-
- Academic Editors
-
-
-
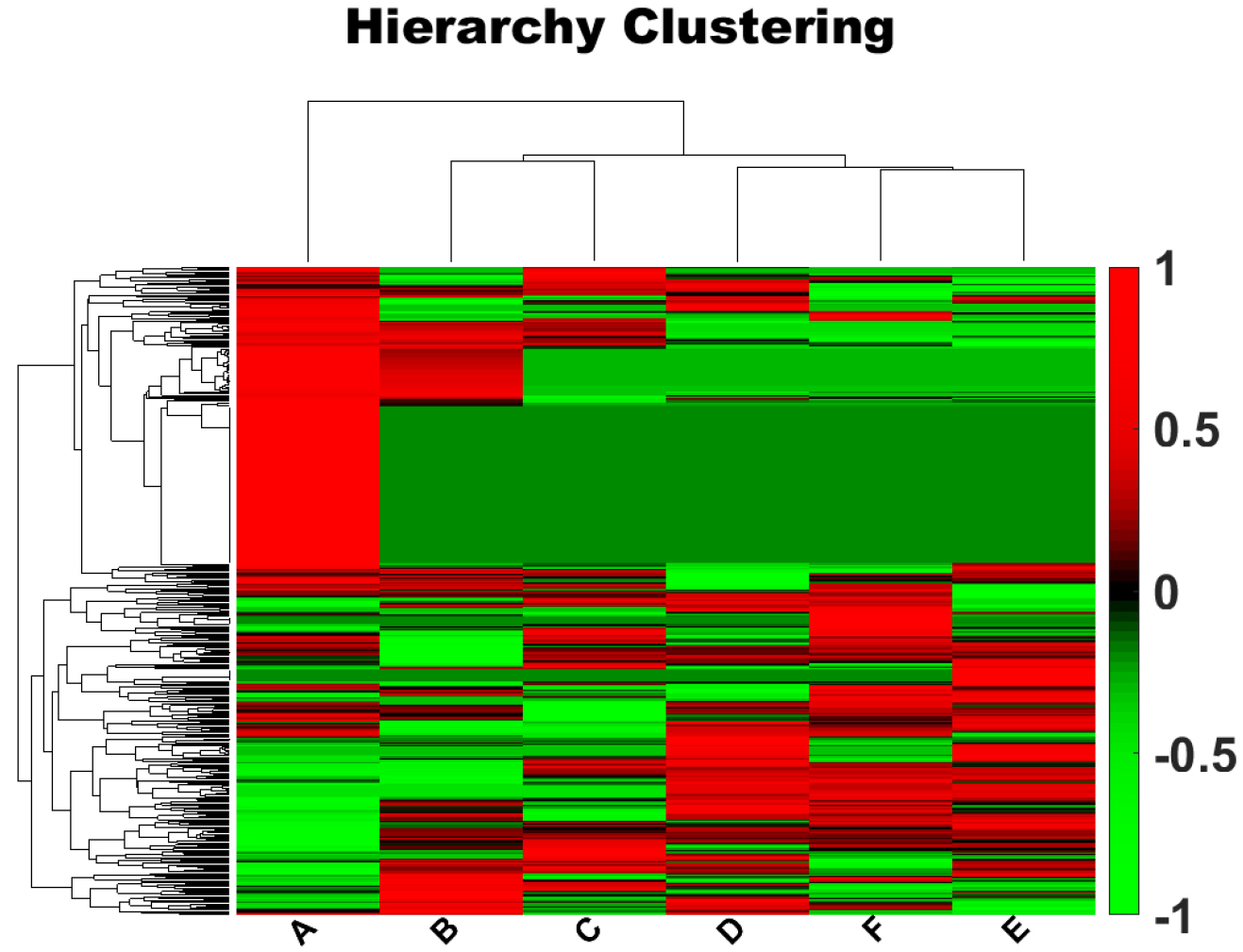
Background: Methamphetamine (METH) is a highly addictive drug that directly affects the central nervous system. METH use not only harms the user’s health but also poses risks and costs to society. Prolonged METH dependence has been shown to impair cognition, which may be the primary factor in impulsive drug-seeking behaviors and high relapse rates. However, the molecular mechanisms underlying METH addiction and METH-induced cognitive decline remain poorly understood. Methods: To illuminate the potential molecular mechanisms underpinning METH addiction, we compared serum protein expression levels between 12 long-term METH users and 12 healthy controls using label-free quantitative proteomics. Bioinformatic analyses were conducted to determine functional networks and protein-protein interactions. Results: In total, 23 differentially expressed proteins were identified between the two groups. The differentially expressed proteins were related to cognitive dysfunction, neuroinflammation, immune impairment, metabolic disturbances, and calcium binding and regulation. Conclusions: These 23 proteins may underpin the multi-system damage induced by chronic METH exposure. Our findings provide novel insights into the molecular basis of METH addiction and inform potential prevention and treatment strategies for individuals with METH dependence.