Academic Editor: Yoshihiro Noda
Background: Anxiety disorders are an important not only medical, but also social problem, affecting approx. 300 million people worldwide in 2019. Medications used in the treatment of anxiety are associated with many adverse reactions, which explains the increased use of herbal products as anxiolytics. Methods: An anxiolytic activity of Satureja montana, rosmarinic acid and carvacrol after 14-day long administration on an animal model of acute stress was studied. For measurement of anxiolytic effect elevated plus maze, social interaction and Vogel tests were provided as well as examination of locomotor activity. Results: The dry extract of Satureja montana at both tested doses significantly increased locomotor activity as well as the time spent in the social recognition, compared to the control groups. The extract reduced the time in the closed arms and the proportion of entries into open arms to total entries and increased the time in the open arms of elevated plus maze compared to the positive control group. Likewise, rosmarinic acid and carvacrol increased significantly the time spent with a new congener in the social interaction test. Both compounds reduced the ratio of entries into open arms to total entries similarly to the dry extract of Satureja montana. Only rosmarinic acid increased the time in the open arms and reduced the time in the closed arms. Conclusions: Satureja montana at both experimental doses exerted a significant anxiolytic activity in almost all the tests employed for evaluating anxious behavior. Carvacrol and rosmarinic acid showed a moderate anxiolytic effect.
The prevalence of anxiety disorders for 2019 is approx. 300 million people worldwide (4.05%), compared to 264 million in 2015 with economy costs more than US$ 1 trillion per year [1]. For the Eastern Mediterranean region, to which Bulgaria belongs, the estimated frequency is 3.6% for 2015 [2]. This psychiatric disorder affects predominantly the female sex compared to males (4.6% compared to 2.6%) [1]. The current Covid-19 pandemic and related social restrictions during the last two years have led to an increase in anxiety disorder incidence with more than 25% than normal anxiety rate in the previous years [3, 4, 5].
The presence of anxiety disorders is associated with the development of somatic diseases later in the patients’ lifetime [6]. Furthermore, the presence of an anxiety disorder has a huge impact on the course of socially important chronic somatic diseases. A significant correlation has been established between anxiety and cardiovascular complications [7]. However, the presence of such a psychiatric disorder is very often not recognized during the treatment of cardiac diseases [8].
The comorbidity between anxiety and/or depression and diabetes is also associated with increased risk of lethal outcome [9]. Patients with insulin resistance and a concomitant anxiety disorder are exposed to increased risk of developing diabetes type 2 compared to non-comorbid patients [10].
There is evidence about a genetic link between anxiety and depression [11]. This comorbidity in psychiatric patients is associated with increased risk of suicidal behavior [12].
All these data justify the need for timely treatment of anxiety disorders.
The traditional treatment of anxiety disorders is with anxiolytics (mainly the group of benzodiazepines) and different groups of antidepressants [13]. The use of benzodiazepines has good clinical efficiency but is associated with tolerance, dependence and abuse with these medications [14]. Some antidepressants like tricyclic antidepressants or monoamine oxidase inhibitors are associated with many adverse drug reactions [13]. Possible alternatives are selective serotonin and serotonin/norepinephrine reuptake inhibitors, which are better tolerated, but their delayed onset of action is another problem in the treatment of anxiety disorders and often requires co-medication with anxiyolitics [15]. The unfavorable safety profile and slow onset of action of the most commonly used drugs explains the increased interest in herbal remedies for reducing anxiety symptoms [13].
There exists a huge range of plants which exert anxiolytic activity. The most commonly used plants are Melissa officinalis (Lemon balm), Passiflora incarnata (Passionflower), Valeriana officinalis (Valerian), Humulus lupulus (Hops), Matricaria chamomilla (Chamomile) [13, 16]. Potential phyto remedies with anxiolytic activity are also extracts from plants like Ginkgo biloba (Maiden hair), Piper methysticum (Kava), Magnolia officinalis L., Achillea millefolium L. and others [13, 16]. All of them exert such a therapeutic effect due to their composition and the ability of the contained active ingredients to affect GABA mediation [13]. However, the discovery of even more herbs with anxiolytic activity is of interest to the scientific community around the world, due to their good safety profile.
Satureja montana, also known as winter savory, is a medical plant belonging to genus Satureja [17, 18]. This genus and its representative Satureja montana are widespread in South Europe — the Mediterranean, Balkans and particularly in Bulgaria [19, 20, 21]. This medical plant is used in folk medicine for treatment of gastrointestinal and pulmonary diseases [19]. It is one of the most pharmacologically active representatives from genus Satureja and antibacterial, antiviral, antioxidant and antitumor effects of Satureja montana are wellknown [22]. However, insufficient data on the its anxiolytic activity have been found in the available literature.
Satureja montana is rich source of active compounds [23]. In the essential oil from aerial parts of this medical plant are found phenols like carvacrol [24]. Analysis of aqueous and alcoholic extracts of the herb have found the presence of phenolic acids, one of which is the rosmarinic acid [25, 26]. Both active ingredients exert organ protective, antioxidant and anti-inflammatory effect [27, 28, 29, 30, 31].
The available literature data on the composition of Satureja montana as well as insufficient data on the effect of this medical plant and its active ingredients — Rosmarinic acid and Carvacrol on the mood and behavior, were the reason to conduct the present study to determine the presence or absence of anxiolytic effect of Satureja montana and to compare it with that of self-administration of both active compounds — Rosmarinic acid and Carvacrol, found in the composition of this medical plant.
The Wistar rats used in the experiment were obtained from the Vivarium of the Medical University of Plovdiv. All animals were housed under conventional conditions — standard room temperature — 20–22 °C, 12-hour light/dark cycle (from 07:00 to 19:00), with free access to food and water. In each home cage were housed 8 animals corresponding to experimental groups. All experiments were performed between 08:00 and 13:30 o’clock.
For investigating the anxiolytic effect of the dry extract of Satureja montana we used 64 male, 8-week-old Wistar rats with average body weight at the beginning of the experiment 150 g (140 g–160 g) randomly divided in 8 groups (n = 8), treated with saline and olive oil (control groups), dry extract of Satureja montana (SM) at doses of 250 and 500 mg/kg b.w., carvacrol 500 mg/kg b.w. and rosmarinic acid (RA) 15 mg/kg b.w. per os.
The dry extract of Satureja montana was prepared by “Veselino EOOD”, Kazanlak, Bulgaria from dried leaves of the medicinal plant, bought from an herbal pharmacy in Plovdiv, Bulgaria. The used method was methanol-aqueous extraction, followed by drying in a spray dryer at 40 °C. Carvacrol and Rosmarinic acid were bought from Sigma-Aldrich (St. Louis, Missouri, USA).
All substances were administered orally by stomach gavage after dissolving in distilled water for the dry extract and rosmarinic acid and olive oil for carvacrol. Doses and volumes of the administered solutions are presented in Table 1.
| Group | Substance and dose | Stress factor |
| Group I – negative control | Saline, 1 mL/100 g b.w | No |
| Saline (–) | ||
| Group II – positive control | Saline, 1 mL/ 100 g b.w. | Yes |
| Saline (+) | ||
| Group III – negative control | Olive oil, 1 mL/ 100 g b.w. | No |
| Olive oil (–) | ||
| Group IV – positive control | Olive oil, 1 mL/100 g b.w. | Yes |
| Olive oil (+) | ||
| Group V | Satureja montana dry extract, 250 mg/kg b.w | Yes |
| SM-250 mg | ||
| Group VI | Satureja montana dry extract, 500 mg/kg b.w | Yes |
| SM-500 mg | ||
| Group VII | Carvacrol, 500 mg/kg b.w | Yes |
| Carv-500 mg | ||
| Group VIII | Rosmarinic acid, 15 mg/kg b.w | Yes |
| RA-15 mg |
The doses of the dry extract of Satureja montana were calculated in
accordance to the results of previous acute and chronic toxicity experiments
conducted by our team. The dose of Rosmarinic acid was determined according to
the content of this phenolic compound in the composition of the extract — 44.730
The rats were divided in eight groups as follows (Table 1):
Anxiety was induced with an acute cold stress experimental model. The Wistar
rats from both positive controls and all test groups were exposed to low
temperature of 4 °C in the refrigerator for 60 min. Rodents were placed in
plastic boxes with dimensions 20
Fifteen minutes after removing them from the refrigerator their behavior was evaluated with the following tests: elevated plus maze, social interaction test, Vogel test and Activity cage. All tests were performed at the same day one after another for every group. The arrangement of the tests is shown on Fig. 1.
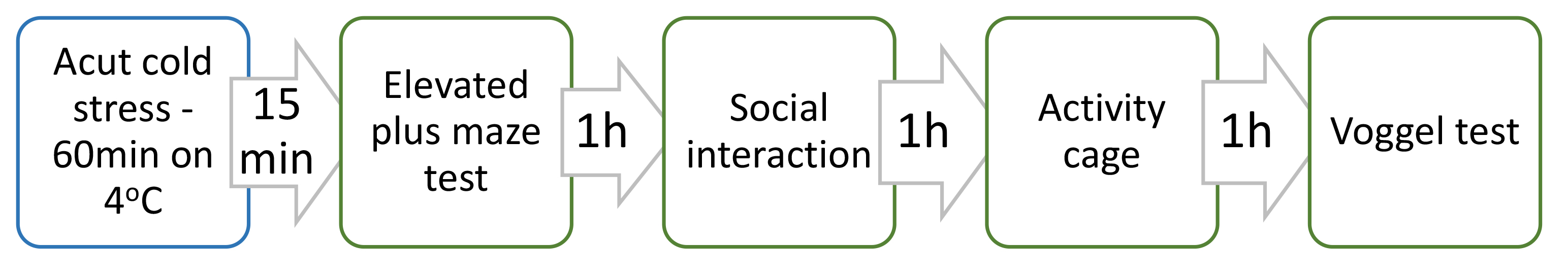 Fig. 1.
Fig. 1.Arrangement of the tests for establishing anxiolytic activity of Satureja montana extract after exposure to the stress factor.
Elevated plus maze and social interaction tests were performed in the presence of two observers in order to increase the in order to obtain accurate data. The Vogel test and activity cage apparatus detect automatically monitored parameters via incorporated in both apparatuses (Ugo Basile, Italy) software.
The test is performed in one test session with a duration of 5 min, detected via chronometer. A training session is not required. The animals were placed at the center of the apparatus. During the test the following parameters were observed: time spent in the open and closed arms, number of entries in each arm, sum of visits of both arms, and the ratio between the entries in the open arms compared to the total movements. A sign for reduced anxiety is increasing the time spent in the open arms and decreasing the total movements of the examined subject.
The sum of visits of both arms and the ratio between the entries in the open arms compared to the total movements were calculated by the following formulas:
where Nop is the number of entries into the open arms of the apparatus and Ncl is the number of entries into the enclosed arms of the apparatus
where Nop is the number of entries into the open arms of the apparatus and Ncl is the number of entries into the enclosed arms of the apparatus.
The apparatus was cleaned with 70% alcohol after each animal.
The social interaction test is performed in one test session. Two animals — the
test subject and a random animal from another group were placed in a plastic test
box (dimensions 60
The investigation of the locomotor activity is also performed in only one test session with a duration of 5 minutes without previous training. For measurement of the locomotor activity, we used Activity cage apparatus (Ugo Basile, Italy). The activity was automatically detected. A sign for reduced anxiety is the increase in locomotor activity. The plastic box of the apparatus was cleaned with 70% alcohol after each animal.
The test is performed in two consecutive days. The animals from all groups were
deprived of water for 24 hours before the first day of the test. On the 24th hour
they were placed in a Lickometer apparatus (Ugo Basile, Italy) to acclimate to the test
cage and were free to drink water for a 3-minute-long training session followed
by another 24 hours of water deprivation. The test session was performed on the
second day, also with a duration of 3 min. For every 15 licks the animals
were exposed to 2 seconds 300
The statistical analyses were processed with One Way ANOVA test with IBM SPSS
19.0 software (IBM, New York, USA). Results were expressed as arithmetic mean and
standard error of the mean (mean
No toxicity, changes in mood and behavior of experimental animals or adverse reactions were observed after treatment with both doses dry extract of Satureja montana, Rosmarinic acid and Carvacrol for 14 days.
The acute cold stress induced anxious behavior in the rodents from both positive control groups compared to the corresponding negative controls.
The rodents from both positive control groups spent less time in the open arms
of the EPM apparatus (p = 0.001 and p
In the social interaction test anxious animals spent less time in investigating
a novel individual with p = 0.036 for both Saline treated controls and
p = 0.033 for both Olive oil treated groups. Decrease in overall
activity was also detected by the Activity cage apparatus in the positive
controls. When comparing both Saline treated control significant decrease on
horizontal and vertical activity was measured with p = 0.009 and
p
The animals from both positive control groups demonstrated significantly less shock licking in the Vogel test compared to the negative control groups with p = 0.027 for both Saline treated control groups and p = 0.030 for both Olive oil treated control groups.
The dry extract of Satureja montana at doses of 250 mg/kg b.w. reduced the time spent in the closed arms of the elevated plus maze (EPM) and the proportion of entries into the open arms to total entries. An increase in the time in the open arms of EPM compared to the positive control group was also observed. For the higher dose of 500 mg/kg b.w significant decrease was measured only to the ratio of entries into the open arms to total entries compared to stressed saline treated group.
Similarly, to the dry extract, rosmarinic acid decreased the time in the closed arms and increased the time in the open arms as well as the ratio of entries into the open arms of the apparatus. However, a significance with both used doses of Satureja montana wasn’t established.
Animals treated with carvacrol only had a significant reduction in the proportion of entries into the open arms to total entries. The dose of 250 mg/kg b.w. Satureja montana significantly reduced the time spent in the closed arms and increased the time in the open arms compared to carvacrol treated group. Mean and SEM values, found in EPM test are presented in Table 2. The statistical significance is shown on Figs. 2,3.
| Time in seconds in enclosed arms | Time in seconds in open arms | Ratio | |
| Mean |
Mean |
Mean | |
| Saline (–) | 288.75 |
11.25 |
0.64 |
| Saline (+) | 294.25 |
2.5 |
0.95 |
| SM-250 mg | 275.25 |
24.75 |
0.53 |
| SM-500 mg | 287.38 |
12.38 |
0.59 |
| RA-15 mg | 276.75 |
23.25 |
0.6 |
| Olive oil (–) | 240.38 |
25.175 |
0.2 |
| Olive oil (+) | 292.63 |
6.38 |
0.77 |
| Carvacrol-500 mg | 286.5 |
13.5 |
0.51 |
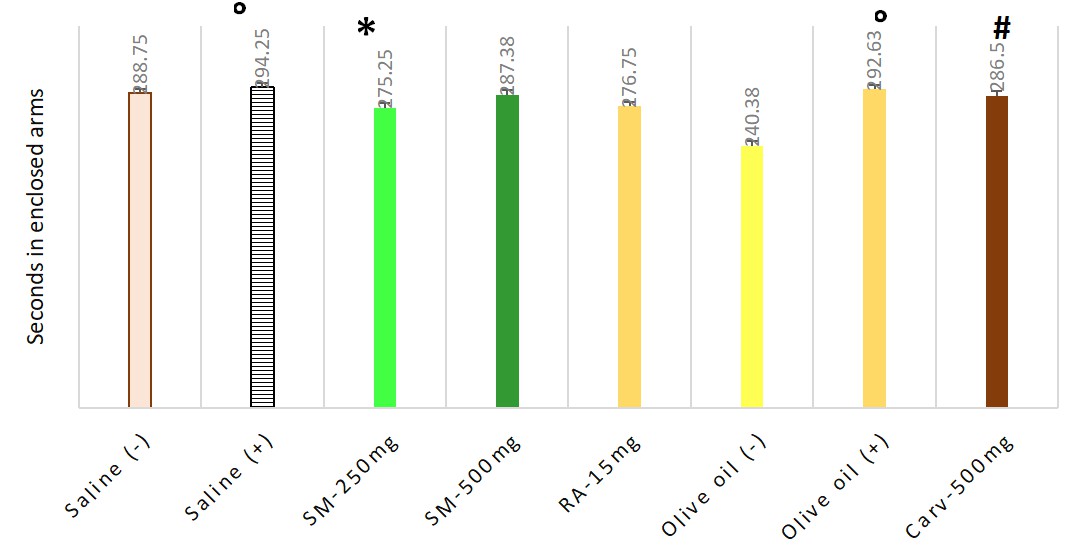 Fig. 2.
Fig. 2.Anxiolytic effect of Satureja montana, rosmarinic acid
and carvacrol, measured in elevated plus maze test via time spent in closed arms
of apparatus. Significant difference for the time in closed arms of EPM (F = 42.034) was
found. Pairwise comparisons with Games-Howell Post hoc test revealed significant
differences between following groups: ° negative — Group I and positive — Group II Saline controls as well
as between negative — Group III and positive — Group IV Olive oil controls with
p = 0.023 and p
 Fig. 3.
Fig. 3.Anxiolytic effect of Satureja montana, rosmarinic acid
and carvacrol, measured in elevated plus maze test via time spent in open arms of
apparatus and ratio of entries into the open arms of the apparatus. Significant main effects for time in open arms (F = 40.697) and for the ratio (F
= 6.967) were found. Pairwise comparisons with Games-Howell Post hoc test
revealed significant differences between following groups: ° negative — Group I and positive — Group II Saline controls with
p = 0.001 for the time in open arms and p = 0.032 for the ratio
respectively as well as between negative — Group III and positive — Group IV
Olive oil controls with p
The dry extract of Satureja montana at both experimental doses increased significantly the time spent investigating a novel individual compared to the positive control group. A similar effect was shown by carvacrol compared to the second positive control group, treated with olive oil. The standalone administration of rosmarinic acid showed only a tendency for increasing the time spent in investigating the new congener. However, a significant difference was not found. Mean and SEM values, found in social recognition test are presented in Table 3. The statistical significance is shown on Fig. 4.
| Seconds of social interaction | |
| Mean | |
| Saline (–) | 18.62 |
| Saline (+) | 10.25 |
| SM-250 mg | 48 |
| SM-500 mg | 35 |
| RA-15 mg | 21.25 |
| Olive oil (–) | 15.37 |
| Olive oil (+) | 6.37 |
| Carvacrol-500 mg | 68.63 |
 Fig. 4.
Fig. 4.Effect of both experimental doses dry extract of Satureja montana, rosmarinic acid and carvacrol on the social interaction. Significant difference for the time spent in social recognition (F = 15.279) was found. Pairwise comparisons with Games-Howell Post hoc test revealed significant differences between following groups: * positive Saline treated control (Group II) and both doses dry extract of Satureja montana treated groups — Groups V — Satureja montana 250 mg/kg b.w and Group VI — Satureja montana 500 mg/kg b.w with p = 0.001 and p = 0.004 respectively. ** positive Olive oil control — Group IV and Carvacrol 500 mg/kg b.w — Group VII with p = 0.001.
The lower dose of 250 mg/kg b.w dry extract of Satureja montana had a significant anxiolytic effect, compared to rosmarinic acid as well — p = 0.004. However, a significant difference between both experimental doses of the extract was not observed. The results are shown on Fig. 5.
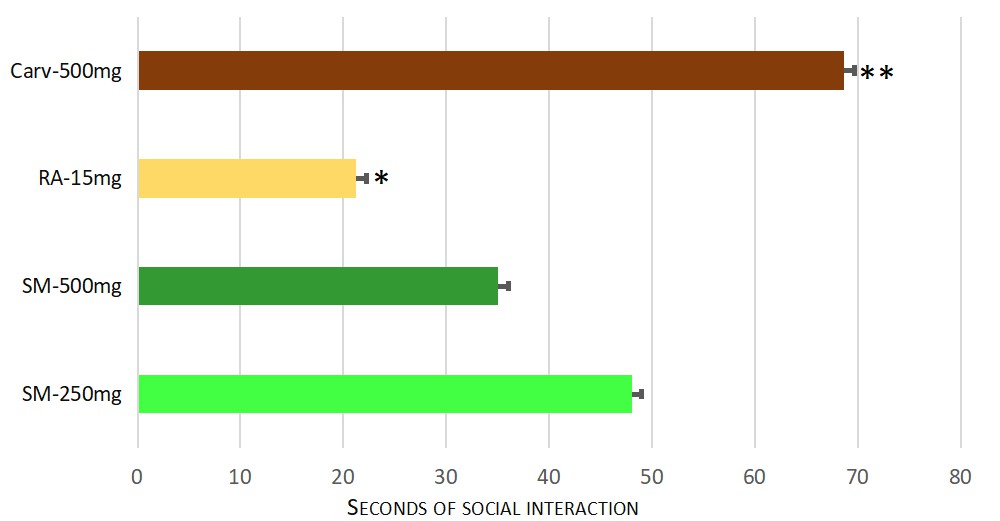 Fig. 5.
Fig. 5.Significant differences in the anxiolytic effect in social interaction between test groups. Significant difference for the time spent in social recognition (F = 15.279) was found. Pairwise comparisons with Games-Howell Post hoc test revealed significant differences between following groups: * Rosmarinic acid 15 mg/kg b.w (Group VIII) and Satureja montana dry extract at dose 250 mg/kg b.w (Group V) with p = 0.004. ** Carvacrol 500 mg/kg b.w (Group VII) and Satureja montana dry extract at dose 500 mg/kg b.w (Group VIII) with p = 0.019.
The dry extract of SM showed a moderate effect on the locomotor activity of the
experimental animals. No significant results were established between the
positive control group, treated with saline and both experimental groups, treated
with the dry extract of Satureja montana at doses 250 mg/kg b.w and 500
mg/kg b.w. in terms of horizontal activity. Both experimental doses
Satureja montana showed a significant increase in vertical activity (SEM
= 81.5
Rosmarinic acid and carvacrol also didn’t show a significant impact on the locomotor activity compared to the positive control groups. The results are presented on Fig. 6.
 Fig. 6.
Fig. 6.Effect on the locomotor activity of both experimental
doses dry extract Satureja montana and Rosmarinic acid, compared to
positive and negative control groups. Significant main effect for vertical activity (F = 4.362) was found. Pairwise
comparisons with Games-Howell Post hoc test revealed significant differences
between following groups: * positive Saline treated control (Group II) and both experimental doses of the
dry extract of Satureja montana (Group VI — Satureja montana
250 mg/kg b.w and Group VII — Satureja montana 500 mg/kg b.w) with
p
The dry extract of Satureja montana at doses of 250 mg and 500 mg/kg b.w did not significantly increase the number of shock lickings in the Vogel test compared to the positive control group. Carvacrol and rosmarinic acid similarly to the dry extract did not show a significant increase in the shock lickings. The results are presented on Fig. 7.
 Fig. 7.
Fig. 7.Effect of Satureja montana, Rosmarinic acid and carvacrol on the total and shock licks in Vogel conflict test. * Significant difference (F = 1.479) compared to the corresponding negative control group was found — positive Saline control (Group II) has significant decrease in the number of shock licks compared to negative Saline control (Group I) as well as Olive oil with stress (Group IV) compared to negative Olive oil control (Group III) with Games-Howell Post Hoc test with p = 0.027 and p = 0.030 respectively.
Inducing stress in experimental animals can be caused by different models, including physical, social and psychological stressors [32]. The following methods belong to these groups: deprivation from essential for survival food, water and sleep, limitations of free research behavior, exposure to unfavorable environmental conditions and pain stimuli [33]. It is well known that exposure to low temperatures activates systems, mediating the body’s response to stress and is associated with anxiety in male rats [34, 35]. In accordance with the literature data acute cold stress model was chosen for inducing the anxiety behavior in our research.
Use of animal models is crucial for the understanding the pathological mechanisms underlying the development of anxiety as well as in the screening, establishing the toxicity and effectivity of new molecules for development of new anxiolytic drugs [36, 37].
Animal models used for investigating anxiety behavior are based on the conflict caused by situations of avoidance. The most used are those tests that assess unlearned behavior (elevated plus maze, open field, social interaction) as well as experiments, which include learned punished reactions [13, 38]. The combined use of different behavioral tests to assess anxiety allows not only to refine the results, but also to establish the probable mechanisms of action of the tested substances.
The EPM is the one of the most common tests used to assess anxious behavior in
rodents [39]. Its use is mainly to screen the potential anxiolytic activity
of tested drugs [40]. The establishment of possible mechanisms of action
related to influencing the levels of various neurotransmitters requires further
research. For example, the investigation of locomotor activity assumes the
involvement of dopamine and 5-hydroxytryptamine neurotransmitters [41]. Likewise, the social interaction test is associated with serotonin but also
with
In our study the tests described above were used for investigation of the anxiolytic activity of the dry extract of Satureja montana, carvacrol and Rosmarinic acid. Carvacrol and Rosmarinic acid are two of the active ingredients, found in the different extracts of Satureja montana [43, 44].
The exact mechanism of development of anxiety disorders is not fully understood [45]. There are many evidences of 5-hydroxitryptamine and norepinephrine mediation involvement in their pathophysiology [46]. The main changes in the pathways of these two neuromodulators are associated with insufficient activation of the serotonergic function and over activation of the norepinephrine system [45]. Another mediator, found to have an important role in the development of anxiety disorders is GABA [46].
Both of the tested principal ingredients – carvacrol and Rosmarinic acid, also
possess various mechanisms through which they act on the nervous system
[47, 48, 49]. Rosmarinic acid is supposed to exert activity on the T subtype
of Ca
In the last few years there has been an increased interest in establishing the link between anxiety disorders and oxidative stress [50]. Its markers were elevated in patients with such pathology [50, 51, 52]. Various mechanisms are being discussed in relation to this interaction [31], but the exact mechanism has not yet been established [51, 52]. In addition, there are studies which investigate the potential antioxidant activity of some drug classes, used in the treatment of anxiety disorders [53].
Carvacrol and Rosmarinic acid are well known for their antioxidant activity, which probably plays a key role in the anxiolytic activity of both compounds [47, 54, 55, 56].
The screening provided with EPM showed a significant anxiolytic activity of all tested substances. Our results for the carvacrol and Rosmarinic acid treated groups agree with the findings of Noshy P.A and Mirza F.J [57, 58]. Hypotheses about engagement of 5-hydroxytryptamine mediation in social interaction test have been hypothesized in the available literature [42]. The results of this test obtained in the present study are reason to assume that both active ingredients — Rosmarinic acid and carvacrol affect this mediation. The study team of Polli FS found that carvacrol affect several mediators in central nervous system, including serotonin, which supports our hypothesis [49]. On the other hand, confirmation or rejection of such possible mechanism of action of rosmarinic acid will be subject of forthcoming further studies to confirm or refute it. The results from the investigation of the locomotor activity, which is also associated with serotonin mediation [41], didn’t show significance in the present study. Our results from investigating the locomotion for the carvacrol treated group agree with the findings of Melo FHC [59]. We observed a lack of statistical significance in the Vogel test for both tested compounds which excludes the possibility of influencing GABA. These results lead us to believe that the anxiolytic effect is due more to the antioxidant effect of Rosmarinic acid and carvacrol than to the effect on neurotransmitter mediation. In the available literature there is limited data for the anxiolytic activity of both tested compounds which limits our ability to compare our results with those of other research teams.
Like the standalone administration of Rosmarinic acid and carvacrol, the dry extract of Satureja montana also significantly reduced anxious behavior in all used tests apart from the Vogel test. In EPM the demonstrated anxiolytic effect of Satureja montana was stronger than the one presented by the standalone administration of Rosmarinic acid and carvacrol. Significance was also established in the investigation of locomotor activity, which was not found for the other compounds. These findings suggest a synergistic effect of both active ingredients in the composition of the dry extract. The most probable mechanism of the observed anxiolytic action of the dry extract is due to the antioxidant activity of the extract. However, based on the results from all test we could speculate with the engagement of some neurotransmitters like serotonin and lack of activity on GABA mediation. It is possible potential antioxidant or anti-inflammatory activity to participate in the exert anxiolytic effect. Further studies should be undertaken to confirm or reject such hypotheses.
The dry extract of Satureja montana at both experimental doses exerted significant anxiolytic activity in all tests employed for evaluating anxious behavior, with the exception of the Vogel test. Carvacrol and rosmarinic acid showed moderate anxiolytic effect only in EPM and social interaction tests. Further studies should be undertaken to clarify the exact mechanisms of the anxiolytic effect.
SM, Satureja montana; RA, Rosmarinic acid; Carv, Carvacrol; EPM,
elevated plus maze; GABA,
All experiments were designed and performed under the guidance of IvanK, DD and IlinK. NV, MK, HZ, IliaK performed the experiments. Each author has contributed to writing the article.
Animals were raised in vivarium of Medical University of Plovdiv. All experiments are in accordance with protocol No. 01-2/10.04.2020 of the Ethic Committee, Medical University of Plovdiv and Protocol No. 258 from Bulgarian Food Safety Agency based on the position of the Ethic Committee, Bulgarian Food Safety Agency No. 174 from 08. 10. 2019.
We thank to all the peer reviewers for their reviews, options, suggestions and constructive criticism.
This study was supported by Medical University of Plovdiv, Project No. DPDP-15/2020.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
