- Academic Editor
The microbiome composition in dairy cows (Bos taurus) directly impacts on health and reproductive performance. This study aimed to determine the metagenomic composition and predicted microbial community functions in the endometrium and rectal chyme of cows fed a complex feed additive (CFA). The latter included the Bacillus mucilaginosus 159 strain, a short-chain fatty acid, plus essential oils.
Clinically healthy cows were divided into two groups (n = 15 in each): (I) a control group fed the standard diet, and (II) an experimental group. CFA was introduced into the diet of Group II during the entire transit period at a dose of 50 g per animal per day; moreover, all animals received Pen-Strep 400 antibiotics to prevent endometritis and other pathologies. The microbial community composition from the endometrium and rectal chyme biotopes was assessed using targeted next-generation sequencing.
Significant changes were observed in the composition and predicted metabolic pathways due to the CFA administration, with the endometrial microbiota being more responsive to CFA than the intestinal chyme microbiome. Remarkably, the Actinobacteriota representatives disappeared in the endometrium of Group II animals compared to controls, whose content ranged from 0.34 to 3.3%. The use of CFA also resulted in a less pronounced effect in four predicted metabolic pathways for microbial degradation of catechol in the endometrium compared to controls (p < 0.05).
Our findings support the concept of a relationship between the gut microbiome and the reproductive system microflora of cows, as we observed changes in the composition and predicted metabolic pathways of the endometrial microbiota after orally administering CFA. This emphasizes the need for an integrated approach combining the correction of microecological disorders in the intestines and the reproductive system simultaneously.
Managed livestock represents an essential cornerstone for human nutrition, with cattle (Bos taurus) being used both for meat and milk [1]. In recent years, much attention has been paid to the comfort of the animals’ habitat during the transit period, i.e., the 21 days immediately preceding and the 21 days following calving. This is the most critical period in the life of a productive dairy cow, shaping a state of homeostasis of the animal throughout the next physiological cycle and is the time of greatest susceptibility to disease [2]. A disparity between the need for energy, the level of its supply through feed and the failure of internal pathogenetic mechanisms can inhibit the functionality of the reproductive system [3]. It can lead to disruption of the delicate balance of the microbiota, including microbial populations living in both the digestive and reproductive systems [4].
Modern treatment of postpartum endometritis is based on complex treatment regimens that include antibacterial drugs, often administered in combination [5]. The use of antibiotics, however, while suppressing the symptoms of endometritis, often has no effect on improving the reproductive capability of the cow, and can often lead to a recurrence of infection. In our opinion, this may be due to the suppression of the normal microbiota composition of the reproductive organs, thereby perpetuating the initial problem it was designed to improve [6, 7]. Treatment regimens for endometritis thus require adjustments that include the development of new treatment regimes, alongside effective preventive measures. The concomitant use of biological products to restore the microbiota before, during and after antibiotic treatment [8, 9] can help normalize the microecological status of the cow’s reproductive system and increase the effectiveness of standard therapy.
In terms of improving the gut health of livestock and poultry, therapeutic and
nutritional interest in commercially produced biological feed additives, such as
prebiotics [10, 11] and probiotics [9, 12, 13, 14, 15], is rapidly growing. The microbiome
of the cattle digestive system has been repeatedly confirmed to be associated
with health and productivity of animals [16]. Probiotics have been shown to have
significant potential in altering the composition of the rumen microbiota
beneficially, with some probiotics colonizing the vagina after oral
administration [17]. In a human study, a meta-analysis of 22 randomized
controlled trials found that probiotics are very effective when used in
combination with antibiotics to treat and prevent relapses of bacterial vaginosis
[8]. When conducting studies of the vaginal mucosa of cows using quantitative PCR
(qPCR), we discovered a relationship between the rumen microbiota and the
reproductive system [18, 19]. Based on the results obtained, the spectrum of
action of the probiotic Provitol composed of a strain of the microorganism
Enterococcus faecium and essential oils for oral administration has been
expanded. This enabled us to recommend this developed biotechnological product
for regulating the composition of the vaginal microbiota of cows through
optimizing the composition of the rumen microbiome [20]. Previously, we [16, 21]
also used metagenomic next-generation sequencing (NGS) to explore the functional
potential of metabolic pathways,
In addition to microorganisms with probiotic properties, essential oils, which are natural substances produced by plants, are widely used in human and veterinary medicine due to their antioxidant, antiviral, and antibacterial properties [23]. The efficacy of essential oils against mastitis [24], respiratory diseases [25], and reproductive pathologies in cattle [26] has been reported. For example, a recent study showed that cinnamon essential oil had high antibacterial activity against Trueperella pyogenes and Escherichia coli that are pathogens associated with inflammatory diseases of the uterus in dairy cows [27]. Short- and medium-chain fatty acids are also considered as an effective means for restoring the composition of the microbiome and reducing inflammatory reactions in the body [28]. Antimicrobial activity is manifested due to the ability of these substances to penetrate the bacterial cell wall and inhibit enzymatic reactions and nutrient transport systems [29]. Moreover, the anti-inflammatory, antioxidant and immunomodulatory properties of organic acids are manifested both at the local and systemic levels [30]. This demonstrates the high potential of using essential oils and fatty acids as alternative antibacterial agent for endometritis treatment in dairy cows.
It has been shown that disturbances in the intestinal microbiota can cause diseases of the extraintestinal system, such as diabetes, mastitis, and reproductive pathologies [31]. On the other hand, immune homeostasis is fundamentally maintained by the immune system’s interaction with the gut bacteria [31]. Therefore, further in-depth studies are required to explore the relationship between the composition of the intestinal microbiota and endometrium of cows [32] when administering oral preventive supplements.
The purpose of this study was to examine the metagenomic composition and predicted functions of the microbial communities of the endometrium and rectal chyme biotopes of cows. We specifically investigated the effect of a complex feed additive (CFA) containing the microorganism strain Bacillus mucilaginosus 159, a short-chain fatty acid and essential oils was introduced into the cow diet during the transit period.
The experiment was carried out including a total of 2261 animals, including Holstein cows of the third lactation with an average milk production of 12,819 kg per lactation and an average live weight of 715 kg. Animals with similar characteristics, in terms of productivity during the previous lactation, age, live weight, and date of calving, were subjected to clinical and gynecological examination [33] during the transit period conforming to interlactation (i.e., 21 days before calving). The criteria for selecting animals for the experiment were the absence of treatment with antibiotics and hormonal drugs or other drugs in the previous lactation. The live weight of cows in the experiment and control averaged 712–715 kg. The animals were kept in the same free-stall conditions, had the same diet composition, and had good reproductive history. Rations were calculated according to the feeding standards recommended by the National Academies of Sciences, Engineering, and Medicine [34] taking into account the physiological needs of animals during the whole transit period (i.e., 21 days before calving and 21 days after calving). The standard (base) diets were completely balanced in nutrients, and all components of the diet were studied for quality and safety. In particular, the formulation of the base diet in the second phase of the dry period included (in kg per cow per day): straw, 1.3, silage, 18.0; corn, 2.4; soybean meal, 0.2; rapeseed cake, 0.4; flattened barley, 1.5; beet pulp, 2.7; spent grain, 1.0; glycerin, 0.1; plus vitamin and mineral microsupplements. The composition of the base diet in the fresh calving period included (in kg per cow per day): hay, 0.9; silage, 27; wheat, 1.2; corn, 4.2; soybean meal, 1.3; rapeseed cake, 0.5; crushed barley, 1.6; beet pulp, 3.0; spent grain, 0.5; glycerin, 0.2; plus vitamin and mineral microsupplements. Daily monitoring of the correct loading of diet components, weekly assessment of dry matter intake and assessment of digestibility of diets were carried out.
Based on the results of medical examination during the interlactation period (i.e., 21 days before calving), a total of 30 clinically healthy cows were selected and divided into two groups (n = 15 in each): control group (I), without the CFA (BIOTROF Ltd, St. Petersburg, Russia) introduction into the diet; and experiment group (II), with the CFA introduction into the diet at a dose of 50 g per animal per day during the entire transit period (i.e., 42 days). The CFA dosage was determined according to the manufacturer’s instructions and recommendations, calculated earlier based on the initial concentration of active compounds, ease of administration when mixing in feed shops, cost of the additive, previous experience in developing similar supplements, and the results of preliminary experiments. In order to prevent endometritis and other possible pathological conditions, all animals from both groups, without exception, received the antibiotic Pen-Strep 400 (ImmCont GmbH, Eberswalde, Germany) based on benzylpenicillin-procaine and dihydrostreptomycin sulfate intramuscularly at the rate of 1 mL per 20 kg of animal weight once per day for 5 days, starting from the 5th day after calving. The animals did not receive any other drug or probiotic supplements.
The CFA included the following natural substances of antibacterial nature: the
strain of probiotic bacteria B. mucilaginosus 159, a short-chain fatty
acid, essential oils of plants eucalyptus and cinnamon, and wheat bran filler.
The B. mucilaginosus 159 strain was obtained from the collection of
BIOTROF LLC (St. Petersburg, Russia) and had been originally isolated from the
chyme of the cow’s rumen. The cells of the strain are motionless rods with
rounded ends; it forms oval spores located subterminally and is not a genetically
modified organism. The B. mucilaginous 159 strain has no toxicogenic or
virulent properties; the strain cell components are not toxic to laboratory
animals [35]. The B. mucilaginosus 159 strain was cultured at 37 °C in a
broth medium of the following composition: enzymatic peptone, pancreatic
hydrolysate of fish meal, and sodium chloride, at pH = 7.2
At the end of the experiment (i.e., on Day 21 after calving), endometrial and rectal chyme samples were taken from three clinically healthy cows from each group to analyze the composition and functions of the microbiota. Cows with abnormal calving, post-calving complications, or other clinical diseases before or during the study period were not included in the study. The collection of biological material was carried out in compliance with aseptic conditions to ensure individuality and prevent cross-contamination of samples. The tail of the animals was covered with sterile gauze, and the perineum and vulva were washed with soap and water until completely clean, then treated with an antiseptic solution. Collection of scrapings from the surface of the endometrium was carried out using a cytobrush. Digestive chyme samples were collected directly from the rectum of each animal using disposable gloves. Overall, the sampling methods complied with the standards of humane treatment of animals. These methods were based on the use of a special veterinary probe and a specialist who was proficient in this technique. No painful manipulations were applied to the animals; therefore, the use of anesthesia was not required. A lubricative (vaseline oil), to facilitate the insertion of the probe, and an antiseptic were used as recommended and was successively washed off to prevent contamination.
Endometrial scraping samples for microbiota analysis were immediately frozen at –20 °C and sent to the laboratory for DNA extraction. Total DNA from the samples was isolated using the Genomic DNA Purification Kit (lot No. 10571031; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions.
The metagenomic composition of the microbial communities in scrapings from the cow endometrium and rectal chyme biotopes was assessed using the targeted next-generation sequencing (NGS). The latter was performed using the MiSeq platform (Illumina, Inc., San Diego, USA) and the following primers for the V3–V4 region of the 16S rRNA gene: forward, 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′; reverse, 5′- GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′. NGS was carried out using the respective reagents for preparation libraries (Nextera® XT Index Kit, lot No. FC-131-1001; Illumina), purification of qPCR products (Agencourt AMPure XP, lot No. A63881; Beckman Coulter Inc., Brea, CA, USA), and sequencing (MiSeq® Reagent Kit v2, 500 cycles, lot No. MS-102-2003; Illumina). Raw 16S rRNA sequence dataset was uploaded to the NCBI Sequence Read Archive (SRA; BioProject Number: PRJNA1102864) [36]).
The functional potential of the microbiota was assessed using the appropriate bioinformatics methods. In particular, bioinformatics analysis of NGS data was performed using a metagenomic analysis platform Quantitative Insights Into Microbial Ecology (QIIME2, Version 2022.2), version 2020.8 [37, 38]. After the initial import of sequences into QIIME2 format, paired strings of reads were aligned. Sequences were then filtered by quality using the default settings. Noise sequences were filtered using the Deblur method. To construct a de novo phylogeny, the MAFFT software package, version 7 [39, 40] was employed followed by the masked sequence alignment. The SILVA 138 reference database [41, 42, 43] was used for taxonomy analysis.
Based on the resultant table of operational taxonomic units (OTUs), biodiversity indices (Shannon, Simpson, Chao1, and Fisher alpha) were calculated using plugins of the QIIME2 software package [37, 38]. For the statistical analysis of diversity indices, no additional transformation was performed. That is, no further standardization or scaling was applied to the calculated diversity data. The analysis result was presented in the respective graphs as actual values of diversity indices.
Reconstruction and prediction of the functional content of gene families and enzymes was carried out using the PICRUSt2 software package, version 2.3.0 [44, 45]. The MetaCyc database [46, 47] was employed to analyze metabolic pathways and enzymes. Predicted MetaCyc metabolic pathway profiles were assessed by the amplicon sequence variants (ASV)-based abundance [48, 49].
Using Microsoft Excel XP/2003 (Microsoft Corporation, Redmond, WA, USA) and
RStudio (version 1.1.453, Boston, MA, USA [50]), multivariate analysis of
variance (multi-factor ANOVA) was used to process the results mathematically and
statistically. The mean (M) and standard errors of the mean (
As a result of the metagenomic NGS analysis, a total of 33,523 16S rRNA gene
sequences were generated for the bovine intestinal chyme microbiome and 1367 for
the endometrium microbiome. Bioinformatics data processing revealed differences
(p
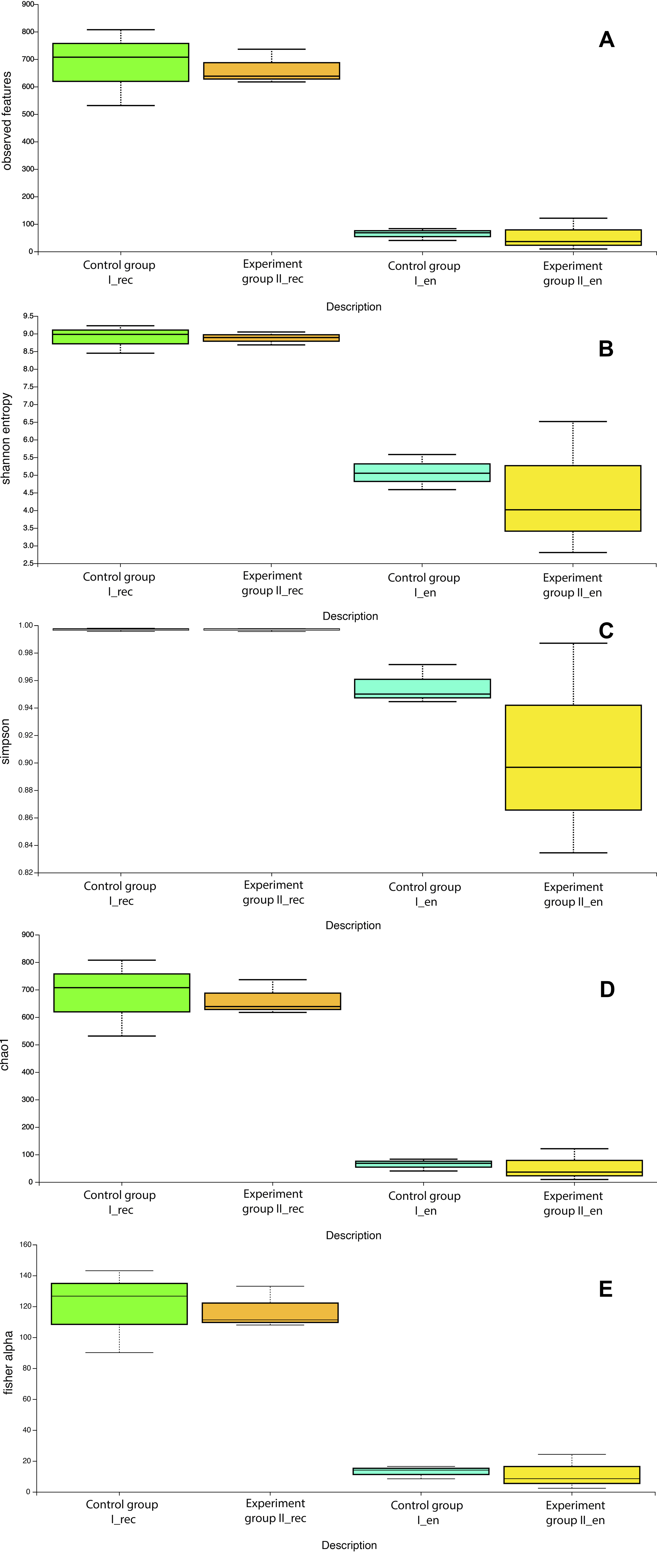 Fig. 1.
Fig. 1.
Graphs of the microbial biodiversity distribution based on the NGS data for samples from the rectal chyme (_rec) and endometrium (_en) of dairy cows of the Holstein breed. Biodiversity indicators were as follows: (A) number of operational taxonomic units (OTUs), (B) Shannon entropy, (C) Simpson index, (D) Chao1 index, and (E) Fisher alpha index. The bottom of the horizontal bar represents the bottom value of the group, the top of the bar reflects the top value of the group, the dividing line within the bar conforms to the median, and the whiskers are the minimum and maximum values of the data. NGS, next-generation sequencing.
In the rectal chyme microbiota of the cows, 15 superphyla and phyla of microorganisms were discovered, and slightly less (12) in the endometrium (Fig. 2). Herewith, the phylum Firmicutes and superphylum Bacteroidota can be considered the dominating bacteria among both the rectum (respectively up to 73.4 and 26.4% in Group I and up to 77.8 and 28.2% in Group II) and endometrium (respectively up to 59.6 and 68.6% in Group I and up to 26.5 and 21.3% in Group II) biotopes. However, representatives of the phylum Proteobacteria in the endometrial microbiota were also represented in significant numbers (up to 33.1% in Group I and up to 28.2% in Group II). This was in contrast to the rectal chyme microbiota, where representatives of this phylum were detected in a trace amount not exceeding 0.64%.
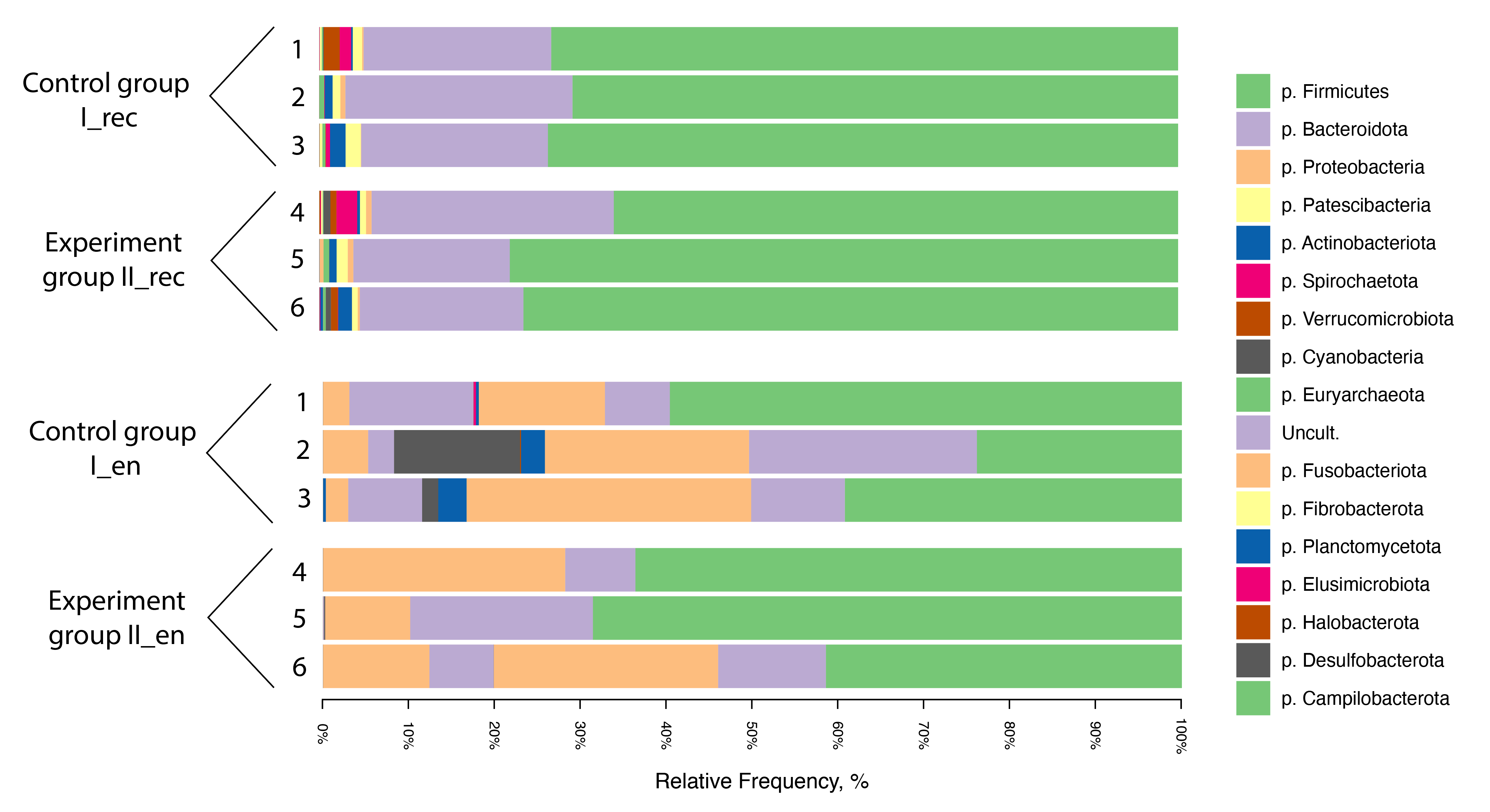 Fig. 2.
Fig. 2.
Taxonomic composition of the microbiota at the phylum level based on the NGS data for samples from the rectal chyme (_rec) and endometrium (_en) of dairy cows of the Holstein breed. 1–6, animal IDs; p., phylum.; uncult., uncultured.
Interestingly, the proportion of Firmicutes to Bacteroidetes both in the chyme of the digestive system and in the endometrium of cows and in both groups (Fig. 3) was quite high (up to 4.27 in the rectum and up to 7.9 in the endometrium). The exception was animal No. 2 in Group I, in whose endometrial microbiota this ratio was below 1. According to Miranda-CasoLuengo et al. [53], a low ratio of Firmicutes to Bacteroidetes in the reproductive system of cows was an early signal of the subsequent development of inflammatory diseases. Our data on the ratio of Firmicutes to Bacteroidetes, in general, coincided with the clinical observation of the absence of endometritis signs in the studied animals.
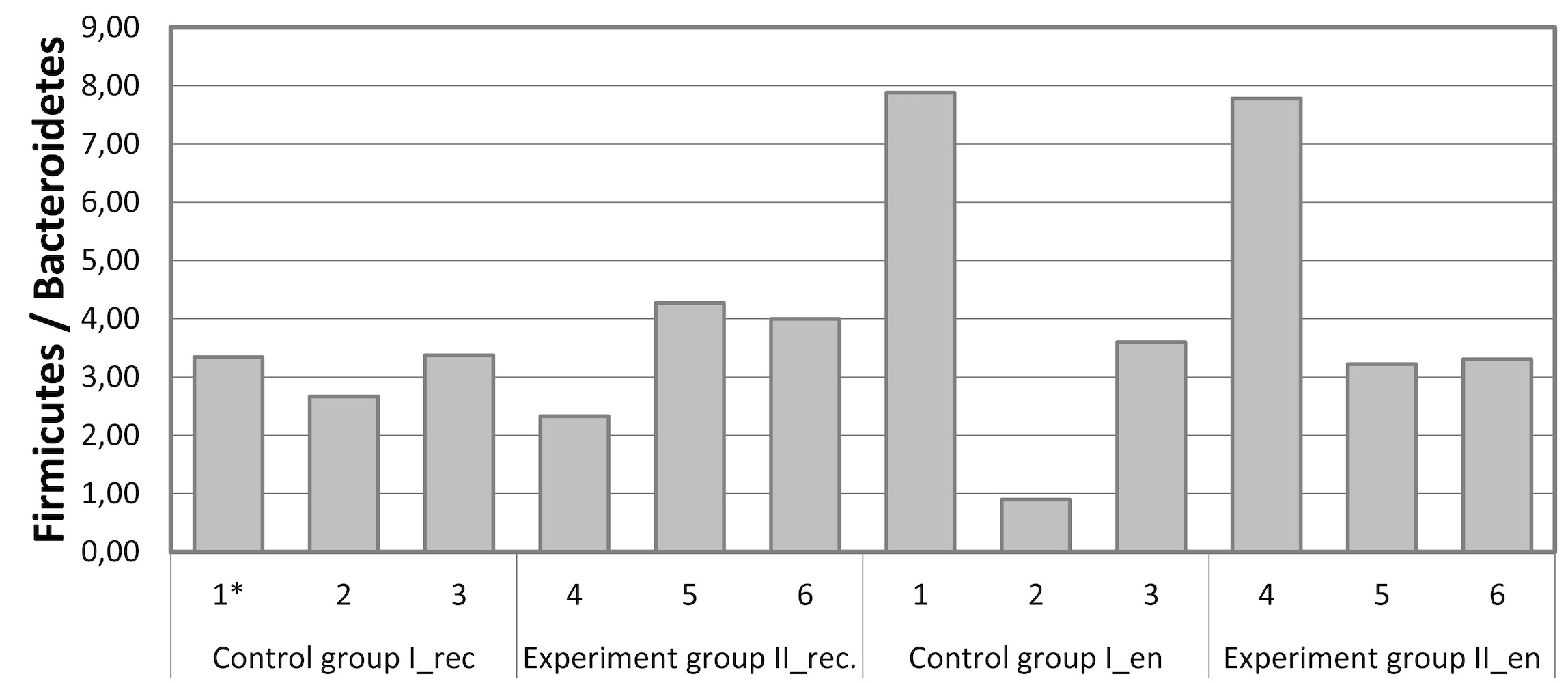 Fig. 3.
Fig. 3.
The ratio of Firmicutes to Bacteroidetes in the microbiota at the phylum level based on the NGS data for samples from the rectal chyme (_rec) and endometrium (_en) of dairy cows of the Holstein breed. *1–6, animal IDs.
Another difference between the two studied biotopes was the established fact that the microbiota of the chyme of the rectum included representatives of the phyla Euryarchaeota, Patescibacteria and Fibrobacterota (Fig. 2) that were not identified in the endometrium of cows. It is widely known that archaea are a specialized group of microorganisms including methanogenic euryarchaeota, one of the main habitats of which is the digestive system of ruminants [54, 55]. Members of the superphylum Patescibacteria symbiotically interact with archaea, have been commonly found in anaerobic environments [56, 57, 58], including the digestive tract of ruminants [59], and are associated with anaerobic enzymatic metabolism [60]. Members of the superphylum Fibrobacterota are cellulolytics in the herbivore gut and other cellulolytic ecosystems [61].
More pronounced changes (p
Interestingly, in the endometrium of two Group I cows and two Group II cows, a significant presence (up to 47.2%) of the Mycoplasmataceae family bacteria was revealed (Fig. 4). The data presented were in agreement with the results obtained by Knudsen et al. [63] who concluded that Mycoplasmataceae was one of the most abundant families in uterine lavage samples from clinically healthy cows.
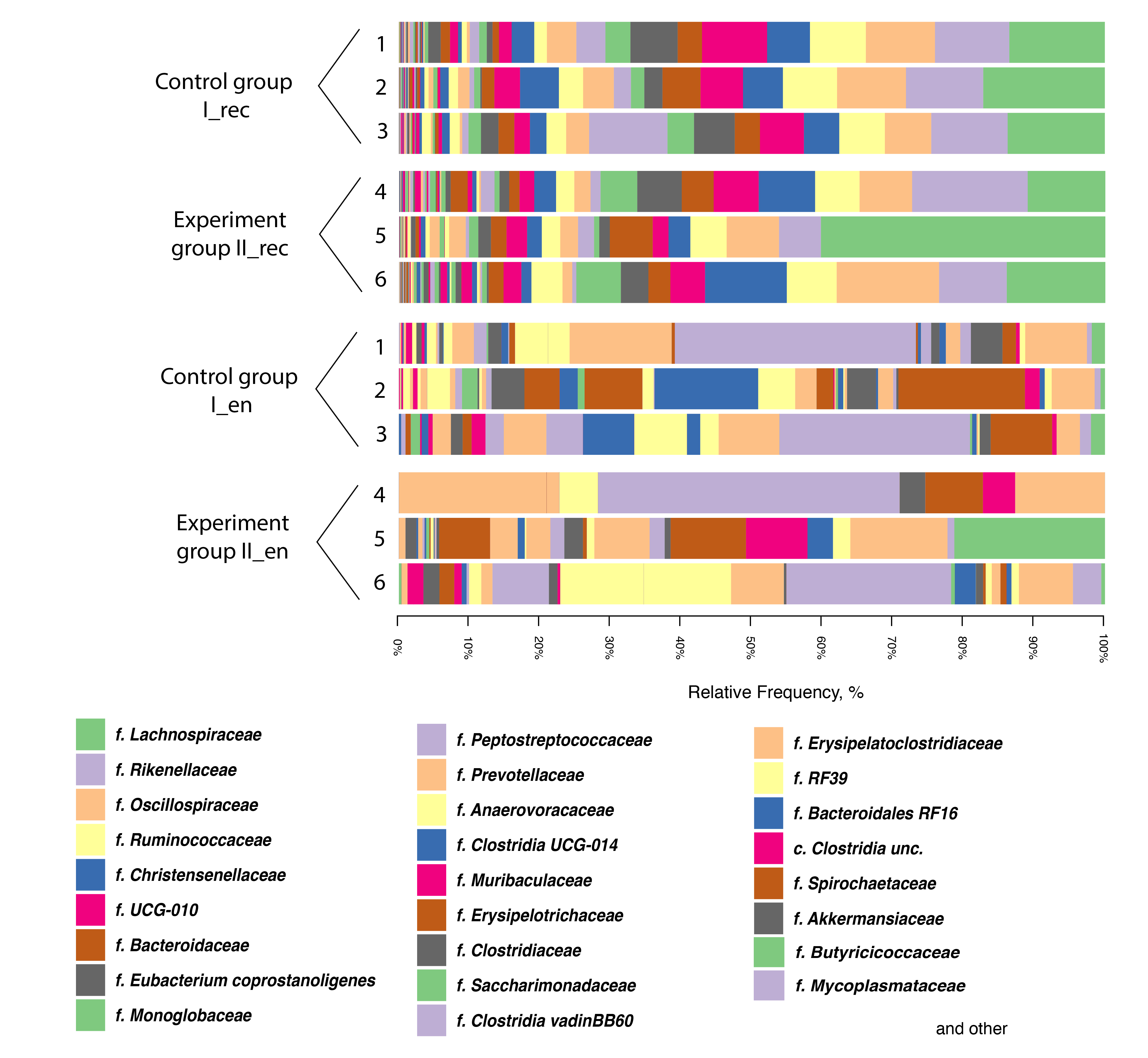 Fig. 4.
Fig. 4.
Taxonomic composition of the microbiota at the family level based on the NGS data for samples from the rectal chyme (_rec) and endometrium (_en) of dairy cows of the Holstein breed. 1–6, animal IDs; f., family; c., class.
The content of the Lactobacillaceae family bacteria in the intestinal chyme and endometrium of cows in both groups was at a very low level: no more than 0.08% in the chyme and no more than 2.6% in the endometrium (Fig. 4). On the one hand, the low number of normal flora representatives of lactobacilli in the endometrium of cows could be a consequence of antibiotic therapy, since antibiotics suppress some representatives of the normobiota [64]. On the other hand, the importance of lactobacilli in the reproductive tract of cows remains an open question. Their clear positive role in maintaining homeostasis and dominance in the reproductive system in healthy individuals has been established only in humans [65]. Indeed, a previous study of the vaginal microbiota of cows has demonstrated a unique composition with a low content of lactobacilli and a nearly neutral pH [66]. Unlike humans, the presence of the genera Enterococcus and Streptococcus, with a minimal proportion of Lactobacillus spp., has been demonstrated for cows [66], which is presumably associated with a higher (almost neutral) pH level compared to humans [67]. It is still not clear, however, whether this pH level is caused by a difference in the genotype or whether it is a consequence of the widespread reproductive system dysbiosis of cows. Indeed, based on human experiments, a decrease in the content of lactobacilli is usually accompanied by an increase in pH [68] — a common symptom of bacterial vaginosis.
While analyzing the effects of CFA, it is worth noting that the content of
representatives of Porphyromonas spp. decreased in the endometrium of
Group II cows by 5.3 times compared to Group I (p
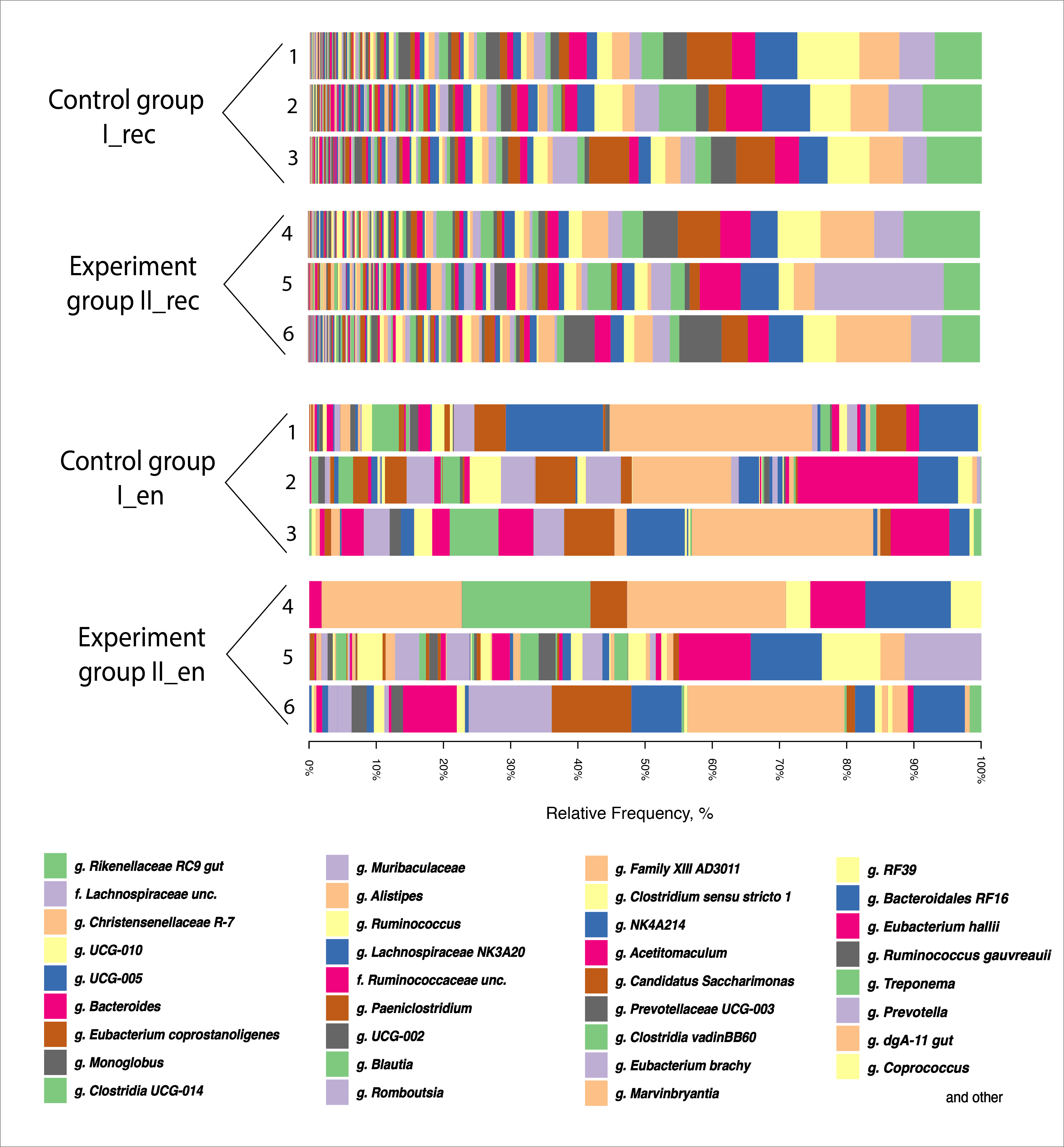 Fig. 5.
Fig. 5.
Taxonomic composition of the microbiota at the genera level based on the NGS data for samples from the rectal chyme (_rec) and endometrium (_en) of dairy cows of the Holstein breed. 1–6, animal IDs; g., genus; f., family; unc., uncultured.
Thus, the endometrial microflora composition was more sensitive to the introduction of CFA into the diet than the intestinal chyme microbiome. On the one hand, this may be due to the fact that the endometrial microbiota is characterized by a high level of response to changes in exogenous factors [73]. The composition of the reproductive system microbiota is extremely dynamic and varies significantly with age, genotype, and physiological factors such as monthly hormonal fluctuations and the immune system [74]. At the same time, the bacterial community of the intestinal chyme is more stable. Species-rich communities have been shown to be less susceptible to invasion because they use limited resources more efficiently, with different species specializing in a potentially limited resource [75]. It has been found that the gut microbiota often exhibits “colonization resistance”, i.e., the indigenous microbiota actively prevents the emergence of new potentially beneficial (probiotic) microorganisms [76]. On the other hand, changes in the composition of the gut microbiome under the influence of CFA could occur at the species level, affecting pathogenic forms present at relatively low concentrations, which is not always detectable by NGS sequencing. This could have a stimulating effect on the immune system, reduce the pathogen load, and have additional beneficial effects on endometrial health via the gut–vaginal axis.
Concerning possible adverse effects of the antibiotic treatment on the results in this experiment, the use of probiotic supplements along with antibiotic treatment is a fairly common practice. Concomitant use of biopreparations to restore the microbiota during antibiotic treatment helps normalize the microecological status of the body and increases the effectiveness of standard therapy. The chromosome of Bacillus spp. usually contains loci to control their resistance to antibiotics, including benzylpenicillin novocaine and dihydrostreptomycin sulfate [77]. In the case of intrinsic or acquired resistance (as a result of chromosomal mutation), the risk of transmitting antibiotic resistance genes is considered very low [78].
To establish whether alterations in the microbiota composition of the intestinal
chyme and endometrium in cows were associated with changes in its functionality,
we reconstructed and predicted the functional content of the microbial community
using the PICRUSt2 software package [44, 45]. As a result, 380 predicted gut
microbiome metabolic pathways and 370 endometrial microbiota pathways were
annotated. In general, the level of the predicted functional potential of 70
pathways changed (p
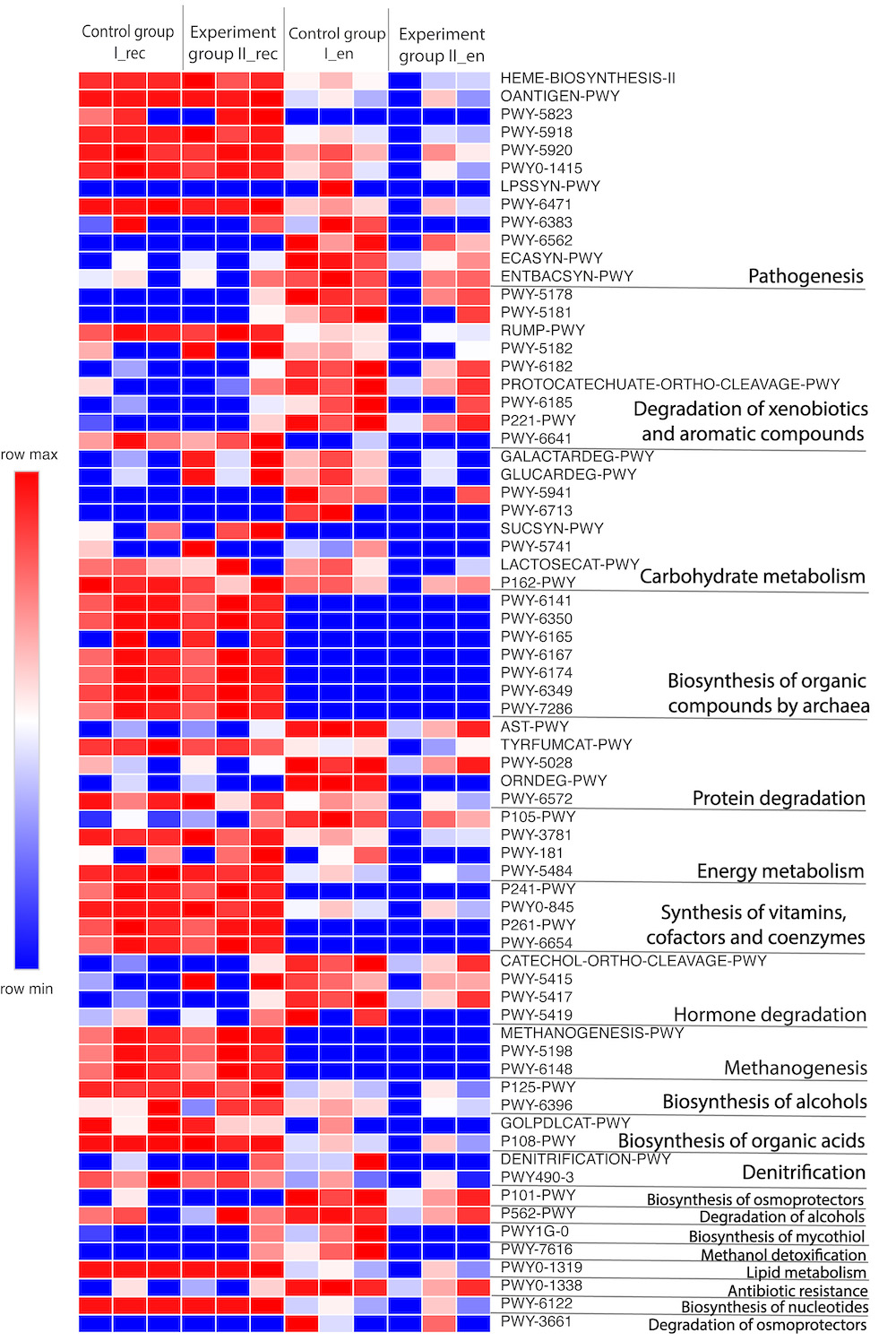 Fig. 6.
Fig. 6.
Data on the functional annotation of the metabolic pathways based on the NGS data for samples from the rectal chyme (_rec) and endometrium (_en) of dairy cows of the Holstein breed as analyzed using the PICRUSt2 software package.
When comparing the expression of the predicted metabolic pathways of the microbiome in different biotopes, it should be noted that some processes, such as methanogenesis (METHANOGENESIS-PWY, PWY-5198 and PWY-6148 pathways), were active only in the intestinal chyme, but not in the endometrium of cows. Indeed, methanogenesis is carried out by methanogenic archaea of the superphylum Euryarchaeota [79] that we did not identify in the endometrium of cows, unlike the digestive system. Therefore, it is natural that the biosynthetic pathways predicted in our study for the formation of archaetidylserine, archaetidylethanolamine, chorismite, flavin, mevalonate, cytidine diphosphate (CDP)-archaeol and 7-(3-amino-3-carboxypropyl)-wyosine, inherent in archaea, also showed activity only in the intestinal chyme, but not in the endometrium.
The most pronounced changes (p
It is known that in cows prone to inflammatory diseases of the uterus in the
early postpartum period, there is a predominance of progesterone concentration in
the blood serum, along with low estradiol levels [82]. On the other hand,
postpartum uterine infections have previously been shown to disrupt the integrity
of the endometrial epithelium by impairing the prostaglandin secretion, thereby
compromising folliculogenesis and impairing fertility [83]. We established in the
endometrium that the use of CFA (Group II) led to a less pronounced effect in of
four predicted metabolic pathways (CATECHOL-ORTHO-CLEAVAGE-PWY, PWY-5415,
PWY-5417, and PWY-5419) by 31–100% compared with Group I (p
In addition, in the endometrium of all Group II cows, no activation of the predicted metabolic pathway PWY1G-0 (mycothiol biosynthesis) was observed. In contrast, we observed the activity of this pathway in the endometrium of all Group I animals. This was probably due to the absence of representatives of the phylum Actinobacteriota in the endometrium of Group II cows (in contrast to Group I), as mentioned above, since mycothiol is a thiol found only in the taxon Actinomycetales (representatives of the phylum Actinobacteriota) [89]. The main role of mycothiol is to maintain intracellular redox homeostasis; it acts as an electron acceptor/donor and serves as a cofactor in detoxification reactions of alkylating agents, free radicals and xenobiotics. In addition, like glutathione, mycothiol may be involved in catabolic processes that play an important role in growth when consuming recalcitrant chemicals such as aromatic compounds [90].
The results of our metagenomic study provide additional information on the mechanisms of the possible influence of biological feed additives administered orally during the transit period on the intestinal and endometrial microbiota of cows (after calving) that are subject to antibiotic therapy. Intravaginal use of agents that affect the reproductive system microbiota usually does not influence the intestinal microbiota that is important for the homeostasis and resistance of the body as a whole. This effect can be achieved through the application of oral probiotics, such as CFA. The use of CFA made it possible to confirm the concept of a interconnection between the intestinal and uterine microbiota, since we observed changes in the composition and predicted metabolic pathways of the endometrial microbiota after oral administration of CFA. Previous studies have also shown that oral probiotics can help restore the reproductive system microbiota [17, 91, 92].
The composition and predicted metabolic pathways of the endometrial microbiota turned out to be more responsive to the introduction of CFA than the intestinal chyme microbiome. This is likely due to the fact that the endometrial microbiota is characterized by a high level of responsiveness to changes in external conditions [74], while the intestinal chyme bacterial community is characterized by a greater stability.
Our data suggest that the integrity of the microbiome of cows is essential for good reproductive health. They emphasize the need for an integrated approach that combines the correction of microecological disorders simultaneously in the intestines and in the reproductive system.
This article contains a presentation of all the experiments and findings from the study. Further details can be obtained upon request from the corresponding author.
Conceptualization, EAY and GYL; methodology, EAY, VAF and LAI; software, EAB and ESP; validation, EAB, ESP and EAY; formal analysis, EAB, ESP, MNR, DKG and EAY; investigation, TSS, AVD, ASD, KAK, IAK, VAZ; resources, TSS; data curation, EAY, NIN and DGT; visualization, ESP and AVD; supervision, GYL and DKG; project administration, EAY, MNR and GYL; funding acquisition, EAY and VAF; writing—original draft preparation, EAY; writing—review and editing, EAY, MNR and DKG. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study received ethical approval from the Bioethical Commission of the L.K. Ernst Federal Research Center for Animal Husbandry (Protocol No. 2023-10/1, dated October 31, 2023).
Not applicable.
The study was financially supported by the Russian Science Foundation (Grant No. 24-16-00131, Development of a New Biotechnological Approach to the Prevention and Treatment of Endometritis in Cows).
The authors declare no conflict of interest. EAY, GYL, LAI, EAB, TSS, VAF, KAK and IAK are employees of BIOTROF+ Ltd. TSS is an employee of JSC Gatchinskoe. The judgments in data interpretation and writing were not influenced by this relationship.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
