-
- Academic Editor
-
-
-
Thalamic hemorrhage (TH) is a severe neurological condition, the molecular mechanisms of which are poorly understood, particularly in clinical settings. N-acetyltransferase 10 (NAT10), a regulator of RNA N4-acetylcytidine (ac4C) modification, has been implicated in cell cycle regulation and identified as a potential therapeutic target. This study explored the effects of NAT10 inhibition on TH pathology using a multi-omics approach.
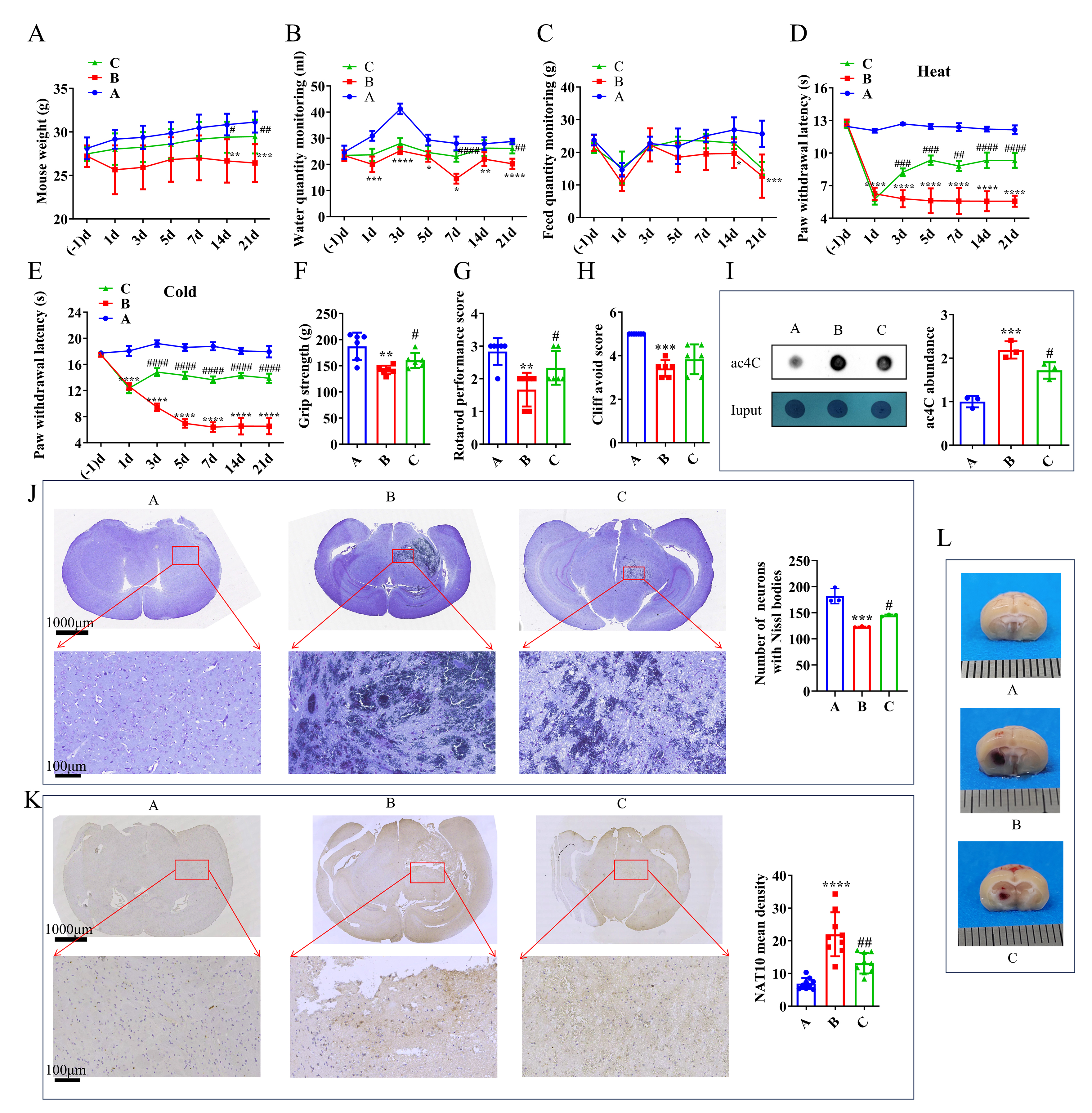
A mouse model of TH was established via collagenase IV injection. NAT10 activity was detected by dot blot and inhibited using Remodelin. Comprehensive multi-omics analyses, including 16S ribosomal Deoxyribonucleic Acid (16S rDNA) sequencing, metabolomics, and transcriptomics, were used. Behavioral, histological, and molecular evaluations were conducted to evaluate the key genes.
A total of 35 hub genes, 30 hub metabolites, and 28 hub microorganisms associated with NAT10 inhibition were identified. Among them, the xanthine dehydrogenase (XDH) and guanine deaminase (GDA) genes were linked to xanthine, which is a key metabolite implicated in TH pathology. Based on these findings, the xanthine oxidase inhibitor febuxostat was tested, demonstrating significant therapeutic benefits in TH-affected mice. Behavioral, histological, and molecular evaluations confirmed that NAT10 inhibition alleviated TH-induced damage.
This study provides the first comprehensive molecular insights into the therapeutic potential of NAT10 inhibition in TH. Moreover, it identified NAT10 inhibitors and febuxostat as promising candidates for TH management, paving the way for future therapeutic development targeting NAT10 in this condition.