-
- Academic Editor
-
-
-
Transcatheter aortic valve replacement (TAVR) has emerged as the preferred treatment for symptomatic severe aortic stenosis (AS). However, China’s unique patient population presents distinct challenges, including a higher prevalence of bicuspid aortic valves (BAVs) and severe valve calcification. This study used real-world clinical data from Chinese patients to assess the safety and efficacy of the SAPIEN 3 balloon-expandable transcatheter heart valve (THV) in TAVR, particularly in patients with BAVs.
This retrospective, multicenter study enrolled consecutive severe AS patients treated with SAPIEN 3 THVs via a transfemoral approach from June 2020 to March 2024. The primary endpoint was 30-day mortality, while secondary endpoints included procedural mortality, procedural success, conversion to surgery, coronary artery occlusion, THV-in-THV deployment, permanent pacemaker implantation, and paravalvular leaks (PVLs).
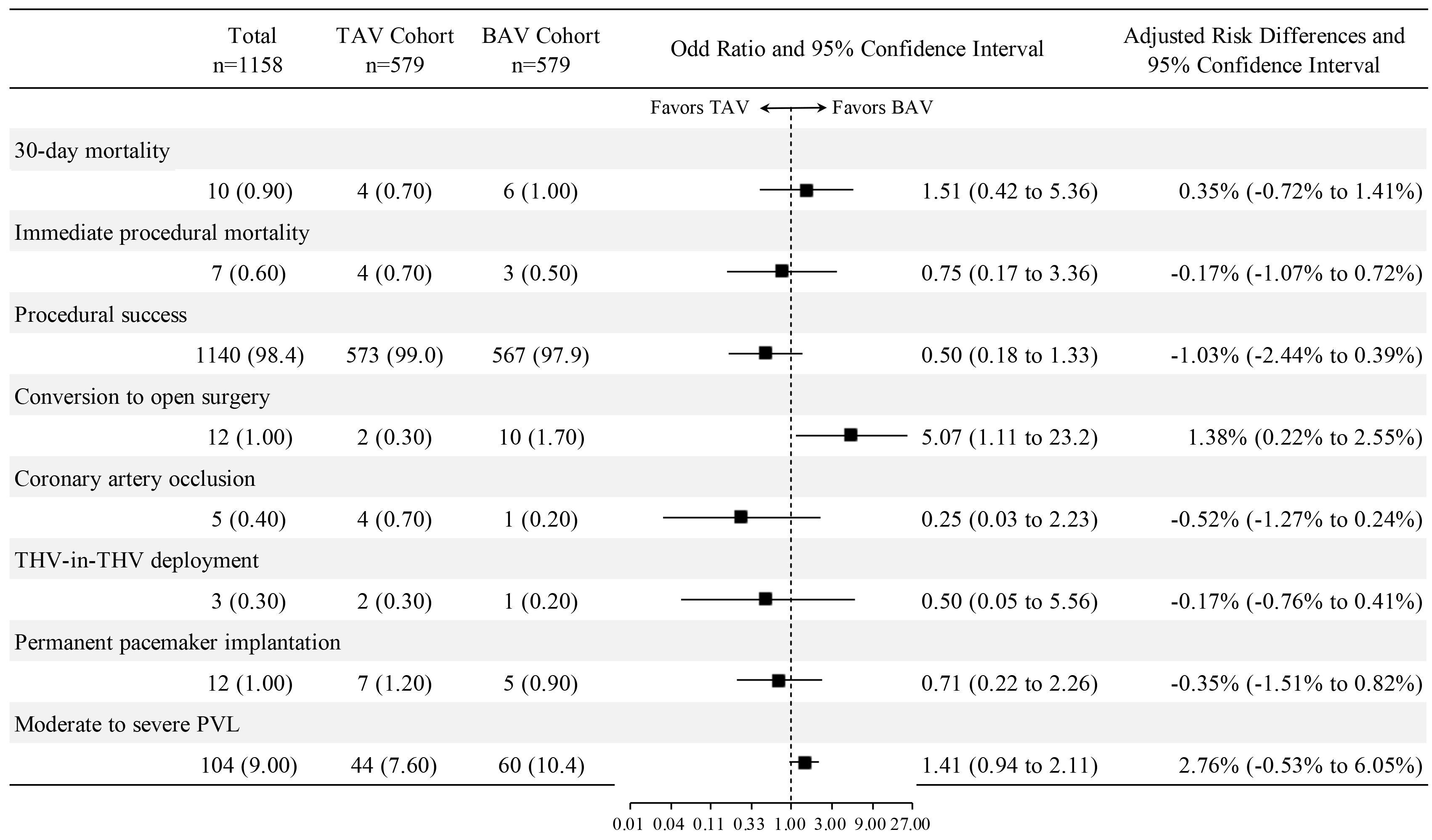
Among the 1642 enrolled patients, 56.0% had BAVs, and 44.0% had tricuspid aortic valves (TAVs). The 30-day mortality rate was 0.90%. Propensity score matching revealed no statistically significant differences between patients with BAVs and TAVs in terms of 30-day mortality (odds ratio (OR): 1.51, 95% confidence interval (CI): 0.42 to 5.36; p = 0.531), immediate procedural mortality, procedural success, coronary artery occlusion, THV-in-THV deployment, permanent pacemaker implantation, or moderate to severe PVLs. However, a significant difference was found in the conversion rate to open surgery (OR: 5.07, 95% CI: 1.11 to 23.2; p = 0.036).
This study demonstrates the safety and feasibility of SAPIEN 3 balloon-expandable THVs in TAVR for Chinese patients with severe AS, including those with BAV stenosis. These findings challenge historical relative contraindications for TAVR in BAV patients and highlight the potential of TAVR in diverse patient populations. Larger prospective studies with extended follow-ups are needed to refine patient selection and evaluate longer-term outcomes.