- Academic Editors
Plant–microbial degradation of organic pollutants occurs in the rhizosphere under the influence of plant root exudates. Similarities in chemical structure to polycyclic aromatic hydrocarbons (PAHs), phenolic compounds and flavonoids released with exudates can determine the ability of rhizosphere microorganisms to degrade hazardous aromatic pollutants.
Here, we analyzed phenolic compounds in the root exudates of alfalfa (Medicago sativa L.) grown in quartz sand uncontaminated and phenanthrene-contaminated quartz sand, a model PAH pollutant, under axenic conditions. The effect of six flavonoids (naringenin, rutin, morin, quercetin, apigenin, and luteolin) on phenanthrene degradation by two PAH-degrading bacteria, Ensifer meliloti P221 and Mycolicibacterium gilvum PAM1, previously isolated from the rhizosphere of alfalfa was also investigated. Ultraviolet (UV)-vis spectroscopy and high-performance liquid chromatography (HPLC) were applied to assay flavonoid and phenanthrene content in cultivation media.
The quantitative and qualitative characteristics of the root-exuded phenolic compounds changed under the influence of phenanthrene. The impact of the flavonoids on PAH biodegradation varied from neutral or even inhibitory to stimulatory. The same flavonoid (quercetin) had opposite effects on the growth of the two bacteria and on phenanthrene degradation. The effect of the flavonoids on bacterial growth did not depend on the presence of PAHs. Using naringenin as an example, we showed that increased PAH degradations could not accompany bacterial growth promotion by any flavonoid. Except for rutin, all flavonoids were subject to bacterial degradation. Inoculation of alfalfa with the competent rhizobacterium Ensifer meliloti increased the contents phenolic compounds in the plant root exudate, promoted qualitative changes in their profile, and increased the rhizodegradation of phenanthrene from 6% and 22% to 57% and 34% at initial phenanthrene concentrations of 50 and 100 mg/L respectively.
Our data suggest a the role for plant flavonoids in the rhizome-mediated degradation of PAHs. The microbe-induced qualitative and quantitative changes in root exudation illustrate the induction of PAH-mediated catabolic activity in the rhizosphere.
Polycyclic aromatic hydrocarbons (PAHs) are common environmental pollutants of both natural and human-caused origin [1]. They are formed as a result of natural phenomena, such as forest fires and volcanic eruptions, and man-made processes such as the incomplete combustion of fossil fuels and organic materials. PAHs make up the bulk of vehicle exhaust gases and industrial emissions. Structurally, PAHs consist of two or more benzene compounds arranged linearly, at an angle, or in clusters. The physicochemical properties of PAHs, such as molecular weight, hydrophobicity, and thermodynamic stability, determine their mobility, distribution in the environment, and toxicity and resistance to degradation [1, 2, 3].
The bioaccumulation ability, toxicity, mutagenicity, and carcinogenicity of PAHs, alongside their resistance to biological degradation, make cleaning up the environment contaminated with them an urgent task [3, 4]. Modern and popular methods for cleaning soil from PAHs include biological remediation technologies, which use the vital activity of plants and microorganisms playing leading parts in the degradation of PAHs in nature to restore natural objects [5, 6]. As organic pollutants, PAHs can be carbon and energy sources for many microorganisms, and the metabolic pathways of microbes are varied and adaptable, allowing the transformation or mineralization of almost all substrates. Plants are implicated in the remediation of PAH-contaminated soils both directly (owing to their own PAH-detoxification activity, associated with bioaccumulation, sequestration, and pollutant-degrading enzyme activity) and indirectly (by increasing the activity of rhizosphere microorganisms). For this reason, plant-microbe associations are considered the most active participants in the self-cleaning of nature and the main components of bioremediation technologies.
Although various degradative microorganisms [7] and promising remediator plants [8, 9] have been described to date, the factors influencing the biodegradation of PAHs are still insufficiently known. Particularly noteworthy are the processes associated with the degradation of PAHs in the plant rhizosphere, a zone of plant–microbial interactions that is rich in various biochemical activities [6, 10]. The selective effect of some plants on the formation of a rhizosphere microbial community of PAH degraders was reported [11, 12, 13]. Presumably, the special composition of plant root exudates and, in particular, the presence in the exudates of such biologically active compounds as flavonoids are of decisive importance for rhizosphere microbiome formation [14]. Flavonoids are an important class of plant secondary metabolites having a polyphenolic structure based on 15-carbon flavone skeleton, C6-C3-C6, with two benzene rings linked by a three-carbon pyran ring [15]. Flavonoids differ in the arrangement of the hydroxyl, methoxy, and glycosidic side groups, as well as the configuration of the pyran ring that connects the benzene rings. They give rise to a variety of different compounds which perform a variety of functions in plants such as cell growth regulation, antioxidant protection against biotic and abiotic stresses, mediation of plant-plant and plant-microbial signaling. The chemical similarity of the flavonoids to some PAHs may presumably determine the selection of degrading microorganisms that are evolutionarily adapted to the degradation of flavonoids in the rhizosphere of the host plant, thereby ensuring successful pollutant degradation [16, 17, 18, 19, 20]. Understanding the relationships between the biochemistry of plant secondary metabolites, the symbiotic microbial community, and the fate of organic pollutants is essential for the successful application of phytoremediation [21].
Alfalfa (Medicago sativa L.) is used widely for the phytoremediation of hydrocarbon-contaminated soils and is a model for studying plant-microbe interactions [9, 11, 13]. The principal flavonoids of M. sativa have been identified mainly in plant tissues, with insufficient information available about phenolic compounds and flavonoids in alfalfa root exudates. On this basis, our first objective was to analyze phenolic compounds in the root exudates of alfalfa and the effect of phenanthrene (a model PAH) and a bacterial inoculant on their exudation. Our second objective was to characterize the effect of plant flavonoids on the bacterial degradation of PAHs, with phenanthrene as an example. The experiments used two PAH-degrading bacteria, Ensifer meliloti P221 and Mycolicibacterium gilvum PAM1, which had previously been isolated from the rhizosphere of alfalfa growing on an oil-contaminated soil [22, 23]. E. meliloti P221 is of interest as a natural symbiont of alfalfa, evolutionarily adapted to the signal flavonoids of the plant, whereas M. gilvum PAM1 is considered a nontarget free-living rhizobacterium that probably does not respond specifically to the presence of flavonoids in the rhizosphere.
Seeds of alfalfa (Medicago sativa L.) of the Diana variety (https://www.arisersar.ru/diana.htm) were obtained from the Federal Center of Agriculture Research of the South-East Region (Saratov, Russia). The
cultivation of sterile plants and collection of sterile root exudations was
adopted from the previously reported study [24]. For an axenic culture, alfalfa
seeds were calibrated, surface sterilized in a sodium hypochlorite solution
(active chlorine content of 5–6%) for 30 min, and then washed with sterile tap
water for at least 10 times. After that, the seeds were plated on the surface of
a doubly diluted nutrient agar medium in petri dishes for germination and
sterility control. Quartz sand (particle size of 1–2 mm) in 0.3-L Erlenmeyer
flasks (150 g per flask) was heat sterilized and sprayed with a 1.5% (w/v)
acetonic solution of phenanthrene (
Alfalfa plants inoculated with the rhizobacterium E. meliloti P221 were grown as described above, but before the seedlings were planted, a bacterial suspension was added to the sand. For the suspension, cells were grown in the Luria-Bertani (LB) medium for 2 days, after which they were pelleted by centrifugation at 9000 rpm for 10 min, washed with sterile saline twice, and centrifuged again under the same conditions. The washed cells were resuspended in sterile Knop’s solution and added to flasks with sand to a final cell density corresponding to 107 cells per gram of sand.
After the plants had been removed from the flasks, the roots were dipped gently in tap water and the shoots were separated from the roots. For analysis of the root morphometric variables, the washed and separated roots were dried with filter paper and scanned with a BROTHER DCP-7065DNR MFP scanner (Brother International Co., Ltd., HCMC, Vietnam). The images were analyzed using RhizoVision Explorer v2.0.3 (https://zenodo.org/records/5121845) [25] using algorithms described by Seethepalli et al. [26] to estimate the total length (in millimeters), volume (in cubic millimeters), and surface area (in square millimeters) of the alfalfa root samples.
For measurement of wet weight, the shoots and roots were weighed, and then shoot and root biomass was dried at 70 °C until constant dry weight was achieved. From the obtained data, the ratio of root biomass to shoot biomass (R/S) was calculated.
Before collection of root exudates, the rhizosphere solution from each planted
flask was checked for sterility by plating a 0.1-mL aliquot of the solution on
nutrient agar. Thereafter, the sand in each flask was flooded with sterile
distilled water (
Phenolic compounds were determined by the Folin-Ciocalteu spectrophotometric method [27], with some modifications. Gallic acid was used as a standard to calibrate the method. A 3.5-mL aliquot of each exudate sample was added to 0.5 mL of the Folin-Ciocalteu reagent (2 N; CDH, New Delhi, India), and the mixture was vigorously stirred. The solution was left to stand for 5 min before being added with 1 mL of a 20% sodium carbonate solution and stirred again. The resulting mixture was incubated at 30 °C for 2 h in the dark. The absorbance of the reaction mixture was determined at 750 nm in an Evolution 60 ultraviolet (UV)-vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). For obtaining data on the phenolic compound concentrations, a calibration curve was constructed by using different concentrations of gallic acid (40 µg/L–40 mg/L). The total content of phenolic compounds was expressed in µg of gallic acid equivalents per L of sample.
Phenolic compounds were obtained from root exudates as follows: 20-mL exudate
samples were acidified with 1 N HCl to pH 3 and were extracted twice with an
equal volume of ethyl acetate (Vekton, Saint Petersburg, Russia). The extracts
were combined, and the solvent was evaporated. The dry residue was redissolved in
1 mL of acetonitrile (99.9%, Carlo Erba Reagents, Milan, Italy) and analyzed by
HPLC on an Agilent Technologies 1220 Infinity II LC chromatograph (Agilent
Technology, Waldbronn, Germany). Flavonoids were determined with a ZORBAX Eclipse
Plus C18 4.6
UV detection at 254 nm was used to detect the phenolic components in the root
exudates. The compounds were identified on the basis of the retention times (RT)
of the peaks detected in the chromatogram with the retention times of standard
samples. The standards used were as follows: Flavonoids: apigenin (
Ensifer meliloti P221 (IBPPM 383) and Mycolicibacterium gilvum PAM1 (IBPPM 589) were from the Collection of Rhizosphere Microorganisms of the Institute of Biochemistry and Physiology of Plants and Microorganisms (WFCC no. 975, WDCM no.1021; http://collection.ibppm.ru). Both microorganisms have been isolated from the rhizosphere of Medicago sativa L. and have been well characterized as PAH degraders [22, 23].
Flavonoid effect on the growth and phenanthrene-degrading activity of the rhizobacteria was investigated with the following compounds (Fig. 1): rutin, morin, quercetin, naringenin, luteolin, and apigenin. Each flavonoid was added to the medium as an ethanol solution to a final concentration of 10 µmol/L. The flavonoid concentration in the medium was chosen on the basis of literature data and preliminary experiments [28]. Disappearance of flavonoids in the medium was calculated from the final flavonoid content in the abiotic controls. The degradation of flavonoids in the abiotic controls ranged from 10 to 30%.
 Fig. 1.
Fig. 1.
Flavonoids used in this study. Illustration has been created using ChemDraw®Ultra v. 8.0 software (Cambridge Soft Corporation, Cambridge, MA, USA).
For examination of growth and phenanthrene-degrading activity, the rhizobacteria were grown in 0.2-L Erlenmeyer flasks containing 50 mL of a malate-salt medium (MSM) for PAH degraders [22] for 14 days. The pH of the medium was adjusted to 6.8, and 200 mg/L of phenanthrene was supplemented. Incubation was done at 29 °C with rotary shaking (150 rpm) for up to 14 days. Growth was assessed turbidimetrically at 600 nm on an Evolution 60 UV-vis spectrophotometer (Thermo Scientific). Phenanthrene degradation was determined by the elimination of the PAH from the medium after culturing of the bacteria.
The residual content of phenanthrene in the sand or in the cultivation medium was measured after preliminary extraction with a nonpolar solvent. A sand sample (25 g) was extracted twice with 13 mL of chloroform (Vekton, Saint Petersburg, Russia) for 20 min on a shaker; then, the extracts were combined and filtered through a Schott glass filter, dried by solvent evaporation, and redissolved in acetonitrile (99.9%, Carlo Erba Reagents, Milan, Italy) for HPLC analysis.
After the cells had been grown in the liquid medium, phenanthrene was extracted with chloroform (5 mL per 50 mL of the culture medium, three times for 15 min each). The extracts were combined, dried by solvent evaporation, and redissolved in acetonitrile for HPLC analysis.
The phenanthrene content in the extracts was measured with an Agilent
Technologies 1220 Infinity II LC high-performance liquid chromatograph (Agilent) fitted with a UV detector at 245 nm and a ZORBAX Eclipse PAH 4.6
The loss of phenanthrene in the experimental variants was always calculated from the final concentration of phenanthrene in the abiotic control.
All experiments were carried out in triplicate. All data were checked for normal
distribution by the Kolmogorov-Smirnov test. Means were compared by Fisher’s
test, and the least significant difference was determined in a one-way ANOVA at
p
The effect of various concentrations of phenanthrene on the accumulation of alfalfa shoot and root biomass was investigated, and the ratio between root biomass weight and shoot biomass weight (R/S) was calculated. Table 1 presents the results.
| Phenanthrene, (mg/kg) | Wet weight | Dry weight | ||||
| Shoots (%) | Roots (%) | R/S | Shoots (%) | Roots (%) | R/S | |
| 0 | 100 a | 100 a | 0.21 | 100 a | 100 a | 0.21 |
| 50 | 103.0 |
105.1 |
0.22 | 101.2 |
103.3 |
0.21 |
| 100 | 84.0 |
78.2 |
0.23 | 80.5 |
82.9 |
0.26 |
| 200 | 78.1 |
58.0 |
0.26 | 68.2 |
85.0 |
0.31 |
The values in a column that are marked with the same letters are not
significantly different at p
At low concentrations (50 mg/kg), phenanthrene did not have a significant effect on the accumulation of both above- and belowground alfalfa biomass. At 100 and 200 mg/kg of phenanthrene, the amount of wet shoot biomass was reduced by 16 and 22% and that of wet root biomass was reduced by 22 and 42%, respectively. The decrease in the dry shoot weight was more noticeable and reached 20 and 32%, whereas the decrease in the dry root weight amounted to 17 and 15%, respectively, at 100 and 200 mg/kg of phenanthrene. The R/S index increased with increasing phenanthrene concentration in the sand. This was especially noticeable when the index was calculated on dry biomass basis, which may indicate suberization of the roots [29].
Table 2 presents changes in the root morphometric variables under the influence of phenanthrene.
| Phenanthrene (mg/kg) | Total length (mm) | Total volume (mm3) | Surface area (mm2) |
| 0 | 80.8 |
15.9 |
113.5 |
| 50 | 68.6 |
8.6 |
79.3 |
| 100 | 67.0 |
10.3 |
85.6 |
| 200 | 56.8 |
28.0 |
127.5 |
The values in a column that are marked with the same letters are not
significantly different at p
Under the influence of phenanthrene, the root length was significantly reduced (by 15, 17, and 30%, respectively, at 50, 100, and 200 mg/kg of phenanthrene). The decrease was maximal at 50 mg/kg (by 46 and 30%, respectively) and smaller at 100 mg/kg (by 35 and 25%, respectively). By contrast, at the highest phenanthrene concentration (200 mg/kg) the volume and surface area of the roots even increased (by 76 and 12%, respectively). Under the influence of phenanthrene, alfalfa roots noticeably changed their morphology and structure, thickening and coarsening as a result of physiological and biochemical processes such as suberization and lignification, which could be seen by the naked eye (Fig. 2).
 Fig. 2.
Fig. 2.
Changes in alfalfa root morphology under the influence of phenanthrene. (A) Roots of plants grown in uncontaminated sand. (B) Roots of plants grown in phenanthrene-contaminated sand (200 mg/kg).
Within 14 days, alfalfa roots released 1.1 to 1.6 mg/L of phenolic compounds
(Fig. 3). In the presence of phenanthrene, the exudation of phenolic compounds
tended to increase, and at 100 mg/kg of phenanthrene, the content of phenolic
compounds in the exudates increased significantly (p
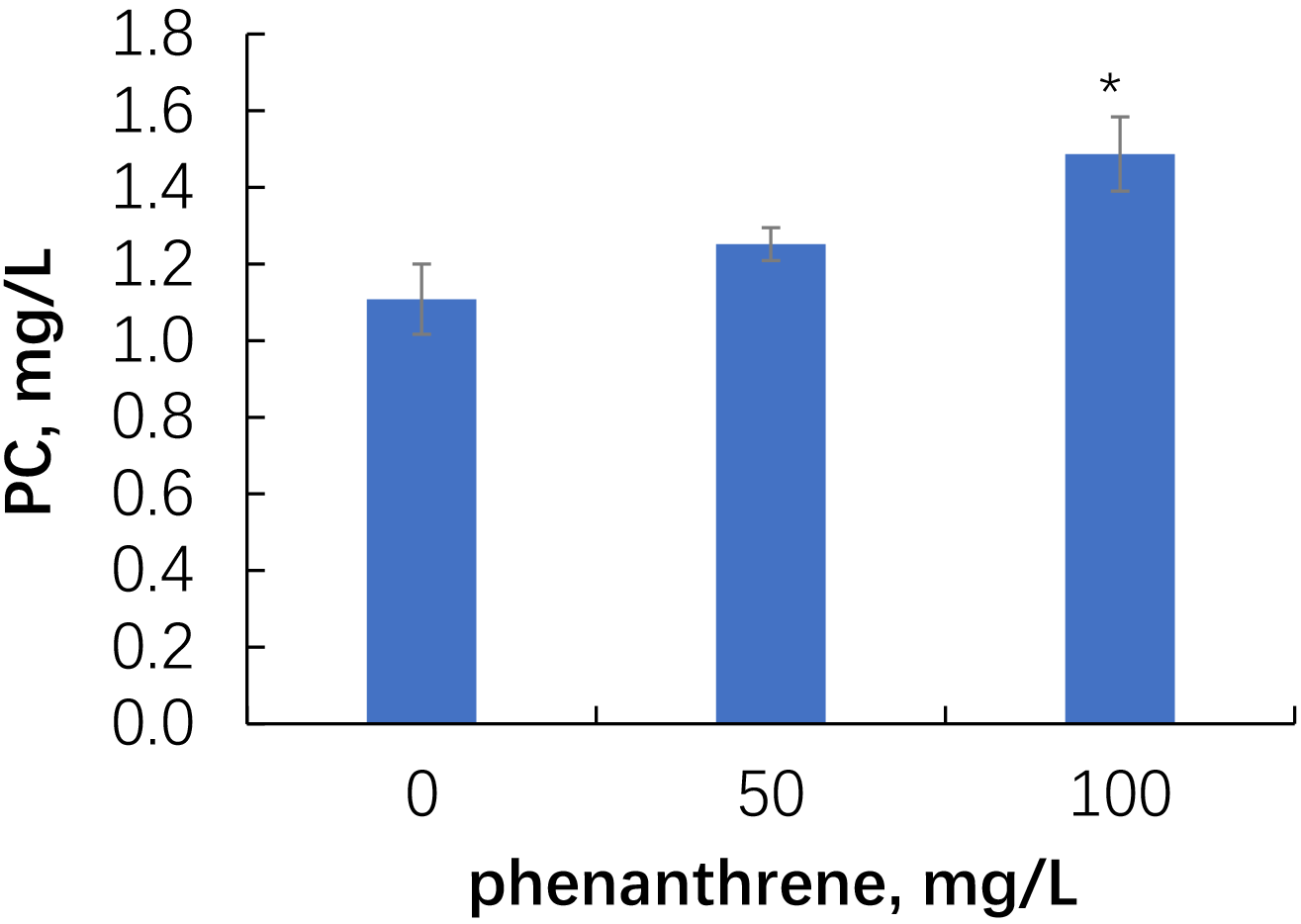 Fig. 3.
Fig. 3.
Phenolic compound content in the root exudates of alfalfa grown
in uncontaminated and phenanthrene-contaminated sand. Single asterisks indicate values
that are significantly different from the uncontaminated control at p
HPLC chromatograms of the phenolic compounds of root exudate from alfalfa grown in uncontaminated sand and in the presence of 100 mg/L of phenanthrene are given in Supplementary Fig. 1. Component (5) with RT = 8.40 min dominated in the spectrum of the phenolic fraction of alfalfa root exudates (Supplementary Fig. 1A). The same component was also present at comparable concentrations in the root exudates from contaminated soil (Supplementary Fig. 1B). Using standard solutions, we were able to identify naringenin (2) in the root exudates, whose RT coincided with that of the standard used (RT = 6.04 min; Fig. 4) but whose concentration was too low. In the presence of phenanthrene, an additional peak (7) corresponding to the phenanthrene standard (RT = 9.65) appeared in the spectrum of the analyzed root-exudate fraction. Unfortunately, efforts to reliably identify any other compounds in the root exudates were unsuccessful.
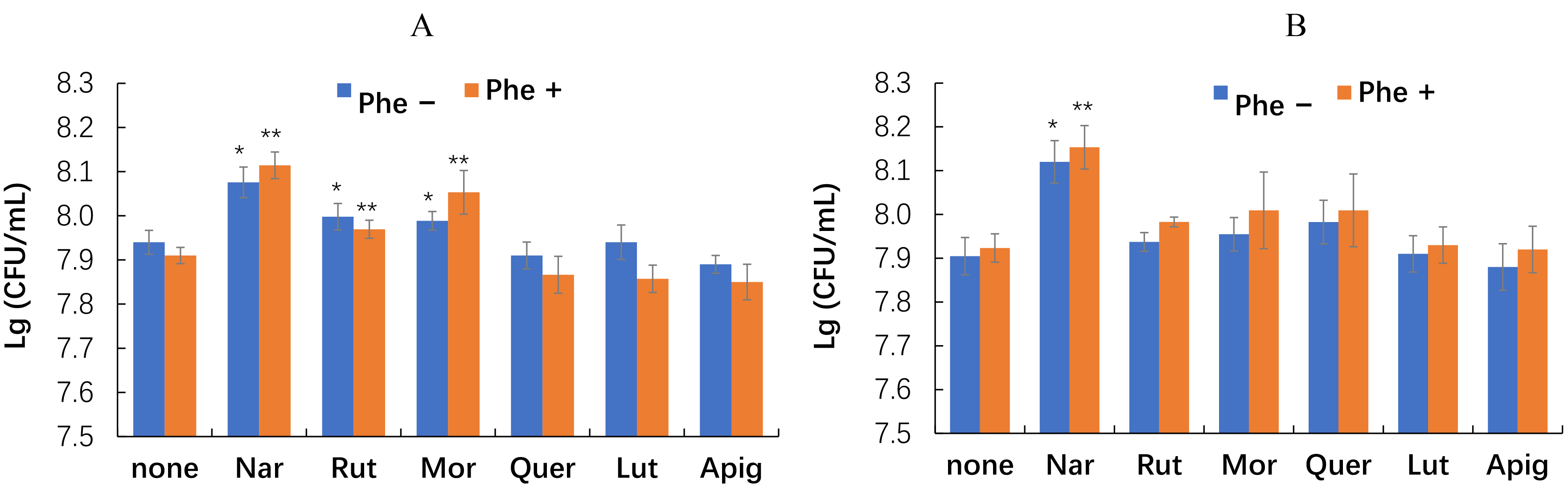 Fig. 4.
Fig. 4.
Effect of phenanthrene and flavonoids on the growth of the
rhizobacteria E. meliloti P221 (A) and M. gilvum PAM1 (B). Single asterisks indicate values that are significantly different from the flavonoid-free control without phenanthrene at p
On the basis of literature data, we chose a series of flavonoids including one flavanone (naringenin), two flavones (apigenin and luteolin), and three flavanols (morin, quercetin, and rutin). We investigated the effect of these flavonoids on the growth and phenanthrene degradation by the rhizospheric bacteria E. meliloti P221 and M. gilvum PAM1.
Our data show that naringenin promoted the growth of both microorganisms most significantly (Fig. 4). The growth of E. meliloti P221 was also enhanced by rutin and morin but to a lesser extent. Other flavonoids tested did not have a noticeable effect on the growth of both rhizobacteria.
Despite the distinct promotion of bacterial growth, naringenin did not affect the bacterial degradation of phenanthrene (Fig. 5).
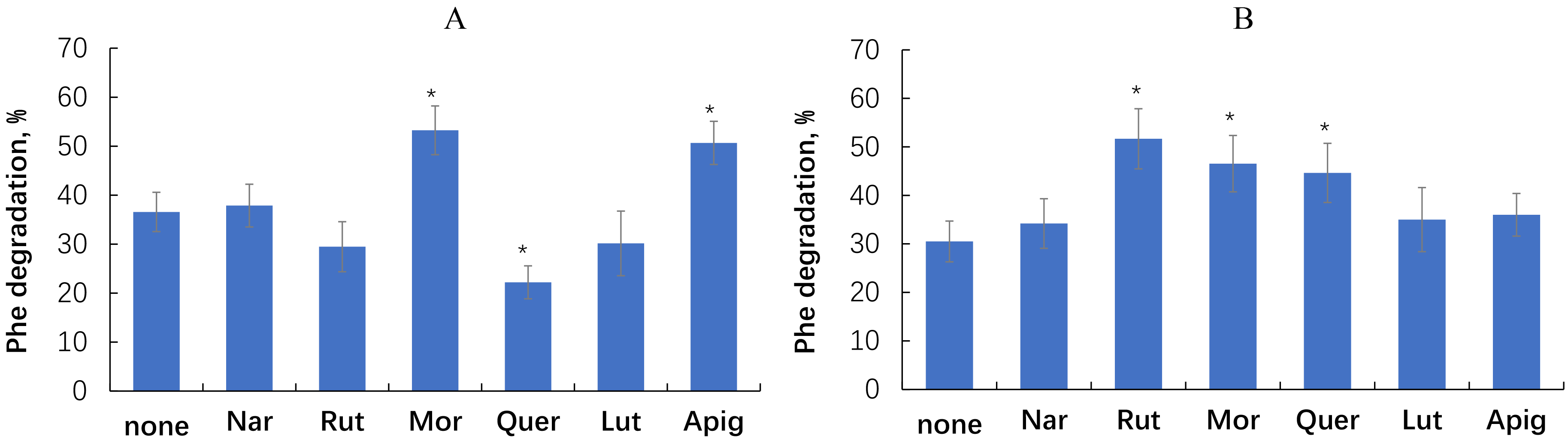 Fig. 5.
Fig. 5.
Effect of flavonoids on phenanthrene degradation by the
rhizobacteria E. meliloti P221 (A) and M. gilvum PAM1 (B).
Single asterisks indicate values that are significantly different from the
flavonoid-free control at p
Phenanthrene degradation by E. meliloti P221 was most effectively promoted by morin (+46%) and apigenin (+39%), whereas quercetin inhibited phenanthrene degradation by 39%. With M. gilvum PAM1, the most pronounced stimulatory effect (+69%) was exerted by rutin, followed by morin (+53%) and quercetin (+46%).
Evaluation of the postincubation flavonoid content showed that with the exception of rutin, all the flavonoids were utilized by 35–65% (Fig. 6).
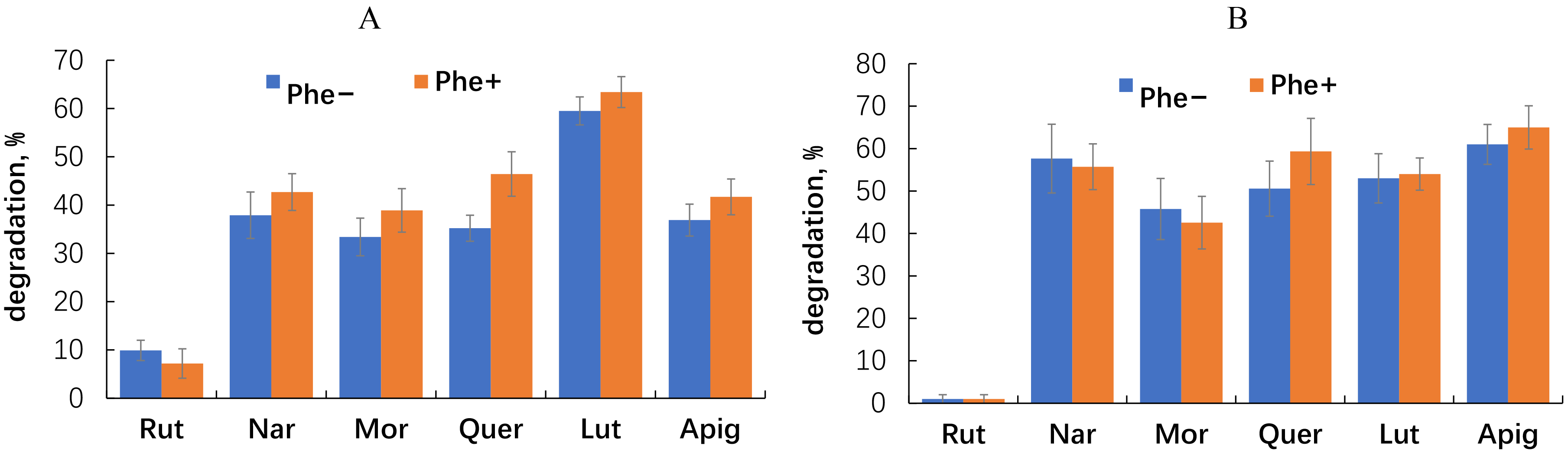 Fig. 6.
Fig. 6.
Disappearance of flavonoids from the medium after culturing of
E. meliloti P221 (A) and M. gilvum PAM 1 (B) in the medium
without and with phenanthrene. The error bands represent the 95% confidence interval. Statistical significance was determined using a threshold of p
Inoculation of alfalfa with the plant-competent phenanthrene-degrading rhizobacterium E. meliloti P221 led us to observe increased degradation of phenanthrene in contaminated soil, both at 50 and 100 mg/kg (Fig. 7).
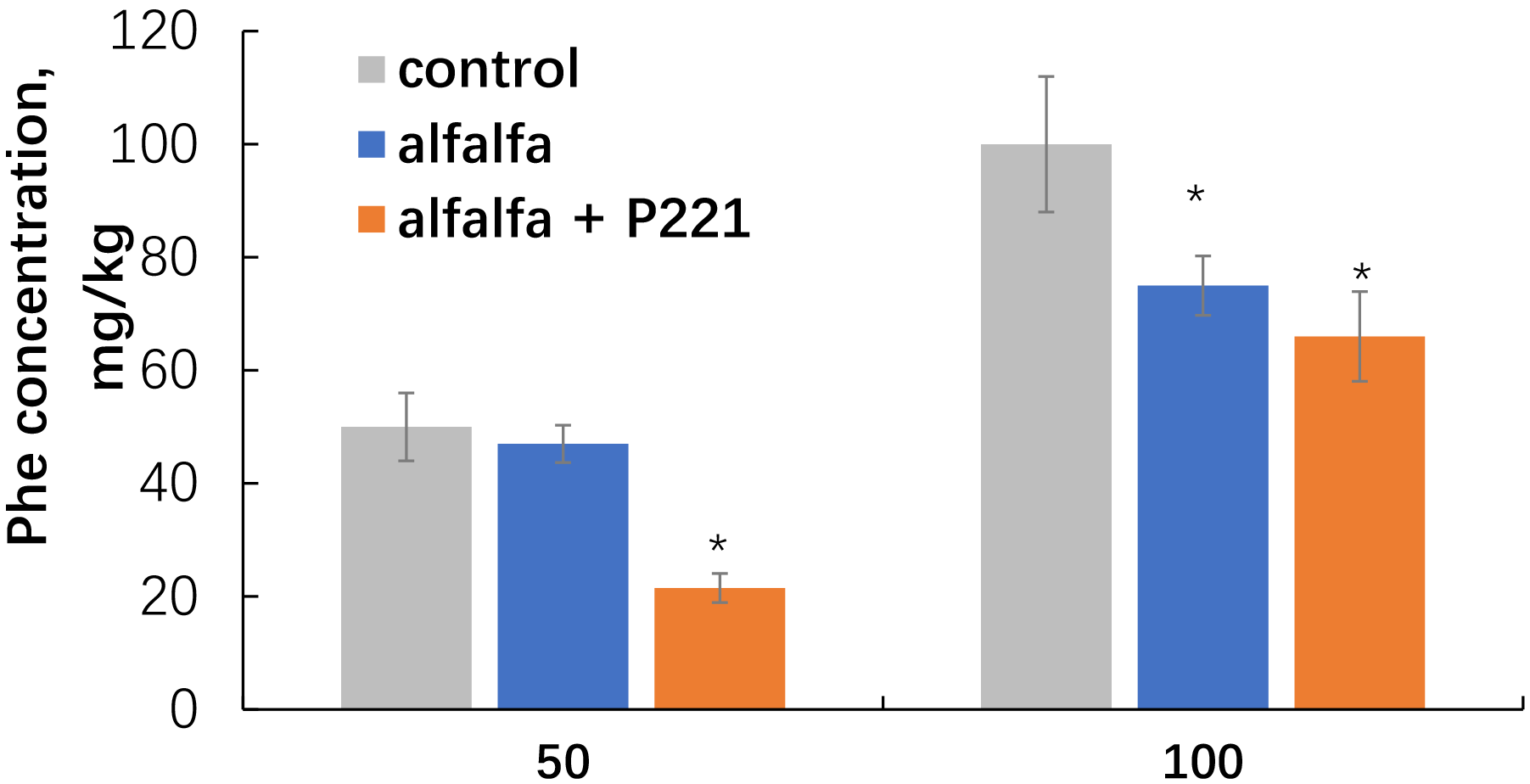 Fig. 7.
Fig. 7.
Disappearance of phenanthrene from the sand after cultivation of
alfalfa inoculated with E. meliloti P221. Single asterisks indicate values
that are significantly different from the flavonoid-free control at p
When alfalfa was grown sterile, the sand phenanthrene content decreased in all treatment options. Elimination of phenanthrene from sand with noninoculated alfalfa was 6 and 22%, respectively, for the 50 and 100 mg/kg treatments. Inoculation with the PAH-degrading rhizobacterium enhanced the remediation effect of the system, in which the degradation of phenanthrene reached 57 and 34%, respectively, for the 50 and 100 mg/kg treatments.
Analysis of the root exudates of the inoculated alfalfa plants made it possible to observe an increase in the content of phenolic compounds under the influence of the microorganism (Fig. 8).
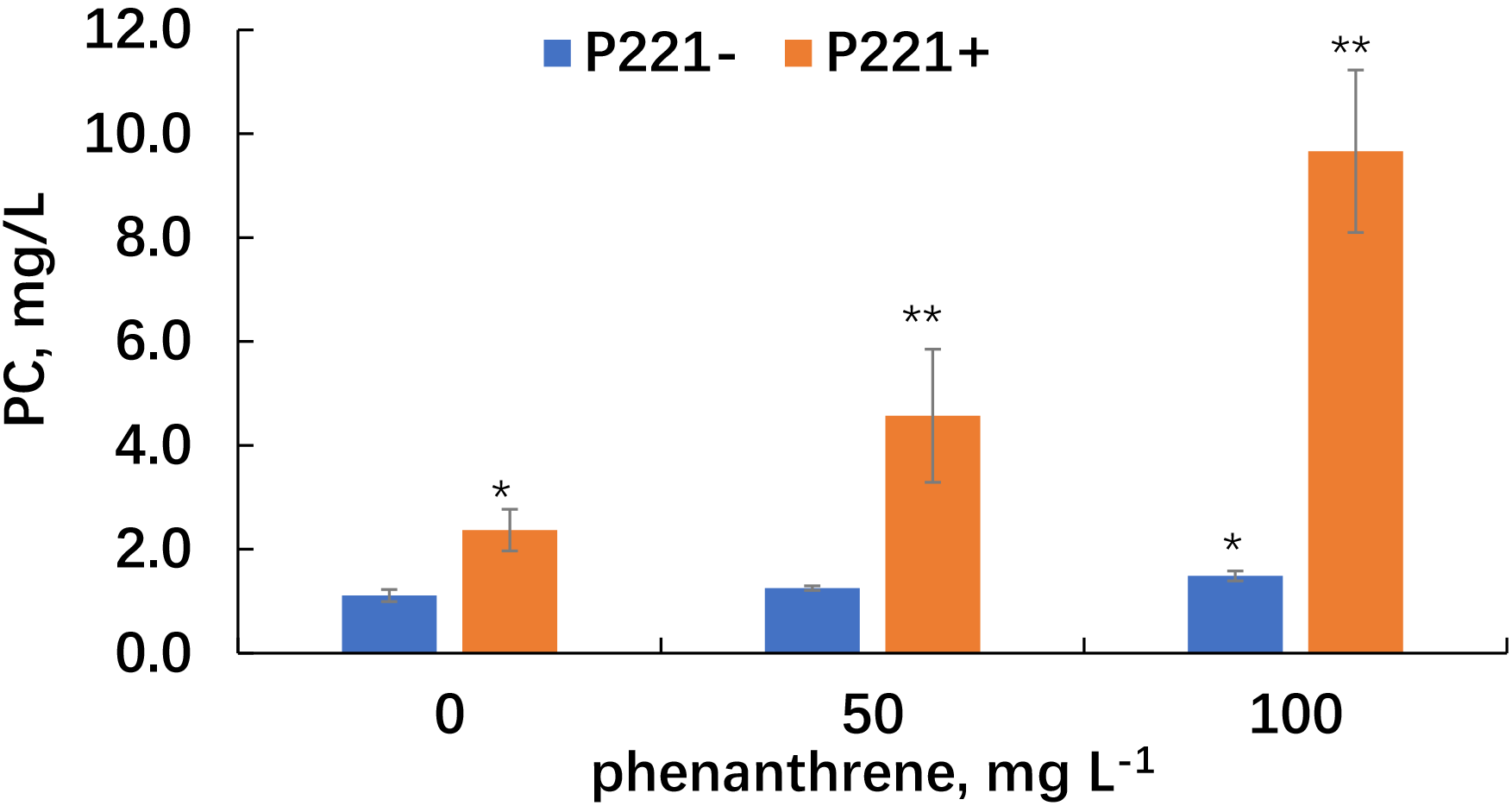 Fig. 8.
Fig. 8.
Phenolic compound content in the root exudates of alfalfa grown
in uncontaminated and phenanthrene-contaminated sand. Single asterisks indicate
values that are significantly different from the uncontaminated and noninoculated
plants at p
In uncontaminated sand, the content of phenolic compounds in the alfalfa root exudates, as affected by the inoculant, increased by more than two times. In phenanthrene-contaminated sand, the content of phenolic compounds in the root exudates of inoculated alfalfa increased by 3.6 and 6.5 times, respectively, at 50 and 100 mg/kg of phenanthrene, respectively.
HPLC analysis of the root exudates of alfalfa inoculated with E. meliloti P221 showed pronounced qualitative and quantitative changes in the fraction of phenolic compounds under the influence of the microorganism, both in the presence and in the absence of phenanthrene (Supplementary Fig. 2). Along with the previously identified predominant component (5) (Supplementary Fig. 1), in response to inoculation, another major component (11) with RT = 7.34 min appeared in the alfalfa root exudates. Naringenin (2) was also detected in the chromatograms. In the exudates collected from contaminated sand, bacterial metabolites from phenanthrene degradation were detected. The RT of component (8) coincided with that (RT = 5.82 min) of the oxidized phenanthrene derivative 1-hydroxy-2-naphthoic acid.
It is now known that PAHs have multilevel toxic effects on plants, causing morphological, physiological, and biochemical changes and also damage at the molecular level [30, 31, 32]. This study of the interaction of young alfalfa (Medicago sativa L.) plants with phenanthrene detected a dose-dependent effect of the PAH on plant biomass accumulation and on morphometric variables of the root system. This effect ranged from nonsignificant promotion of root and shoot growth at 50 mg/kg of phenanthrene to pronounced inhibition at 200 mg/kg of phenanthrene. Overall, our results are consistent with the previously reported data on the dose-dependent effect of PAHs on biomass accumulation in Phaserolus nipponessis and Zea mays [33] and in Brassica campestris [34]. For alfalfa plants both a stimulatory and an inhibitory effect of PAHs, depending on the concentration and number of aromatic rings, were also reported previously [9]. The negative effects of PAHs include impaired seed germination; impaired plant growth, development, and photosynthetic activity; and changes in the physiological and biochemical status of alfalfa [35, 36, 37, 38]. The PAH-caused changes in the antioxidant defense system of alfalfa were also identified [39, 40]. On the other hand, increased growth of alfalfa biomass in the presence of three-, four-, and five-ring PAHs was reported [41, 42].
Roots are critical for water absorption and mineral nutrition, and they interact directly with pollutants in soil. PAH toxicity to plant roots is manifested by changes in their morphometric and physiological–biochemical variables [29, 33, 34, 36, 42, 43, 44]. In our experiment, under the influence of phenanthrene, alfalfa roots decreased in wet weight rather than in dry weight (Table 1) and noticeably changed their morphology and structure, thickening and coarsening as a result of physiological and biochemical transformations in root tissue. These changes could be seen by the naked eye (Fig. 2) and may indicate extensive root suberization under the influence of the pollutant. The lignification and suberization of plant tissue cell walls are promoted by various abiotic and biotic factors [45, 46]. Dupuy et al. [29] also found that phenanthrene activates the suberization of endo- and exodermal cells in maize, which is a protective strategy to prevent pollutant penetration into plant tissues. The formation of dense suberin layers strongly reduces the absorption capacity of the root system and, as a result, inhibits the intensity of plant growth, which too was observed in our experiment.
Suberization and lignification are associated with increased synthesis of phenolic compounds in response to stress [47, 48]. Phenolic compounds, including simple phenols, coumarin, lignin, condensed and hydrolyzable tannins, and flavonoids, play an active part in plant responses to environmental stress [49]. These compounds are also believed to be important for the detoxification of environmental pollutants by promoting the production of peroxidases and oxidases, involved in the metabolism of phenolic compounds [50], and by influencing the biological translocation of pollutants in plants [51]. There is limited evidence for increased production of phenolic compounds in plants in response to the presence of PAHs. For example, Jiang et al. [52] noted that phenanthrene causes a significant increase in the content of total phenolic compounds in the tissues of leaves and roots of the mangrove plant Aegiceras corniculatum L.
Along with other plant metabolites, some of the synthesized phenolic compounds can be released into the environment with root exudates. The role of root exudates in soil remediation has been repeatedly emphasized [19, 53, 54]. However, few studies have addressed changes in plant root exudation under the influence of PAHs [55, 56, 57]. In this work, we also attempted to characterize the phenolic compound fraction of alfalfa root exudates and its changes under the influence of phenanthrene and the inoculated bacteria. Unfortunately, only naringenin was presumably identified in the root exudates, and a more scrupulous qualitative analysis will be the subject of further work. The total content of phenolic compounds in the exudates increased in response to increasing phenanthrene concentration (Fig. 3), which is consistent with the data showing an increase in the tissue content of phenolic compounds as a stress response to phenanthrene [52]. Of course, in the presence of an aromatic pollutant, we cannot rule out a possible contribution of oxidized phenanthrene derivatives, formed as a result of the enzymatic activity of the root exudates [58]. Nevertheless, HPLC analysis of the phenolic components of the exudates made it possible to clearly observe a major compound with RT = 8.40 min, which was present in the exudate from uncontaminated sand and, in no smaller quantity, in that from phenanthrene-containing sand.
Flavonoids are important specific secondary metabolites of plants, exuded by roots along with other phenolic compounds [14, 59, 60]. In plants, they play different important parts, including in transport of auxin, development of roots and shoots, pollination, and modulation of reactive oxygen species. Flavonoids are involved in the regulation of nodulation in legumes; contribute to stress-induced morphogenic responses under a variety of biotic and abiotic conditions; and possess antibacterial, antifungal, antiviral, and anticancer activities [60]. They are transported within and between plant tissues and cells and are specifically released by roots into the rhizosphere, where they act as communication signals in plant–plant (allelopathy) and plant–microbe interactions [61, 62]. Flavonoids affect the structure and function of the root-associated microbiome; mediate root associations with soil microorganisms, including signaling of competent bacteria in the legume-Rhizobium symbiosis; can act as quorum-sensing inhibitors for phytopathogens; and can be carbon and energy sources for nontarget microorganisms [14, 60, 63]. There is the opinion that some flavonoids exuded by roots are structurally similar to aromatic pollutants [64, 65], which may contribute to the formation in the rhizosphere of a specific microbiome enriched with aromatic-degrading microorganisms, thereby influencing the intensity of pollutant rhizodegradation [16, 17, 18, 19, 20].
In alfalfa, flavonoids are represented mainly by glycosides of four flavone aglycones: apigenin, luteolin, tricine, and chrysoeriol [66, 67]. In addition, alfalfa flavonoids include naringenin [68], morin [69], and quercetin [68, 69, 70]. The same compounds may be expected to be in the alfalfa root exudates; however, data about exuded flavonoids are scarce.
We investigated the effect of flavonoids on bacterial growth and phenanthrene degradation in two rhizobacteria isolated from the alfalfa rhizosphere. One, E. meliloti P221, is a natural alfalfa symbiont, whereas the other, M. gilvum PAM1, is a member of a nontarget microbiota associated with alfalfa roots. The growth of both rhizobacteria was significantly promoted by naringenin, and the growth of E. meliloti P221 was also promoted by rutin and morin but to a lesser extent. Similar growth promotion was previously noted for other rhizobacteria, for example, Herbaspirillum seropediceae [71] and Bradyrhizbium sp. ORS285 [72]. Nouwen et al. [72] showed that the naringenin-enhanced growth of Bradyrhizbium sp. strain ORS285 was accompanied by activation of glycerol and fatty acid metabolism and by increasing in 3-hydroxybutyrate dehydrogenase enzyme activity. Strain ORS285 did not degrade the flavonoid but transformed it into a hydroxylated and methylated derivative compound with growth-promoting activity. This bacterial strategy may serve not only as a protective mechanism against the antimicrobial effects of flavonoids [73] but also as a competitive advantage for rhizosphere colonization [72]. As our data showed, both rhizobacteria, isolated from the alfalfa rhizosphere, had precisely this adaptation strategy.
The ability of E. meliloti P221 and M. gilvum PAM1 to degrade flavonoids may also be a manifestation of their adaptation strategy for colonization of the rhizosphere. Countering the antimicrobial activity of plant flavonoids through their catabolism may provide a selective advantage to the rhizobacteria under study over other soil microorganisms in their interaction with alfalfa [18]. Despite their well-known antimicrobial activity [72], flavonoids are actively degraded in the rhizosphere by a wide range of microorganisms [61, 74, 75, 76]. In our experiment, the rhizobacteria vigorously utilized all the flavonoids tested, with the exception of rutin. This glycosylated flavanol was not at all degraded by M. gilvum PAM1 and only slightly degraded by E. meliloti P221.
Phenanthrene metabolism in E. meliloti P221 and M. gilvum PAM1 has been described in detail earlier [22, 23]. This study attempts to demonstrate the influence of plant flavonoids on PAH degradation by these bacteria. The effect of the flavonoids on phenanthrene degradation varied from neutral or even inhibitory (e.g., E. meliloti P221 with quercetin) to stimulatory (E. meliloti P221 with morin and apigenin; M. gilvum PAM1 with rutin, morin, and quercetin). The increased degradation of phenanthrene in the presence of morin, apigenin, rutin, and quercetin could presumably be associated with stimulation by these flavonoids of the activity of the enzymes involved in PAH degradation. It is known that under the action of monooxygenases, dioxygenases, and PAHs, many flavonoids can be biotransformed to epoxides and diols [21]. Ely and Smets [77] suggested that phenolic compounds such as morin, caffeic acid, and protocatechuic acid are associated with bacterial degradation of three- and four-ring PAHs in the rhizosphere. Intracellular enzymes such as dioxygenase and dehydrogenase are responsible for the bacterial degradation of PAHs [78]. Catechol dioxygenases, including catechol 1,2-dioxygenase (C12O) and catechol 2,3-dioxygenase (C23O), are key enzymes for aromatic hydrocarbon ring cleavage, which is a limiting factor in PAH biodegradation [79]. As reported by Lu et al. [80], the presence of rutin in low concentrations enhanced the activity of C12O, C23O, and dehydrogenase in Methylobacterium extorquens strain C1. This may explain the stimulatory effect of flavonoids on the bacterial degradation of phenanthrene in this study.
PAH oxidation can also be inhibited by quercetin and morin [21, 81]. The opposite effect of quercetin on the growth and degradation of phenanthrene in the two bacteria may indicate the specificity of the indirect effect of the flavonoid on PAH catabolism in different microorganisms. With naringenin as an example, it was shown that promotion of bacterial growth by a flavonoid could not be accompanied by increased degradation of PAHs, which probably indicates that the enzymes of PAH catabolism were not a target for this flavonoid.
Inoculation of alfalfa with the competent rhizobacterium E. meliloti led to an increase in the content of and qualitative changes in the profile of the phenolic compounds in the root exudates. It also enhanced phenanthrene rhizodegradation, which could be associated with bacterial metabolic activity.
Microorganisms, rhizobia in particular, increase root exudation in plants [82, 83]. In this case, they influence the production of root exudates by their very presence in the environment, degrading the root-secreted substances, and also through their metabolites and enzymes. The qualitative and quantitative changes in plant root exudation caused by a single microorganism, which we observed in an initially sterile model system, illustrate the induction of metabolic activity in the rhizosphere. The intensification of plant–bacterial metabolic activity in the rhizosphere resulted in phenanthrene decomposition, which was confirmed by the detection of one of the key metabolites specific for phenanthrene degradation by E. meliloti P221 [22].
We characterized, quantitatively and qualitatively, the root exudation of phenolic compounds by young alfalfa plants in a sandy culture, and we examined the effect of the three-ringed PAH phenanthrene and an alfalfa-associated rhizobacterium on this process. Unfortunately, only naringenin was presumably identified in the alfalfa root exudates. Phenanthrene at 50, 100, and 200 mg/kg was toxic to alfalfa and increased the exudation of phenolic compounds. The effect of individual flavonoids on the growth and degradation of phenanthrene in PAH-degrading rhizobacteria was also investigated. Naringenin promoted bacterial growth regardless of the presence of phenanthrene. The effect of flavonoids on the bacterial degradation of phenanthrene varied from inhibitory to stimulatory and differed between the bacteria. With the exception of rutin, all flavonoids tested were subject to bacterial degradation. Inoculation of alfalfa with the competent rhizobacterium E. meliloti led to an increase in the content of and qualitative changes in the profile of the phenolic compounds in the root exudates. It also enhanced phenanthrene rhizodegradation, which could be associated with bacterial metabolic activity.
The data and materials generated during the current study are available from the corresponding author.
LP and AM designed the research study. DK, LP and NP performed the research. NP provided help and advice on flavonoids analysis. DK, LP and AM analyzed the data. DK, LP and AM wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Seeds of alfalfa (Medicago sativa L.) of the Diana variety (https://www.arisersar.ru/diana.htm) were obtained from the Federal Center of Agriculture Research of the South-East Region (Saratov, Russia).
We gratefully acknowledge the assistance and instructions in HPLC analysis of flavonoids to Dr. Vyacheslav S. Grinev from Laboratory of Biochemistry of IBPPM RAS. We are also sincerely grateful to Mr. Dmitry Tychinin, Leading Translator of IBPPM RAS (https://orcid.org/0000-0001-7680-453X) for his help in preparation of English version of manuscript.
The work was carried out within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation for the Saratov Scientific Center of the Russian Academy of Sciences, topic no. 124020100146-9.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/FBE25779.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
