules were generated with the help of AutoDock Tools v.1.5.6 (https://ccsb.scripps.edu/mgltools/1-5-6/). The ligand binding site was already known so grid box was set at this point, all other parameters were assigned to their default values, the best conformations were selected based on docking energy scores and visual inspection of the docked molecules within catalytic pocket.
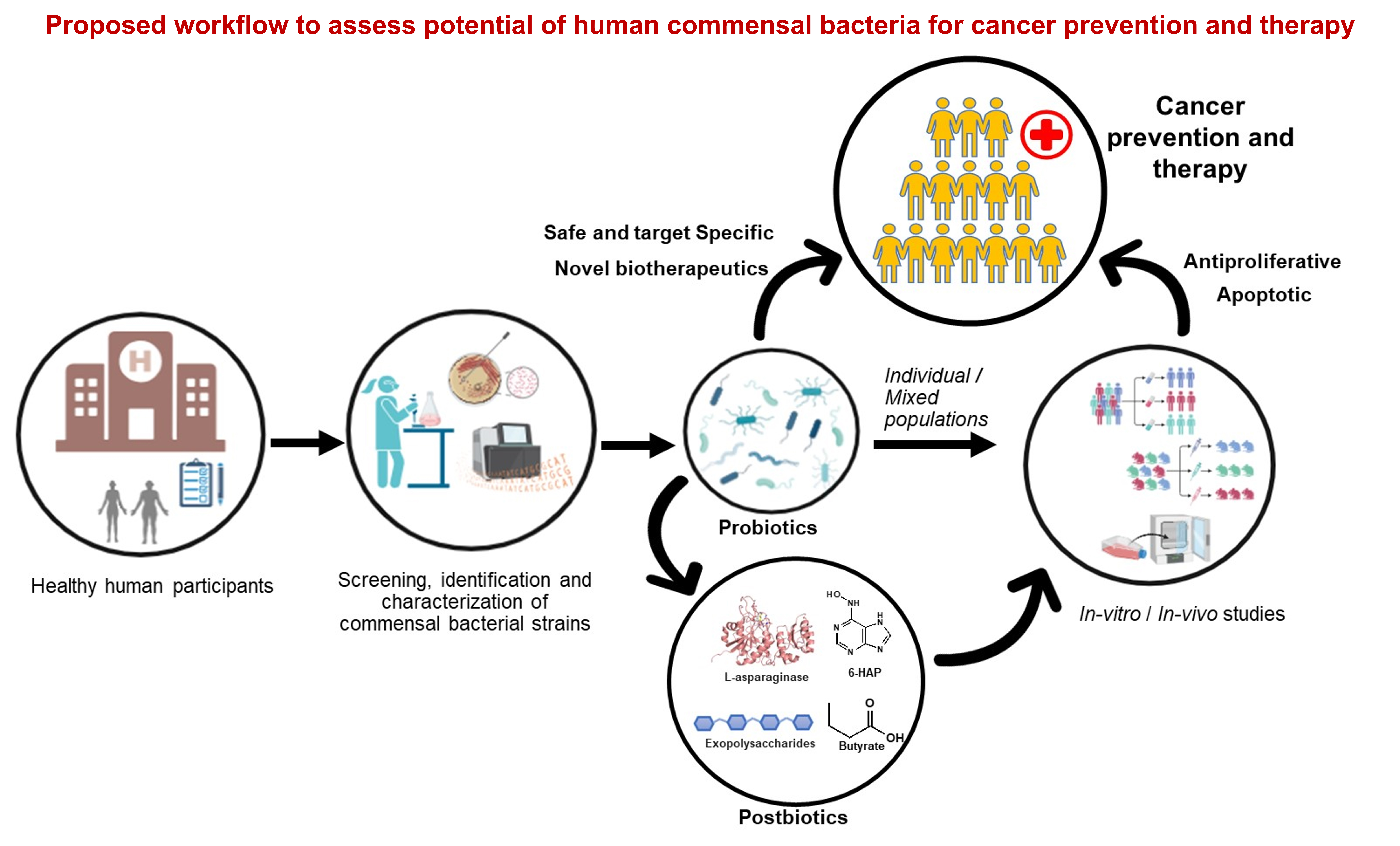
As discussed in previous sections, human-derived, non-lactic acid fermenting bacteria can provide novel probiotic candidates and novel metabolites for anticancer therapy. In this context, we proposed the possible overall working protocol to obtain such strains and exploring them for their anticancer potential (Fig. 4). For instance, based on study’s objectives, first, to get the required number of samples, identification of the volunteers within the framework of inclusion and exclusion criteria should be done [84]. Prior Institutional ethical clearance (IEC) is a must for a study involving human participants. Once the specimens are obtained one would follow a standard protocol to isolate, screen, identify and characterize the desired bacteria at the strain level. Further, the selected bacterial strains and their metabolites can be checked in vitro for effectiveness and preliminary safety assessment, followed by validation through in vivo experiments (using experimental animal models) and human trials. Finally, upon approval from regulatory authorities, lead human commensal bacterial strains and their metabolites can be recommended for use in humans as cancer therapeutics.
 Fig. 4.
Fig. 4.
The overall workflow to obtain human commensal bacterial strains from healthy individuals and exploring them as pro- and post-biotics in prevention and treatment of cancer.
A number of selected bacterial strains obtained from various sources and their metabolites have been recognized for the health benefitting properties. However, the repository of desired bacterial isolates obtained from human commensal pool is still relatively small and even more so with reference to cancer prevention and therapy perspectives. Due to pre-established association with human tissues, the commensals and their metabolites would have a better biocompatibility than the ones from other sources. Identification of bacterial strains present in healthy individuals but particularly absent in cancer patients would help in enriching the pool of therapeutic probiotics with potential application for the cancer treatment. Additionally, the human commensal sources of metabolites proven to have anti-cancer activity should be actively explored. For instance, new commensal source of L-asparaginase—a FDA approved anticancer drug would help in addressing the toxicity issues associated with the currently available L-asparaginase. In this regard, L-asparaginase producing human commensals recently isolated by us is a significant step forward, however, further studies are essential to prove its utility in treatment against cancer. Additionally, the modifications at molecular level using available protein engineering tools, as exemplified for L-asparaginase in this review, would also significantly help in eliminating the unwanted side effects associated with therapeutic biomolecules already in use. Importantly, the effective workflow as suggested in this review would help the exploration aimed to identify, characterize and employ the next-generation pro- and post-biotics for anticancer therapy.
HS - Data curation (Lead), Formal analysis (Lead), Investigation (Lead), Methodology (Lead), Software (Lead), Validation (Supporting), Visualization (Supporting), Writing - original draft (Lead), Writing - review & editing (Supporting). SD - Data curation (Supporting), Formal analysis (Supporting), Methodology (Supporting), Software (Equal), Validation (Supporting), Visualization (Supporting), Writing - review & editing (Supporting). BR - Formal analysis (Supporting), Validation (Supporting), Visualization (Supporting), Writing - review & editing (Supporting). EKP - Conceptualization (Lead), Data curation (Supporting), Formal analysis (Equal), Funding acquisition (Lead), Investigation (Supporting), Methodology (Supporting), Project administration (Lead), Resources (Lead), Supervision (Lead), Validation (Equal), Writing - review & editing (Equal). All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
HS thanks symbiosis international (Deemed University) for the award of junior research fellowship.
EKP is a recipient of the major research project (MJRP) grant (SIU/SCRI/MJRP- Approval/2024/1397) from the SIU.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.