Although the knowledge of sports cardiology advanced significantly in the recent
years, the molecular mechanisms by which exercise training augments cardiac
performance is poorly understood. Here we aimed at determining left ventricular
(LV) myocardial sarcomeric protein modifications in a rat model of exercise
training and detraining. Young male Wistar rats were divided into exercised (Ex)
and control (Co) groups. Trained rats swam 200 min/day for 12 weeks. Detrained
(DEx) and control (DCo) rats remained sedentary for 8 weeks after completion of
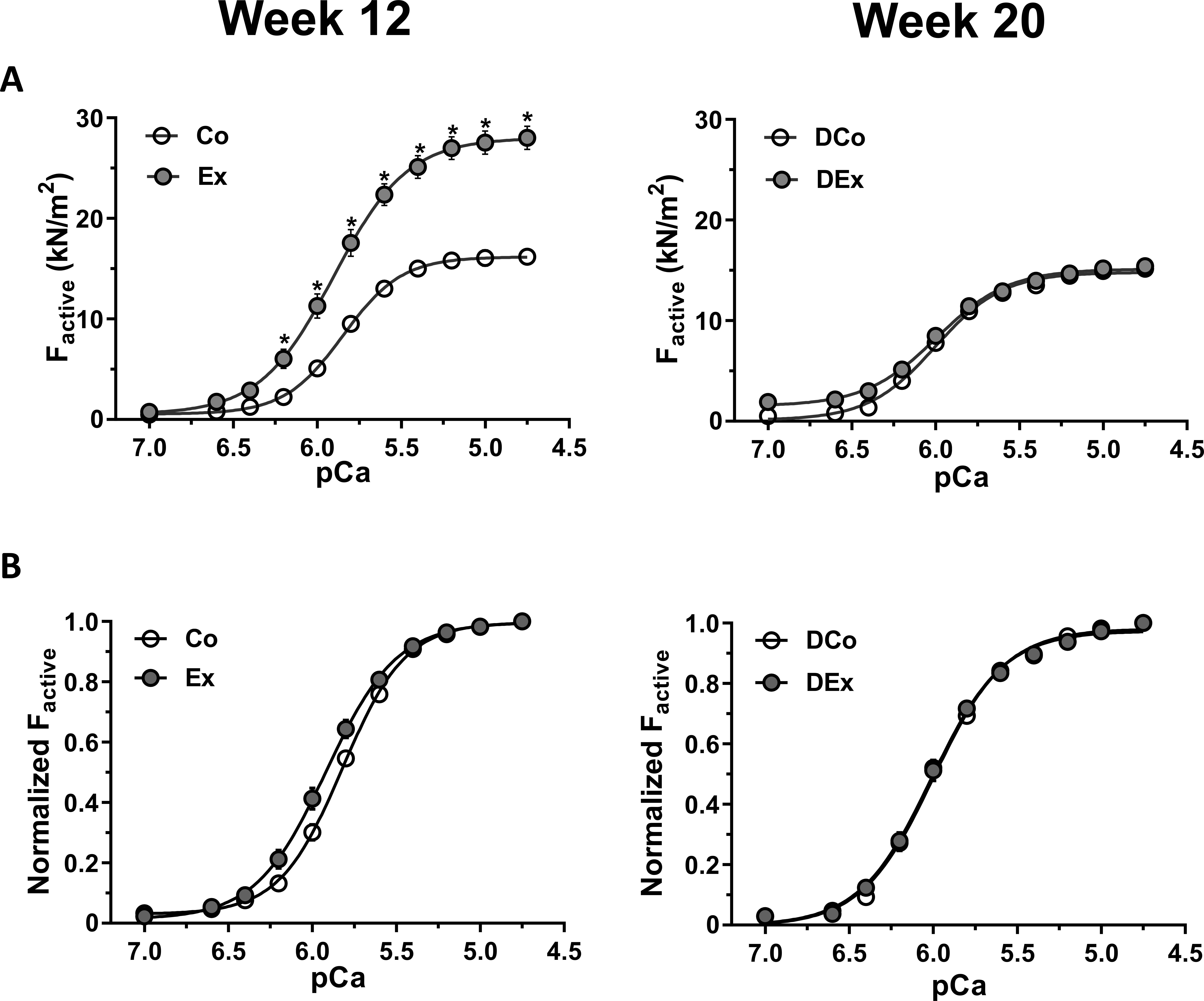
the 12-week-long protocol. Ca-regulated active force production
(F), its Ca-sensitivity (pCa) and Ca-independent
passive tension (F) were determined in isolated permeabilized
cardiomyocytes and phosphorylation levels of sarcomeric proteins were assayed by
biochemical methods. Means of maximal Ca-activated isometric force
(F) and pCa values were higher (p 0.05) in the Ex
group (28.0 1.4 kN/m and 5.91 0.03, respectively, mean
SEM) than those in the Co group (15.8 0.8 kN/m and 5.81
0.03, respectively). F did not differ between these two
groups. The level of cardiac troponin I (cTnI) phosphorylation decreased upon
exercise (from 1.00 0.02 to 0.66 0.06, p 0.05; in
relative units). Site specific phosphorylation assays revealed cTnI
hypophosphorylations at the protein kinase A (PKA)-specific Ser-22/23 sites and
at the protein kinase C (PKC)-specific Thr-143 site. Mechanical and biochemical
parameters of the DEx and DCo groups did not differ from each other following the
detraining period. Exercise-induced hypertrophy is associated with reversible
increases in Ca-dependent force production and its Ca-sensitivity
in LV cardiomyocytes, which can be associated with changes in cTnI
phosphorylation.