- Academic Editor
Climate change affects life on Earth. Meanwhile, microorganisms (unlike plants and animals) are usually not considered when studying climate change, particularly due to the impact of climatic fluctuation on them. A substantial variety of microbes and their responses to changing environmental conditions make determining their role in the ecosystem functioning very difficult. Nevertheless, microorganisms support the existence of all life forms on the planet. It is also important to know how microorganisms affect climate change and how this subsequently then affects microorganisms. Previous research demonstrates the leading role and importance of microorganisms in studying the biological aspects of climate change. Thus, this paper aimed to examine the correlation between nitrogen cycle microorganisms and climate change.
The nitrogen cycle microorganism (NCM) soil formed the primary research object, which, simultaneously, is not associative microflora and belongs to the following groups: amino heterotrophs using organic forms of nitrogen, aminoautotrophs using mineral forms of nitrogen, and diazotrophs fixing nitrogen in the air. The response of NCMs in simultaneously increasing atmospheric CO2, precipitation, temperature, and nitrogen in an artificially created agricultural soil ecosystem was investigated.
The NCM number and their structure responded to these simulated changes. The increased volume of nitrogen significantly changed the NCM structure, which depends on temperature and precipitation. The dominance of NCMs was noted when the temperature and precipitation remained unchanged. However, the number of microorganisms consuming mineral forms of nitrogen increased following a rise in temperature and a reduction in precipitation. Further, the proportion of microorganisms consuming organic forms of nitrogen increased following a decrease in temperature and increased precipitation. Total NCMs reduced significantly when the CO2 increased; this decrease was most pronounced with increased precipitation. Changes in the group composition of the community are associated with an increase in the nitrification process, with no changes in total NCMs.
These results illustrate that the ever-increasing concentration of CO2 in the atmosphere has a direct impact on both Earth’s climate and alters the composition and activity of microbial populations.
Anthropogenic interference with the environment already leads not only to
pollution (chemical, radiation, physical, biological, etc.) but also to climate
change. Under the influence of global climate change, changes occur in the
biosphere, including the soil. Climate possesses a direct impact on soil
formation, being one of the primary soil formation factors. It determines the
hydrothermal regime and energy level of the soil. Furthermore, the climate
indirectly affects other factors of soil formation (parent or soil-forming rocks,
plants and living organisms, etc.). The total number of microorganisms in
terrestrial habitats is approximately 3
The authors have decided to focus on the nitrogen cycle microorganisms (NCMs) freely living in soils as a model. This group of microorganisms has not been chosen by chance. It is an ideal model for studying the global nitrogen cycle. NCMs are widespread in nature. They can be found in soil, freshwater, and seawater. They are of great environmental importance; namely, they play a decisive role in the fate of nitrogen in the ecosystems of Earth. Nitrogen is considered to be the most crucial nutrient in productivity regulation and species diversity in all ecosystems of Earth [4]. Most nitrogen conversions such as nitrogen fixation, nitrification, and denitrification are regulated by microbial-controlled processes. However, as the global population continues to grow, human impact has already substantially altered the global nitrogen cycle [5]. Human impact includes the increase in CO2 in the atmosphere from fuel combustion or agriculture. The latter also leads to the deposition of nitrogen-containing compounds in soils. As a result of vigorous human activity, the air temperature, the amount, and the quality of precipitation change [6, 7]. It is evident that numerous global changes can interact to alter the microbial community of soils. However, how microbial communities respond to such changes is a bit unclear. It has been proven that the set of properties of microbial communities (for example, microbial respiration) can be changed by individual global shifts [8]. There is some evidence that the number of microbial plant symbionts changes with the increase in CO2 [9]. Volatile compounds produced by bacteria are able to act as both intra- and interspecies regulators of microbial communities [10]. It has also been illustrated that the composition and number of soil microorganisms change in response to the application of agricultural fertilizers and substantial fluctuations in soil temperature and moisture [11, 12, 13]. It is unclear, however, whether the NCM abundance and diversity in soils can change under the influence of multifactorial global changes.
The research will help approach the understanding of the contribution of climatic fluctuations to the development of the nitrogen cycle microorganisms (NCM), in particular, and to the development of the microbial community in the soil as a whole. The practical meaning of the paper lies in the ability to regulate NCMs to enrich the soil with available nitrogen, treat it, and, consequently, increase productivity.
The research goal is to detect changes in the structure of nitrogen cycle microorganisms (NCM) in soil. Tasks that contribute to the achievement of the mentioned goal include investigating the effect of carbon dioxide, humidity, nitrogen precipitation, and temperature rise (both individually and in various combinations) on NCMs.
The research object is the NCM soil, which, simultaneously, is not associative microflora and belongs to the following groups: amino heterotrophs using organic forms of nitrogen, aminoautotrophs using mineral forms of nitrogen, and diazotrophs fixing nitrogen in the air.
The authors have investigated the NCM reaction (separately for aminoheterotrophs, aminoautotrophs and diazotrophs) to a simultaneous increase in the atmospheric CO2, precipitation, temperature, and nitrogen in an artificially created ecosystem of agricultural soil.
The authors have artificially created various environmental changes that can
affect the ecosystem in soils, as described in one of the early jobs [14]. The
soil (monolith) was collected in a fiberglass vessel (30
In a hermetically sealed block adding carbon dioxide to the air with a target concentration of 700 ppm. A gas cylinder was used, which was supplied into the vessel through a special hole under the control of a pressure gauge. The plots were fumigated continuously for 30 days. In the Earth’s atmosphere, the concentration of CO2 is, according to various sources, 200–500 ppm [15, 16]. The concentration of carbon dioxide depends on the place and time. In our experiments, the control variants had a CO2 indicator of 350 ppm, so in the experimental variant we doubled the concentration of CO2 to 700 ppm. In addition, we took into account the intermediate forecasting model (RCP 6.0) by 2100 [17] and trends in the concentration of carbon dioxide in the atmosphere according to the National Oceanic and Atmospheric Administration, USA [18].
To moisten the soil, a cap with special holes for water supply was built over the vessel. Quadrants of increased rainfall received increased (by 50% and 75%) moistening of the soil through overhead sprinklers plus drip tubes. Increased hydration was monitored on an ongoing basis and maintained at a given level for 30 days. Volumetric soil moisture was measured using an Aqua-Lab M20SO1 moisture meter (Aqua-Lab, Moscow, Russia) by placing the probe into the soil to a depth of 10 cm (in the middle of the layer being studied). Researchers are actively studying the effects of different levels of soil waterlogging on microbial communities and their functions [19, 20, 21]. We chose two levels (50% and 75%) due to their greatest universality and averageness.
The temperature was increased as suggested in the works of other researchers [22, 23, 24]. Warming of 20 °C relative to the control inside the unit was achieved using infrared ceiling heaters equipped with reflective domes for uniform heat distribution. The soil temperature was measured continuously using a TESTO 103 thermometer (Testo, Lenzkirch, Germany) by placing the probe into the soil to a depth of 10 cm (in the middle of the layer being studied). During 30 days of the experiment.
Determination of the quantitative composition of soil NCM was carried out by sowing dilutions of soil suspension on solid elective media. For one repetition of one experimental group, 10 g of soil was removed sterilely (over an alcohol lamp) and placed in 100 mL of sterile saline solution. The flask was shaken for 20 minutes at room temperature and 150 rpm on an Elmi S-3.02L.A20 shaker (Elmi, Riga, Latvia) to separate microbial cells from soil particles. After completion of the process, the suspension from the flask was plated onto agar selective media: fishmeal hydrolyzate (FHM), starch-ammonia agar (SAA) and Ashby agar, intended for the isolation of aminoheterotrophs, aminoautotrophs and nitrogen fixers, respectively. GRM agar (nutrient agar based on fish meal hydrolysate) used ready-made media produced in Obolensk, Russia. Starch ammonium agar and Ashby agar were prepared independently according to the preparation instructions.
GRM agar, Starch-ammonia agar and Ashby’s agar were prepared independently
according to preparation instructions. GRM agar had the following composition, g:
pancreatic hydrolysate of fish meal—12.0 g, dry enzymatic peptone—12.0 g,
sodium chloride—6.0 g, microbiological agar—10.0
The cultures were incubated in a thermostat for 3–5 days at a temperature of 26 °C. After incubation, the grown colonies were counted.
All calculations were carried out in the STATISTICA v-13.3.0
The NCM number and structure have responded to these simulated changes (Fig. 1).
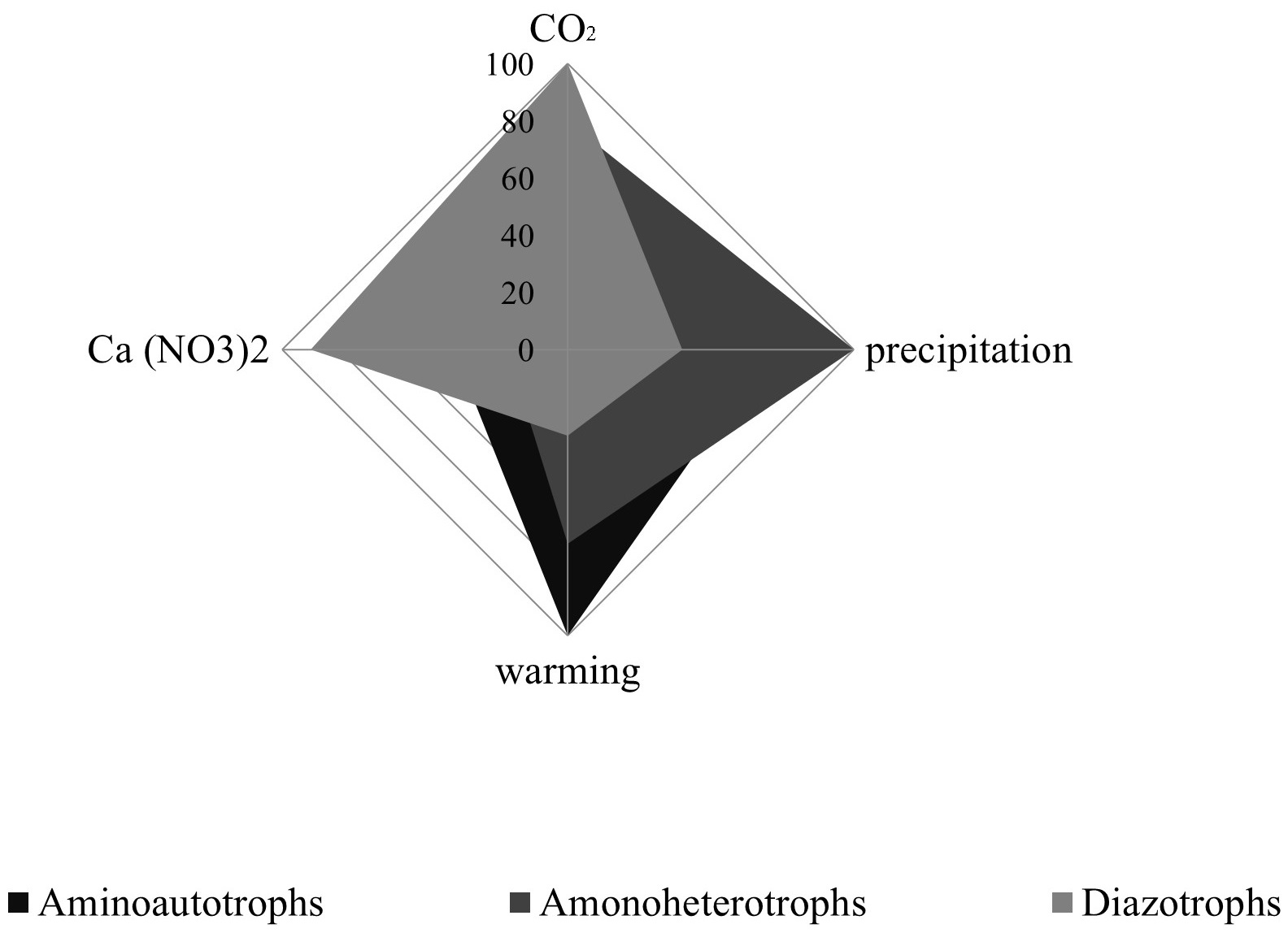 Fig. 1.
Fig. 1.
Multifactorial global induced changes in the nitrogen cycle microorganism (NCM) community.
We observed significant effects of the multivariate global change treatments on the community structure and abundance of NCM. When considering the main effects across all treatments, the abundance of aminoautotrophs tended to increase under elevated temperature (+376.32%) and decreased under elevated precipitation and carbon dioxide (–41.86 and –54.31%, respectively). The effect of N on aminoautotroph abundance was weak and did not increase significantly under high N (+11.11%) (Table 1). In contrast, the amplitude of the response of diazotrophs to the main global change treatments was generally lower than that of aminoautotrophs (Table 1), except for the effect of N. The influence of temperature, precipitation and carbon dioxide on the number of diazotrophs was weak: temperature had a slightly positive effect (+12.39%), while increased precipitation and carbon dioxide had a slightly negative effect, decreasing the number of diazotrophs by –13.51% and –18.92%, respectively. The influence of all the studied factors on the number of aminoheterotrophs was quite strong and relatively equally opposite to each other. Increased precipitation and carbon dioxide contributed to an increase in the number of aminoheterotrophs (+66.67 and +83.33%, respectively), while increased precipitation and nitrogen contributed to approximately the same decrease in the number of aminoheterotrophs (–64.36 and –79.26%, respectively). In addition, it is important to note that for aminoheterotrophs we established effects opposite to those observed on the number of aminoautotrophs.
| Treatments | Aminoautotrophs | Amonoheterotrophs | Diazotrophs |
| Ca(NO3)2 | +11.11 | –79.26 | +476.92 |
| Warming | +376.32 | –64.36 | +12.39 |
| Precipitation | –41.86 | +66.67 | –13.51 |
| CO2 | –54.31 | +83.33 | –18.92 |
| Ca(NO3)2 + warming | +1344.44 | –88.15 | –66.35 |
| Ca(NO3)2 + precipitation | –33.33 | –11.11 | +15.38 |
| Ca(NO3)2 + CO2 | +39.47 | +10.15 | –31.30 |
| Warming + precipitation | +10.15 | –20.63 | +94.33 |
| Warming + CO2 | +54.47 | –44.44 | –12.16 |
| Precipitation + CO2 | –58.14 | +16.67 | –40.54 |
n = 6 per treatment, all the effects are significant (p
The increased nitrogen amount has significantly changed the NCM structure, which depends on temperature and precipitation (Fig. 2). The dominance of nitrogen-fixing microorganisms (up to 80% of the total amount of NCM) is noted when the temperature and precipitation do not increase. With an increase in temperature and a decrease in precipitation, the number of microorganisms consuming mineral nitrogen forms has increased (up to 83% of the total amount of NCM). With a decrease in temperature and an increase in precipitation, the proportion of microorganisms consuming organic nitrogen forms has increased (up to 79% of the total amount of NCM). Total NCMs has decreased significantly with an increase in CO2. This decrease is most pronounced with an increase in precipitation.
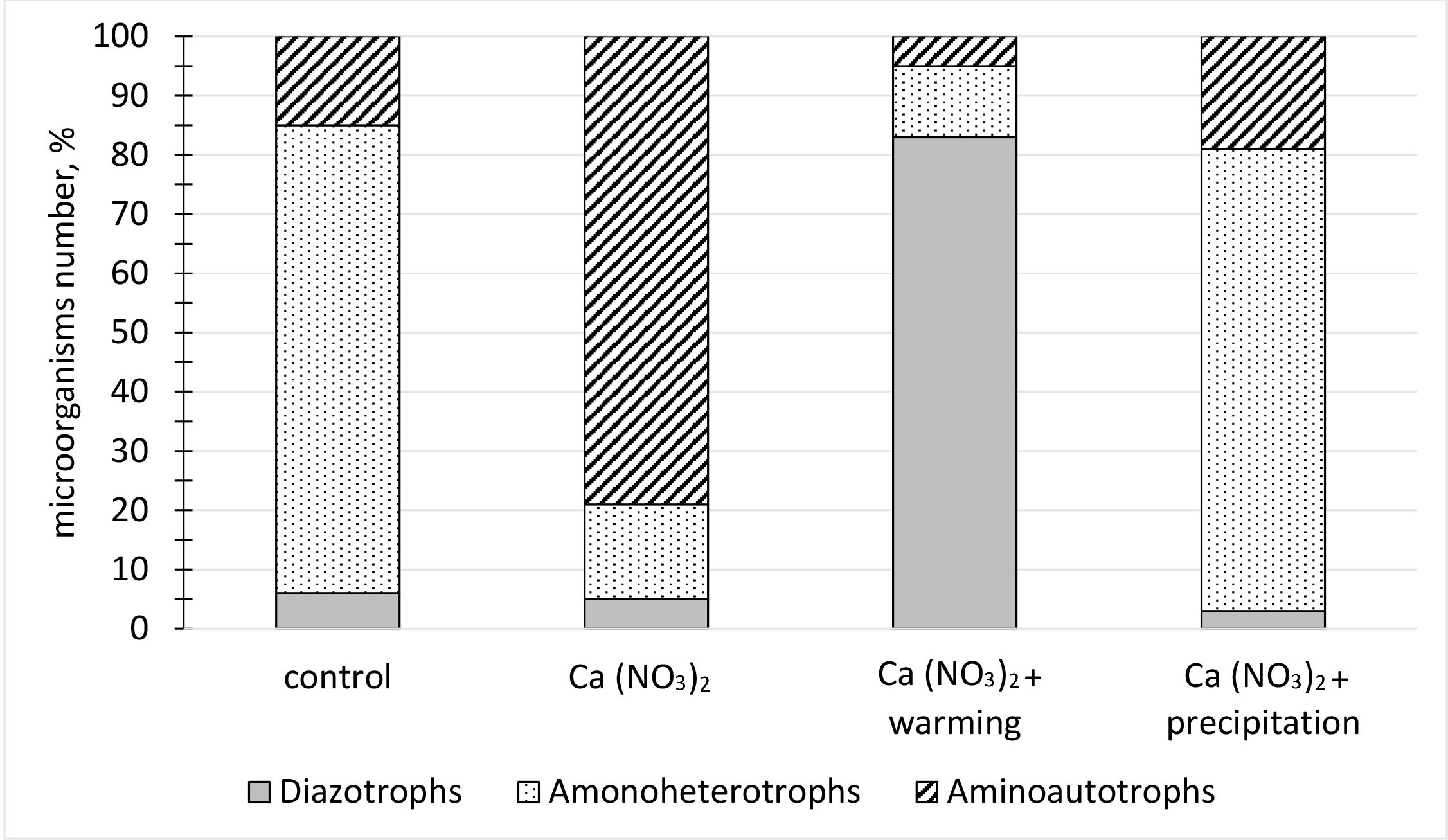 Fig. 2.
Fig. 2.
NCM composition in the studied soils.
According to the calculation of the Wilcoxan T-test, the addition of Ca(NO3)2 alone does not affect the amount of NCM in the soil (does not differ from the control), while Ca(NO3)2 + warming and Ca(NO3)2 differ from the values in the control (Fig. 3).
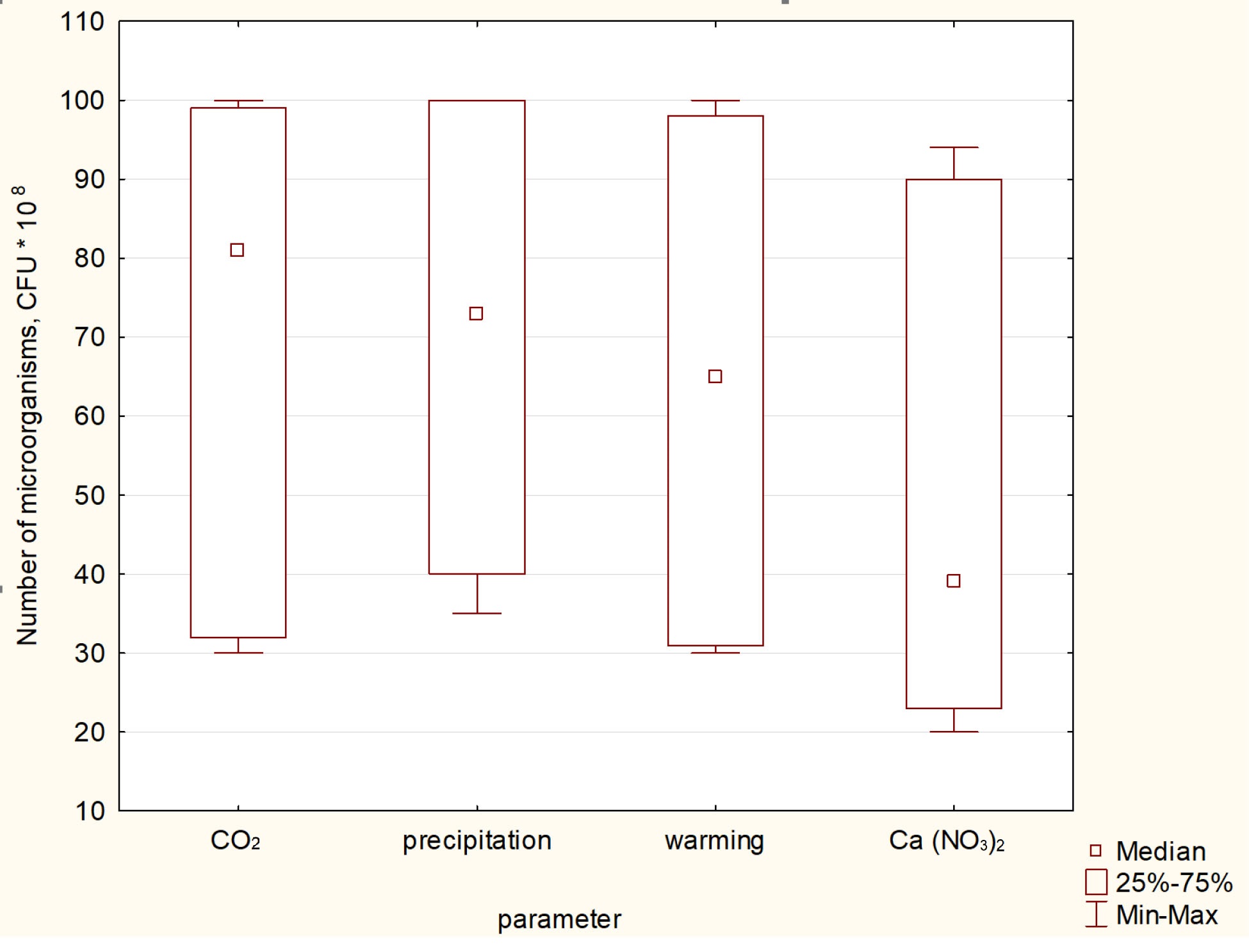 Fig. 3.
Fig. 3.
The Wilcoxan test Mean values are presented with the
corresponding standard errors (n = 6, p
Possible mechanisms for the rational use of the mentioned results. The authors assume that the total microbial population in the soil can indirectly influence the change in the total NCM amount that the authors have observed in response to the introduced factors. The authors have recorded that the increased soil moisture changes the abundance of total NCMs both positively and negatively (Fig. 4). Strong humidification negatively affects the number of microorganisms, but the presence of warming, which obviously leads to the evaporation of excess moisture, contributes to an increase in the number of microorganisms.
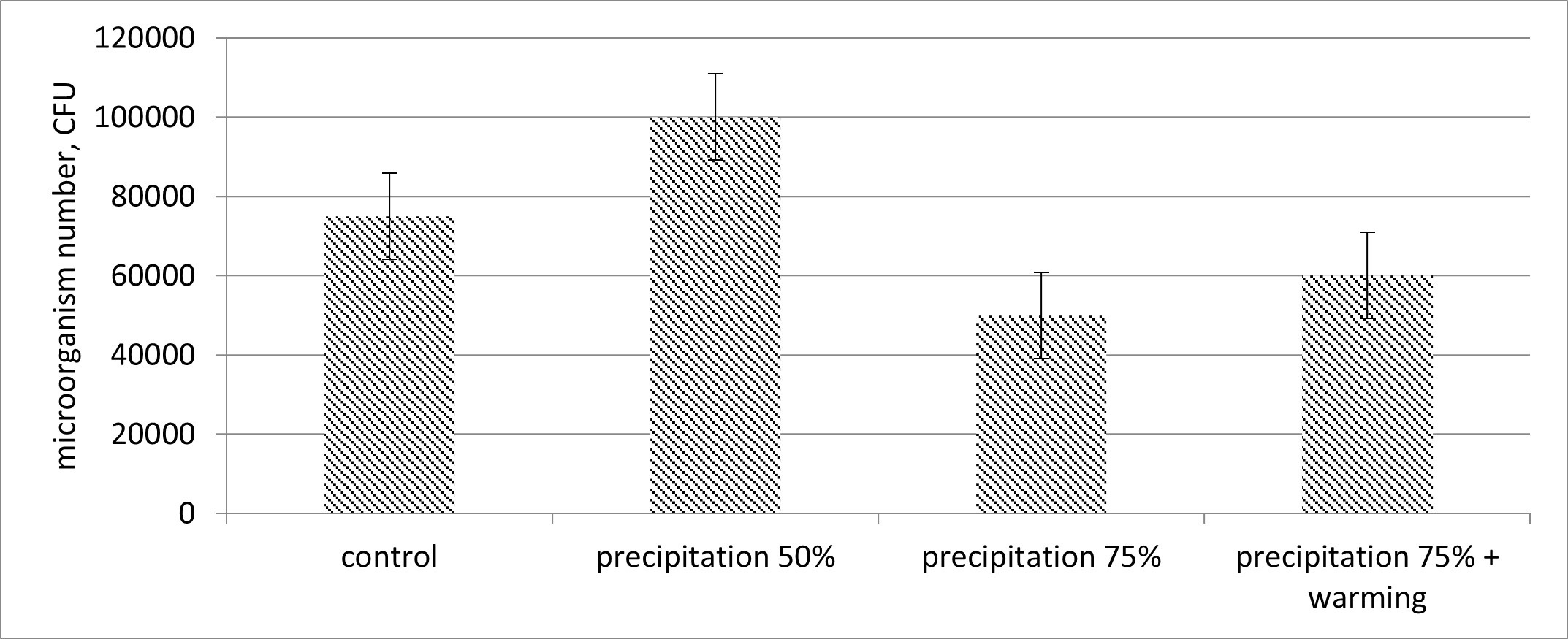 Fig. 4.
Fig. 4.
The level of nitrogen cycle microorganisms in soils depends on
the precipitation amount (colony forming units, CFU). Mean values are presented
with the corresponding standard errors (n = 6, p
An increase in the microorganism number can occur due to a decrease in water stress with a relatively moderate increase in soil moisture [26, 27]. We see this increase at 50% humidity. A greater increase in soil moisture can reduce the abundance by reducing the oxygen diffusion into the soil [26]. Therefore, the optimum soil moisture reflects a steady-state between these two parameters. This steady-state may be the leading one in this interaction, which the authors have established in the process of studying many factors. The elevated temperature reduces water stress and increases the oxygen to the soil access, since a humidity increase leads to a net negative effect on the NCM amount due to the reduced oxygen availability. From Fig. 4 we see that increased temperature reduces water stress (75%) and increases the total amount of NCM in the soil relative to the variant with increased moisture by 75% without increasing the temperature. In general, increasing the moisture by 75% leads to a total negative effect on the amount of NCM.
We found that total NCM decreased with increasing soil temperatures leading us to conclude that both soil moisture and temperature may limit microbial activity in this ecosystem (Fig. 5). Another study found that increased soil temperatures can decrease microbial biomass N and microbial activity measured as microbial respiration [28].
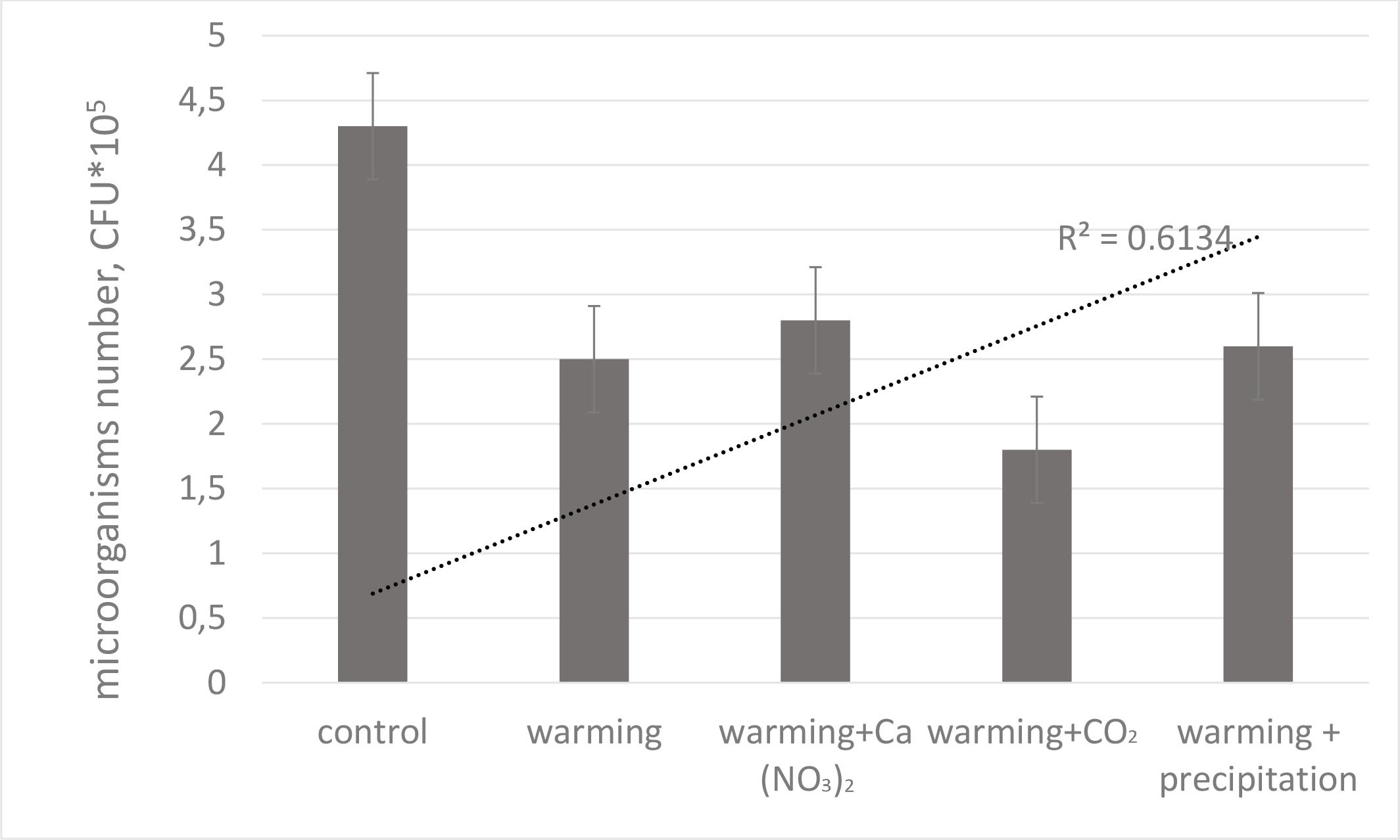 Fig. 5.
Fig. 5.
Reaction of individual microorganism groups to the warming
addition with different treatments (colony forming units, CFU). Mean values are
presented with the corresponding standard errors (n = 6, p
Perhaps, although it is unlikely, the carbon dioxide effect is also mediated through soil moisture. Carbon dioxide adds to soil moisture due to changes in the overall activity of the soil microbial community. Some authors point out that much of the global variability in modeled land carbon uptake is due to temperature and vapor pressure deficit effects, which are controlled by soil moisture [29, 30].
The authors observe a total NCM similar reaction to carbon dioxide and increased soil moisture (Fig. 6). As for individual groups, we see a trend toward an increase in the number of aminoheterotrophs and a decrease in the number of aminoautotrophs. Our approximation reliability values are 0.8057 (aminoheterotrophs) and 0.8784 (aminoautotrophs), which is a fairly acceptable result, characterizing the smoothing as reliable.
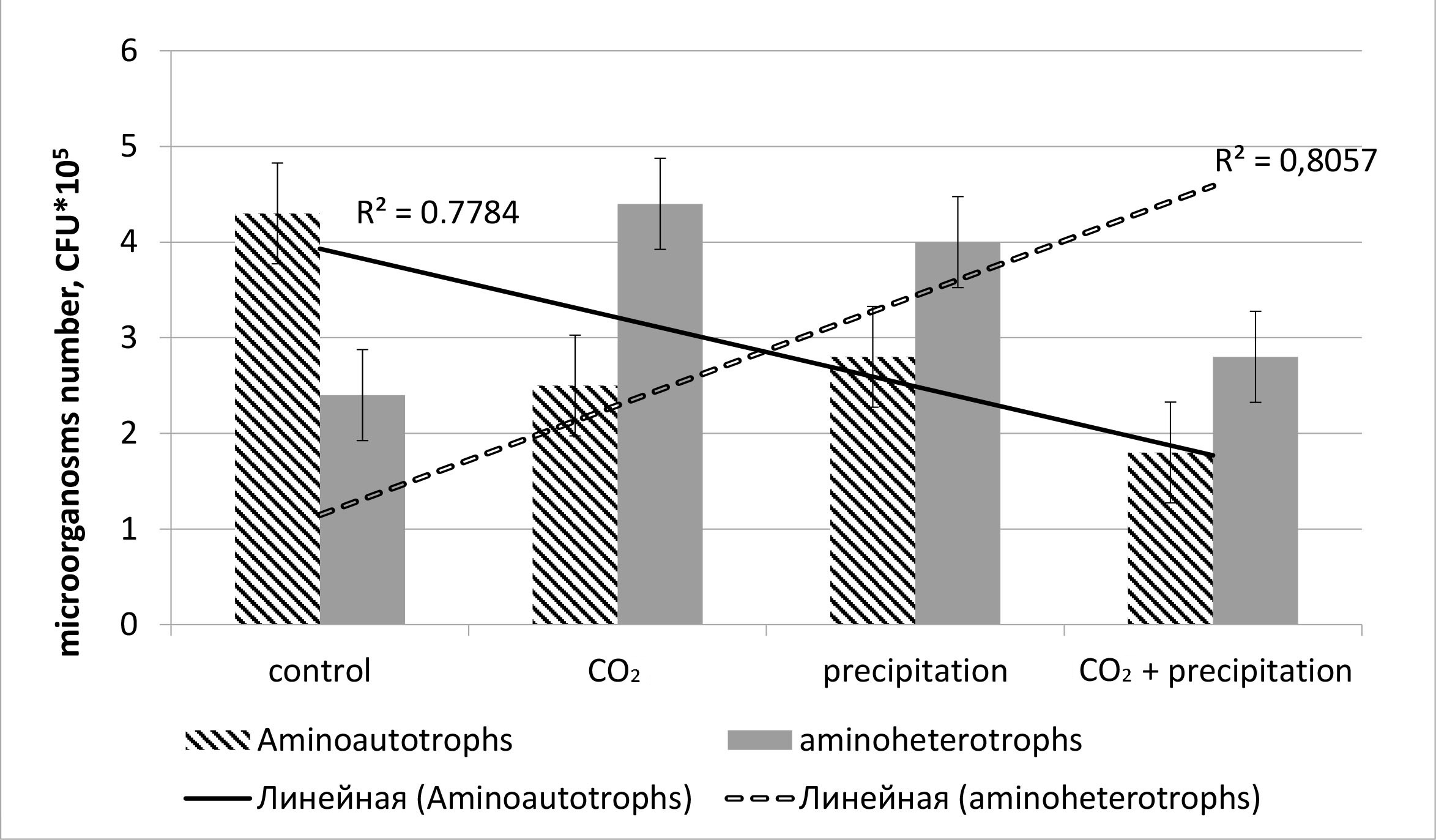 Fig. 6.
Fig. 6.
Reaction of individual microorganism groups to the CO2 or
precipitation addition (colony forming units, CFU). Mean values are presented
with the corresponding standard errors (n = 6, p
The increased carbon dioxide amount changes the resource competition intensity between total NCM and heterotrophic microorganisms. The increased carbon dioxide level adds to the activity and number of heterotrophs in the soil [31, 32]. Total NCMs are inferior competitors for some resources (for example, oxygen) [26] for heterotrophic microorganisms, and exasperating resources competition may cause a decrease in the total NCM number with the elevated carbon dioxide content. This decrease may be aggravated by an addition in the precipitation amount if it also reduces the marginal resource concentration (Fig. 6), either by leaching (for mobile nutrients) [27] or by decreasing diffusion (in the case of oxygen) [26]. Precipitation and high humidity contribute to a two-fold increase in the carbon dioxide content in the surrounding atmosphere [33]. Presumably, it is the primary factor in reducing the aminoautotroph number in a multifactor experiment.
The change in the aminoautotrophs amount that the authors have observed in the nitrogen addition reaction is probably due to the nitrate direct effect on the soil. In the literature, there are indications that elevated precipitation can reduce the available nitrogen [34]. Simultaneously, if the elevated temperature leads to soil moisture decrease [35, 36], then with an increased precipitation amount, the amount of available nitrogen decreases at a fast rate [37]. The loss of soil carbon is also accelerated by the increase in temperature [38]. Warmer soil temperature will accelerate soil processes, rapid decomposition of organic matter, increased microbiological activity, quicker nutrients release, increase nitrification rate and generally accentuate chemical weathering of minerals [39]. Overall, climate change will disrupt the equilibrium, both directly and indirectly, with respect to NCM. These effects can underlie the interactions between precipitation, temperature, and nitrogen introduction.
The results show that the global multifactor changes can consistently change the microbial community. The qualitative and quantitative ratio of NCMs can vary depending on the amount of precipitation, nitrogen and carbon dioxide, and the level of humidity and ambient temperature. Such changes in the microbial community can possess severe responses and disrupt the operation of the entire system. The group of bacteria studied in the paper represents a significant part of the microbial diversity of Earth. Members of this group are the primary shapers of the ecosystem functioning [40]. Changes in the microbial diversity of NCM (both quantitative and qualitative) can be caused by global climate change in general and in particular: increased precipitation, temperature, carbon dioxide, and nitrogen. From the functionality low degree, the nitrogen cycle microorganisms and, in particular, aminoautotrophs are especially vulnerable to the changes. The current research shows that fully understanding the environmental impact of global change requires comprehending the response of the microbial community as one of the most rapidly changing factors. Soil carbon emissions depend on more than just a few parameters, so when conducting these studies, all factors that affect emissions should be taken into account. Only then will the results be definitive. Otherwise, some aspects of the study will be missing.
NCM, nitrogen cycle microorganisms; CFU, colony forming units.
The datasets used and/or analyzed during the current study are available from the author on reasonable request.
MS completed the entire work from research development to writing the manuscript.
Not applicable.
The author thanks the technical service of the Federal Scientific Center for Biodiversity, Far Eastern Branch of the Russian Academy of Sciences, for assistance in creating an artificial ecosystem of agricultural soil.
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme No. 124012400285-7).
The author declares no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
