- Academic Editor
†These authors contributed equally.
Previous studies have indicated that blood lipids can influence skeletal health. However, limited research exists on the impact of serum apolipoprotein B (ApoB) on bone mineral density (BMD); meanwhile, it remains unclear to what extent cardiovascular disease plays in mediating this process.
Therefore, we conducted a cross-sectional analysis involving 2930 participants from the National Health and Nutrition Examination Survey (NHANES) database to explore the relationship between serum ApoB and total body BMD (TB-BMD) and lumbar spine BMD (LS-BMD). We employed a two-step, two-sample Mendelian randomization (MR) analysis using genetic instruments to investigate causality and assess the mediating effects of six cardiovascular diseases.
Multivariable linear regression models demonstrated an inverse linear association between serum ApoB and TB-BMD (β = –0.26, 95% confidence interval (CI): –0.41 to –0.12, p < 0.001; p for non-linearity = 0.771) and LS-BMD (β = –0.53, 95% CI: –0.75 to –0.31, p < 0.001; p for non-linearity = 0.164). The primary analysis utilized the multiplicative random effects inverse variance weighted (IVW-MRE) method for the two-sample MR analysis. The results demonstrated a causal relationship between serum ApoB with TB-BMD (β = –0.0424, 95% CI: –0.0746 to –0.0103; p = 0.0096) and LS-BMD (β = –0.0806, 95% CI: –0.1384 to –0.0229; p = 0.0062). The two-step MR analysis indicated heart failure as a mediating factor in the causal relationship between serum ApoB and TB-BMD, with a mediation proportion of 18.69%.
The results of this study support that lowering serum ApoB levels could enhance BMD while preventing the occurrence of heart failure might reduce the harm caused by the decrease in BMD due to elevated ApoB levels.
Osteoporosis is a widespread condition that impacts a significant portion of the global population [1]. It is characterized by a reduction in bone mass and the degradation of bone tissue microstructure, which significantly increases the risk of a fracture [2]. The diagnosis is established by assessing bone mineral density (BMD) through dual-energy X-ray absorptiometry (DXA) scans [3]. By the year 2030, it is anticipated that the prevalence of osteoporosis or low bone mass will increase from approximately 53 million to over 70 million [4]. Therefore, it is imperative to explore novel biomarkers for bone health.
The relationship between serum lipids and BMD remains inconclusive, with inconsistent findings reported in previous studies [5, 6]. A study suggested that serum ApoB in serum lipids may have a negative correlation with BMD, indicating its potential as an indicator of bone health [7]. In addition, previous studies have mostly ignored the effect of serum apolipoprotein B (ApoB) on bone health. Serum ApoB is considered to be a key indicator of cardiovascular diseases such as atherosclerosis [8, 9, 10]. A previous study indicated a nonlinear relationship between femoral bone mineral density and the risk of cardiovascular disease [11]. Another study reported prospective associations between BMD and cardiovascular diseases, including atrial fibrillation, acute myocardial infarction, stroke, and heart failure [12]. Previous studies have suggested that there is an associations between osteoporosis and deaths from cardiovascular disease [13, 14, 15]. A major limitation in studying the relationship between ApoB and BMD is that hyperlipidemia often coexists with conditions that may affect bone health, such as cardiovascular diseases. Consequently, when an association is observed, it is challenging to distinguish the effects of hyperlipidemia from those of cardiovascular diseases. From 1990 to the subsequent two decades, the prevalence of cardiovascular diseases doubled, with the number of cases increasing by 252 million and deaths rising by 6.5 million [16, 17]. Therefore, serum ApoB may represent a useful indicator for changes in BMD. In addition, investigating the role of cardiovascular disease could further elucidate the pathogenesis of osteoporosis and reveal relevant targets for reducing the risk of osteoporosis.
Mendelian randomization (MR) analysis is a method that employs genetic data to clarify causal associations between exposures and outcomes. Since genetic alleles are randomly assigned during meiosis and not influenced by environmental factors, the genetic associations identified in MR analyses are less susceptible to confounding bias and the potential for reverse causation [18]. Consequently, MR studies offer a valuable complementary approach for investigating the causal link between serum ApoB and BMD.
Currently, there is limited research exploring the correlation between ApoB and BMD, as well as the potential a causal relationship and underlying mediating pathways. To comprehensively investigate the association between ApoB and BMD, we initially conducted a cross-sectional study using the National Health and Nutrition Examination Survey (NHANES) database. Subsequently, we performed MR analysis to assess its causal relationship at the genetic level. We were particularly interested in evaluating the extent to which various cardiovascular diseases mediated these relationships.
This study consisted of two phases: epidemiological observational analysis and
Mendelian randomization analysis, as illustrated in Fig. 1. In the initial phase,
we utilized data from the NHANES database to perform a multivariable linear
regression analysis. This analysis sought to investigate the correlation between
serum ApoB with total body BMD (TB-BMD) and lumbar spine BMD (LS-BMD). During the
second phase, MR analysis was conducted using data from summary statistics
obtained from genome-wide association studies (GWAS). This study aimed to assess
the causal relationship between ApoB and BMD at various sites, including TB-BMD,
LS-BMD, and femoral neck BMD (FN-BMD). Subsequently, a further evaluation was
conducted to assess the mediating role of six cardiovascular diseases (stroke,
coronary artery disease, atrial fibrillation, heart failure, venous
thromboembolism, and peripheral atherosclerosis) in the causal relationship
between ApoB and BMD. All statistical tests were conducted using a two-tailed
approach at a significance level set to p
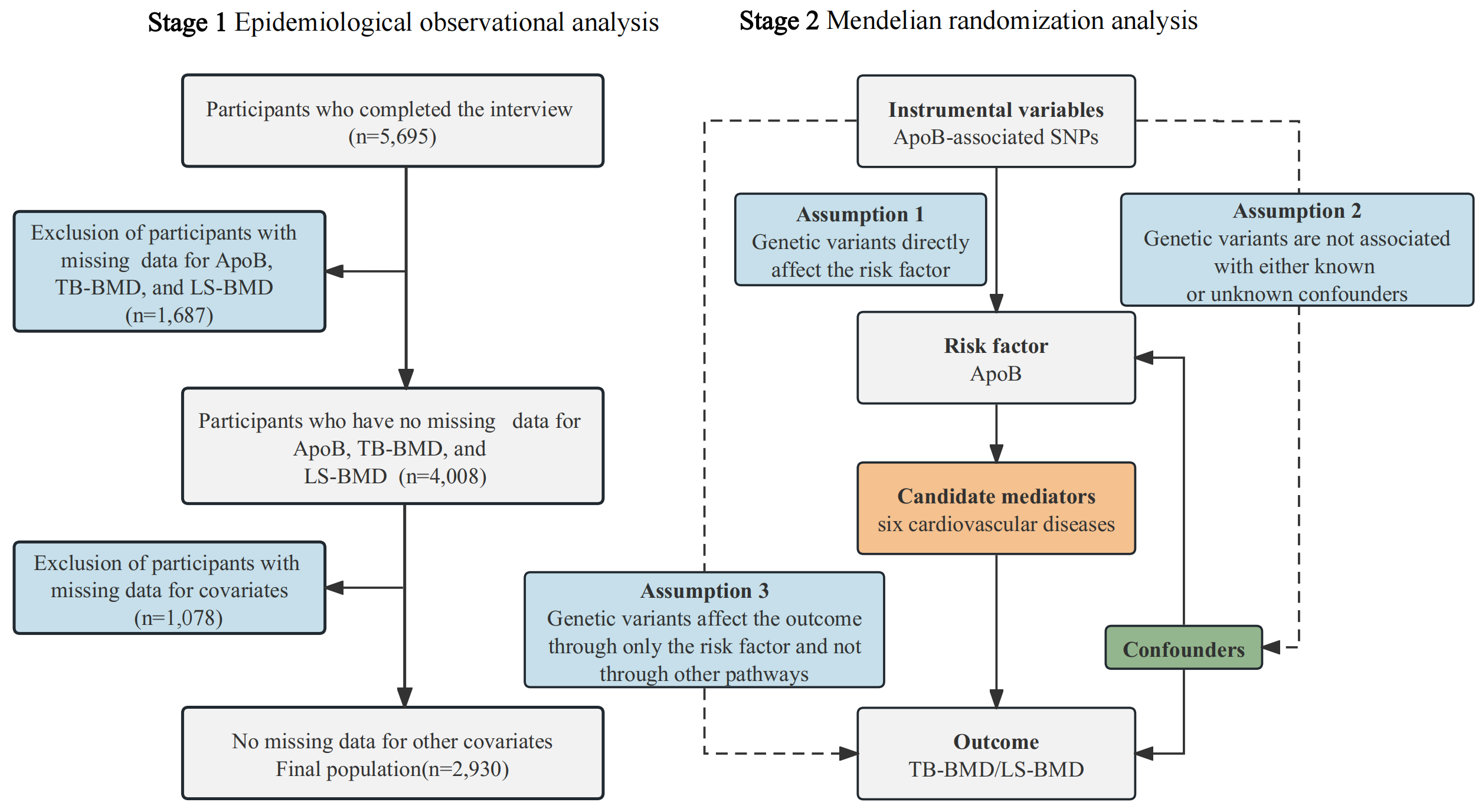 Fig. 1.
Fig. 1.
Study framework. BMD, bone mineral density; SNPs, single-nucleotide polymorphisms; ApoB, apolipoprotein B; TB-BMD, total body BMD; LS-BMD, lumbar spine BMD.
The NHANES study employed a comprehensive multistage survey method that incorporated stratification to enhance precision and accuracy in its outcomes. The research received ethical clearance from the National Center for Health Statistics Research Ethics Review Board, and prior to participation, all individuals provided written informed consent [19]. We acquired data from adult participants (20–59 years) in NHANES from 2011–2016. We excluded participants from the study if they had incomplete data on serum ApoB, TB-BMD, LS-BMD, or covariates (Fig. 1).
The BMD measurements were performed utilizing fan-beam densitometers (Hologic QDR-4500A, Bedford, MA, USA) through DXA scans [20]. ApoB within human serum can engage in an immunochemical reaction, resulting in the creation of immune complexes. These complexes induce alterations in the intensity of scattered light as it traverses through them, and this modified intensity is quantified using a Siemens Prospec chemistry analyzer, enabling precise determination of the ApoB concentration [7]. For further detailed information, please consult the official NHANES website.
We selected these confounders on the basis of judgment, previous scientific literature, all significant covariates in the univariate analysis, or their associations with the outcomes of interest or a change in effect estimate of more than 10% [7, 21]. Standardized questionnaires devised by NHANES researchers collected demographic data, including gender, age, race, education level, poverty-to-income ratio (PIR), marital status, drinking status (determined by whether participants consumed at least 12 alcoholic drinks annually), and smoking status (assessed based on whether participants had smoked a minimum of 100 cigarettes in their lifetime), and physical activity (min/week). Body mass index (BMI) was calculated from weight and height (kg/m2). Co-morbidities diagnosed by physicians, such as liver disease and cancer or malignancies, were determined based on self-reporting by participants. The levels of serum urea nitrogen, serum phosphorus, serum total protein, and serum uric acid were determined using standardized protocols. The intake of magnesium, calcium, sodium, and potassium was obtained through 24-hour dietary recalls conducted by trained interviewers at the mobile examination center.
Categorical variables were presented using frequencies, whereas continuous
variables that followed a normal or skewed distribution were expressed as the
mean or median, respectively. We employed One-Way ANOVA for normally distributed
data, the Kruskal-Wallis H test for skewed distributions, and the chi-square test
for categorical variables to compare the clinical characteristics across ApoB
quartiles. We assessed the relationship between serum ApoB levels and TB-BMD as
well as LS-BMD using linear regression models, presenting regression coefficients
(
The present MR study was composed of two steps (Fig. 1). In the first step, a two-sample univariable mendelian randomization (UVMR) approach was employed to assess the causal relationships between serum ApoB levels and BMD (TB-BMD, LS-BMD, and FN-BMD). The results from the UVMR analysis suggested a causal relationship between ApoB and TB-BMD as well as LS-BMD, while no causal relationship was observed between ApoB and FN-BMD. In the second step, both UVMR and multivariable mendelian randomization (MVMR) were employed to screen for mediators of the association between ApoB and the correlation of TB-BMD as well as LS-BMD among six cardiovascular diseases (stroke, coronary artery disease, atrial fibrillation, heart failure, venous thromboembolism, and peripheral atherosclerosis). Subsequently, a two-step MR analysis was performed to compute the mediating effects.
We utilized summary statistics obtained from extensive genome-wide association studies (GWAS) or meta-analyses of GWAS data to perform estimation of genetic correlations and MR analysis. The summary statistics for the serum ApoB dataset were derived from the UK Biobank GWAS summary statistics, which included 435,744 participants of European ancestry. Basic quality control measures were applied to the serum ApoB trait, including the exclusion of extreme outliers, stratification by gender and menopausal status, inverse normal transformation, removal of covariates, and subsequent reapplication of inverse normal transformation [22].
The summary statistics for TB-BMD were obtained from a meta-analysis of GWAS involving 56,284 participants. TB-BMD was assessed using DXA scans following standard manufacturer protocols [23]. The data is publicly available from the Genetic Factors for Osteoporosis (GeFOS) consortium [24]. The most extensive GWAS dataset for BMD assessed using DXA was acquired from GEFOS, encompassing FN-BMD data from 32,735 European participants and LS-BMD data from 28,498 European participants [25]. Additionally, we selected six cardiovascular diseases including stroke, coronary artery disease, atrial fibrillation, heart failure, venous thromboembolism, and peripheral atherosclerosis as candidate mediators. The GWAS datasets for these conditions were obtained from various organizations or consortia, namely MEGASTROKE, the UK Biobank study, Center for Statistical Genetics, Heart Failure Molecular Epidemiology for Therapeutic Targets, and the FinnGen consortium. All participants included in these studies shared European ancestry. Detailed information can be found in (Supplementary Table 1).
The MR analysis is based on three fundamental assumptions [26]. The initial
assumption necessitates that instrumental variables (IVs) exhibit an association
with the risk factor. Second, these genetic variants should not be connected to
confounding factors. Third, IVs should influence the outcome risk solely through
the risk factor, without involving other pathways. To meet these three
assumptions in the statistical analysis, we conducted quality control procedures
to select appropriate IVs. (1) We opted for single-nucleotide polymorphisms
(SNPs) meeting the genome-wide significance threshold (p
In the two-sample UVMR analysis, we separately evaluated the causal
relationships of ApoB with TB-BMD and LS-BMD, denoting the estimated coefficients
as
For UVMR, we assessed the potential causal associations between the exposure
variable and the outcome variable using the fixed effects inverse variance
weighted (IVW-FE) method. Additionally, we employed three other methods for
additional analysis, including Weighted Median, MR Egger, and MR pleiotropy
residual sum and outlier (MR-PRESSO). Sensitivity analyses were conducted to
validate and ensure the reliability and stability of the results. These analyses
encompassed various tests, including the assessment of heterogeneity using
Cochrane’s Q test, as well as pleiotropy testing through the MR Egger intercept
test [29]. In cases where heterogeneity was detected (p
This study involved 5695 prospective participants (20–59 years) from NHANES
(2011–2016) who completed interviews. We excluded participants with missing data
for ApoB, TB-BMD, and LS-BMD (n = 1687). Finally, 2930 participants with complete
datasets were included (Fig. 1). The mean participant age (years) was 38.9
The results of the univariate analysis for TB-BMD and LS-BMD are presented in
Supplementary Table 4. Following multivariable adjustment, a significant
association was observed between ApoB and TB-BMD. Model 3 revealed an adjusted
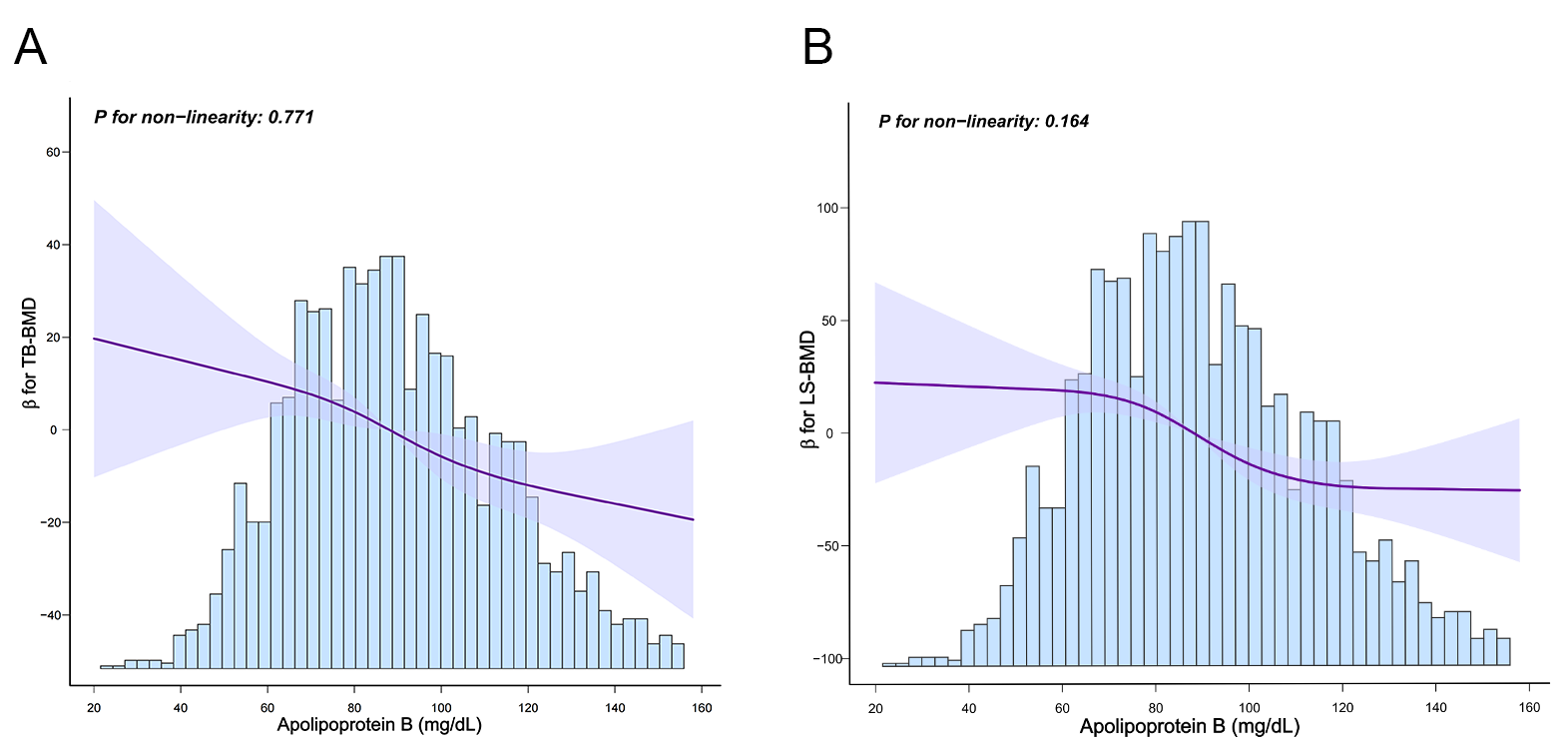 Fig. 2.
Fig. 2.
Restricted cubic spline model of ApoB with TB-BMD and LS-BMD.
(A) Restricted cubic spline model of ApoB and TB-BMD. (B) Restricted cubic
spline model of ApoB and LS-BMD. Solid line represents
| Variable | Unadjusted | Model 1 | Model 2 | Model 3 | |||||
| p-value | p-value | p-value | p-value | ||||||
| TB-BMD, (mg/cm2) | |||||||||
| ApoB (mg/dL) | –0.21 (–0.37 to –0.06) | 0.006 | –0.28 (–0.42 to –0.13) | –0.27 (–0.42 to –0.13) | –0.26 (–0.41 to –0.12) | ||||
| Q1 (20–72 mg/dL) | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) | |||||
| Q2 (73–88 mg/dL) | –1.72 (–12.8 to 9.36) | 0.761 | –1.57 (–11.46 to 8.32) | 0.755 | –1.33 (–11.21 to 8.54) | 0.791 | –0.81 (–10.70 to 9.07) | 0.872 | |
| Q3 (89–106 mg/dL) | –9.53 (–20.73 to 1.67) | 0.096 | –8.97 (–19.23 to 1.29) | 0.087 | –8.50 (–18.76 to 1.75) | 0.104 | –8.21 (–21.09 to 0.88) | 0.119 | |
| Q4 (107–260 mg/dL) | –13.55 (–24.65 to –2.44) | 0.017 | –18.15 (–28.67 to –7.63) | –17.96 (–28.47 to –7.44) | 0.001 | –17.41 (–28.12 to –6.70) | 0.001 | ||
| p for trend | |||||||||
| LS-BMD, (mg/cm2) | |||||||||
| ApoB (mg/dL) | –0.73 (–0.94 to –0.52) | –0.55 (–0.76 to –0.33) | –0.55 (–0.77 to –0.33) | –0.53 (–0.75 to –0.31) | |||||
| Q1 (20–72 mg/dL) | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) | |||||
| Q2 (73–88 mg/dL) | –17.07 (–32.32 to –1.83) | 0.028 | –11.01 (–25.83 to 3.81) | 0.146 | –10.86 (–25.67 to –3.96) | 0.151 | –10.01 (–24.87 to 4.85) | 0.187 | |
| Q3 (89–106 mg/dL) | –38.92 (–54.33 to –23.51) | –28.19 (–43.56 to –12.82) | –28.33 (–43.71 to –12.94) | –27.78 (–43.26 to –12.31) | |||||
| Q4 (107–260 mg/dL) | –52.44 (–67.72 to –37.17) | –38.40 (–54.17 to –22.64) | –38.77 (–54.55 to –22.99) | –37.12 (–53.21 to –21.03) | |||||
| p for trend | |||||||||
Ref, reference; PIR, poverty-to-income ratio; BMI, body mass index.
Model 1 adjust for gender, age, race, education level, marital status, PIR, and BMI.
Model 2 adjust for Model 1 + drinking status, smoking status, physical activity, liver condition, cancer or malignancy.
Model 3 adjust for Model 1 + Model 2 + serum urea nitrogen, serum phosphorus, serum total protein, serum uric acid, calcium, magnesium, sodium, and potassium.
In the UVMR analysis, a causal relationship was observed between a 1-standard deviation (SD) increase
in serum ApoB levels and a subsequent decrease in TB-BMD (
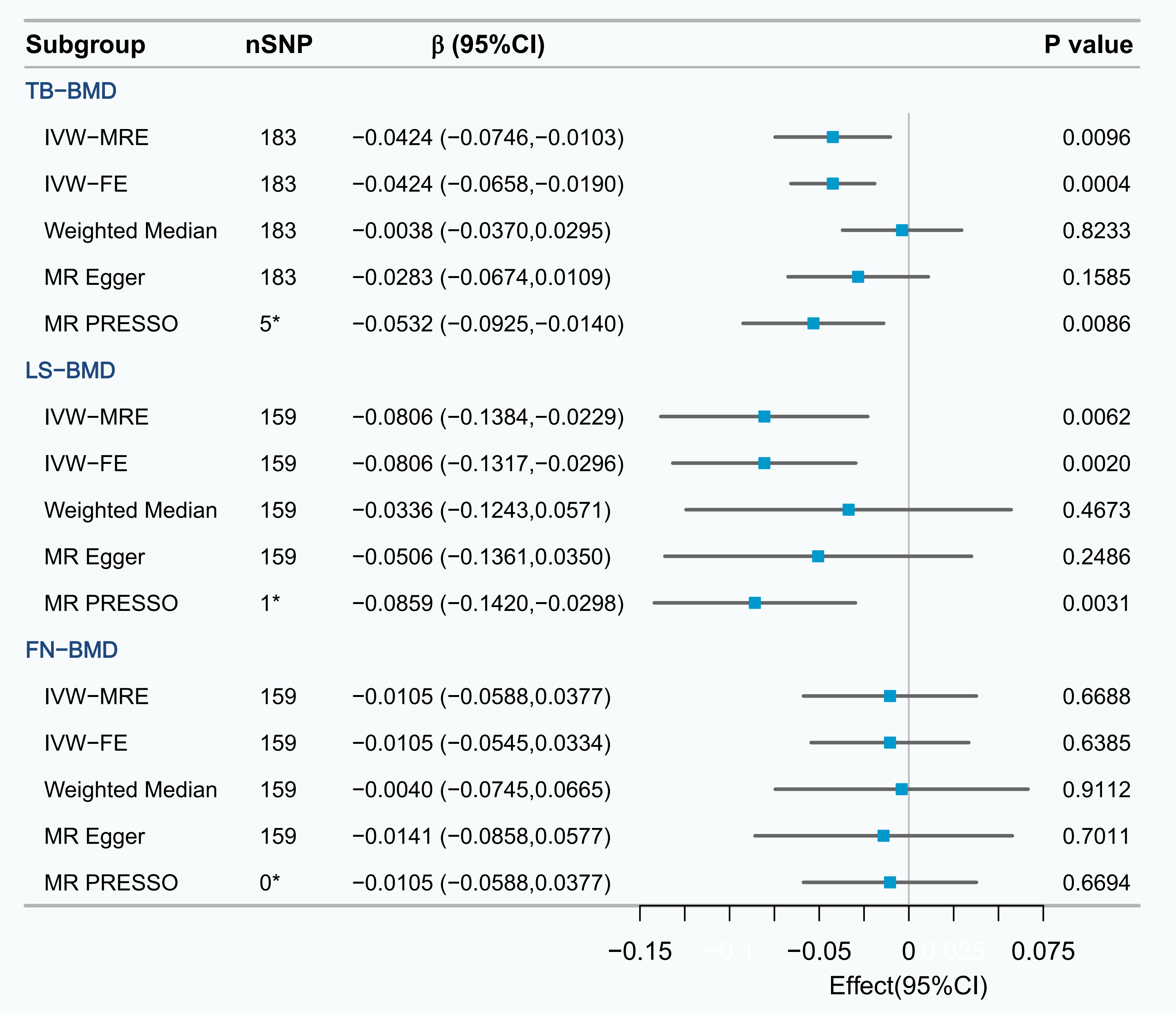 Fig. 3.
Fig. 3.
UVMR analysis of the effect of serum ApoB on TB-BMD, LS-BMD and FN-BMD. *The number of outliers; IVW-FE, fixed effects inverse variance weighted; IVW-MRE, multiplicative random effects inverse variance weighted; MR PRESSO, mendelian randomization pleiotropy residual sum and outlier; UVMR, univariable mendelian randomization; FN-BMD, femoral neck bone mineral density.
The calculations determined that the F-statistic of every SNP exceeded the
threshold of 10 (Supplementary Table 2). Therefore, the likelihood of a
weak IV affecting the results was relatively low. The sensitivity analysis
revealed heterogeneity in the results (Cochrane’s Q test p
In the UVMR, employing the IVW-FE method revealed a causal relationship between
genetically determined serum ApoB levels (per 1-SD increase) and an elevated risk
of all five cardiovascular diseases (except venous thromboembolism) (Table 2).
All SNPs for each variable exhibit sufficient instrument strength, with
F-statistics exceeding 10 (Supplementary Table 2). The MR-Egger
intercept test indicated the absence of horizontal pleiotropy across all analyses
(all p
| Cardiovascular diseases | nSNP | OR (95% CI) | p value | |
| Stroke | ||||
| IVW-MRE | 179 | 1.1068 (1.0505, 1.1662) | 0.0001 | |
| IVW-FE | 179 | 1.1068 (1.0695, 1.1455) | 6.99 | |
| Weighted Median | 179 | 1.0956 (1.0417, 1.1524) | 0.0004 | |
| MR Egger | 179 | 1.1343 (1.0640, 1.2092) | 0.0002 | |
| MR PRESSO | 5* | 1.1142 (1.0647, 1.1661) | 6.22 | |
| Coronary artery disease | ||||
| IVW-MRE | 150 | 1.3693 (1.2649, 1.4822) | 7.80 | |
| IVW-FE | 150 | 1.3693 (1.3036, 1.4383) | 5.27 | |
| Weighted Median | 150 | 1.3621 (1.2307, 1.5076) | 2.39 | |
| MR Egger | 150 | 1.4684 (1.2866, 1.6759) | 6.39 | |
| MR PRESSO | 4* | 1.3875 (1.2901, 1.4923) | 3.30 | |
| Atrial fibrillation | ||||
| IVW-MRE | 184 | 1.0297 (0.9878, 1.0734) | 0.1668 | |
| IVW-FE | 184 | 1.0297 (1.0022, 1.0580) | 0.0339 | |
| Weighted Median | 184 | 1.0148 (0.9768, 1.0542) | 0.4499 | |
| MR Egger | 184 | 1.0541 (1.0023, 1.1085) | 0.0418 | |
| MR PRESSO | 7* | 1.0373 (1.0020, 1.0738) | 0.0397 | |
| Heart failure | ||||
| IVW-MRE | 163 | 1.0729 (1.0202, 1.1284) | 0.0062 | |
| IVW-FE | 163 | 1.0729 (1.0366, 1.1105) | 6.05 | |
| Weighted Median | 163 | 1.0114 (0.9644, 10607) | 0.6394 | |
| MR Egger | 163 | 1.0694 (1.0062, 1.1366) | 0.0323 | |
| MR PRESSO | 5* | 1.1488 (1.0801, 1.2219) | 1.91 | |
| Venous thromboembolism | ||||
| IVW-MRE | 182 | 0.9301 (0.8555, 1.0112) | 0.0894 | |
| IVW-FE | 182 | 0.9301 (0.8703, 0.9940) | 0.0326 | |
| Weighted Median | 182 | 0.8917 (0.8072, 0.9851) | 0.0241 | |
| MR Egger | 182 | 0.9048 (0.8160, 1.0032) | 0.0591 | |
| MR PRESSO | 0* | 0.9301 (0.8555, 1.0112) | 0.0911 | |
| Peripheral atherosclerosis | ||||
| IVW-MRE | 181 | 1.2790 (1.2620, 1.4119) | 4.88 | |
| IVW-FE | 181 | 1.2620 (1.1595, 1.3735) | 7.32 | |
| Weighted Median | 181 | 1.0226 (0.8959, 1.1672) | 0.7406 | |
| MR Egger | 181 | 1.2728 (1.1081, 1.4620) | 0.0008 | |
| MR PRESSO | 4* | 1.4196 (1.2432, 1.6210) | 6.12 | |
*The number of outliers; OR, odds ratio.
The causal relationship between heart failure and TB-BMD remained regardless of
ApoB adjustment (
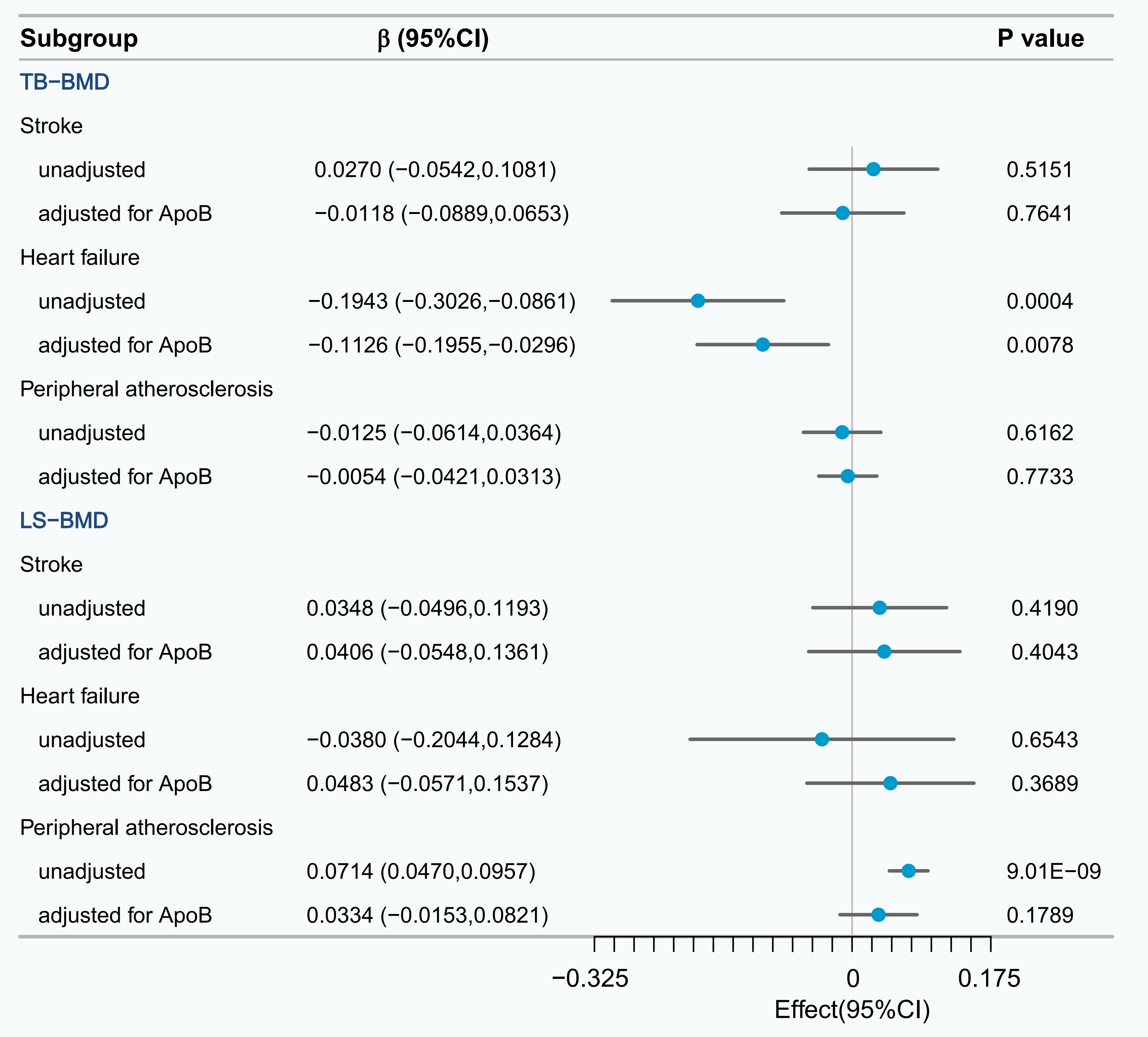 Fig. 4.
Fig. 4.
UVMR and multivariable mendelian randomization (MVMR) analysis of the effect of potential mediator on TB-BMD and LS-BMD.
This study investigated the association and potential causal relationship between ApoB with TB-BMD and LS-BMD, as well as whether cardiovascular diseases could mediate this causal relationship. We initially conducted an epidemiological observational analysis using the NHANES database. The results indicated that, after adjusting for various confounding factors, there was a negative correlation between serum ApoB with TB-BMD and LS-BMD. Further analysis revealed that this relationship was linear. The MR analysis further demonstrated a causal relationship between ApoB with TB-BMD and LS-BMD. Furthermore, we identified heart failure as a mediating factor in the causal relationship between ApoB and TB-BMD, with a mediation proportion of 18.69%.
Previous research has highlighted a correlation between lipid and bone metabolism, via intricate underlying mechanisms [34]. Bone marrow stromal cells require physiologic levels of cholesterol synthesis to undergo the process of osteogenic differentiation [35], while exogenous cholesterol serves as an inhibitor of this differentiation [36]. Cholesterol plays a role in bone metabolism through one of its essential byproducts, vitamin D, which is crucial for maintaining bone calcification [37]. Previous research has observed a negative correlation between serum total cholesterol levels and 25-hydroxyvitamin D (25(OH)D) levels [38]. Even when accounting for age and BMI adjustments, individuals with familial hypercholesterolemia may still encounter a reduction in BMD [39]. While elevated cholesterol levels can lead to arterial calcification, they often exhibit an opposing effect by reducing calcification in bones [40]. The influence of circulating lipids on bone metabolism has been extensively researched. However, studies focusing on ApoB have been relatively scarce. The protein component of lipoproteins, known as apolipoprotein, plays a crucial role in lipoprotein metabolism and has important physiological functions [41]. The liver synthesizes ApoB, which serves as the principal structural protein within low-density lipoprotein (LDL) [42]. ApoB provides an accurate measurement of both very low-density lipoprotein (VLDL) and LDL particle counts, making it a dependable surrogate for the actual count of LDL particles [43]. Therefore, ApoB may also be a valuable indicator for assessing its independent impact on bone health, separate from other lipids.
The evidence directly associating ApoB with bone health is limited. A study from NHANES showed that serum ApoB negatively correlates with lumbar spine BMD in females, which is similar to our findings [7]. Recent studies have shown that ApoB, unaffected by age, demonstrates higher sensitivity and specificity in predicting cardiovascular events. An MR study has indicated that ApoB is a key factor influencing the occurrence of coronary heart disease, distinct from cholesterol and triglycerides [44]. Our MR analysis also confirmed a causal relationship between ApoB and cardiovascular diseases, including stroke, coronary artery disease, heart failure, and peripheral atherosclerosis. An increasing volume of evidence highlights the interconnection between diseases of the vascular system and bone metabolism. Some scholars have introduced the concept of a bone-vascular axis, suggesting the existence of bidirectional endocrine and metabolic signals between the vascular system and bones. Disruptions in the bone-vascular axis, brought about by metabolic abnormalities, may lead to the simultaneous occurrence of vascular and skeletal disorders [45]. Previous studies support this concept, as elderly individuals with osteoporosis often exhibit a higher risk of cardiovascular disease compared to those without osteoporosis [46, 47]. Interestingly, despite increasing evidence of the association between BMD and cardiovascular disease, a prospective cohort study found a lack of clear association between BMD and the risk of cardiovascular disease. Through studying its experimental strategy, we found that this may be related to the shorter follow-up time. Additionally, the study measured the BMD of the non-dominant arm, while our research often focuses on the BMD of the lumbar spine and femur, which may also be a contributing factor. Further in-depth research is needed in this area in the future [12]. In addition, lipid-lowering medications may play a role in preserving BMD and reducing the risk of osteoporotic fractures [48, 49], while high-fat diets have been associated with decreased BMD in animal studies [50]. Some researchers have suggested a potential link between osteoporosis and atherosclerosis [51]. Our MR study found a positive causal relationship between the occurrence of peripheral atherosclerosis and bone density, which disappeared after adjusting for ApoB. Some studies have also reported a positive correlation between the lipid profiles associated with atherosclerosis and BMD [52]. This might be due to the similarity observed between the calcification process of atherosclerosis and processes observed in bone remodeling, as well as the existence of shared regulatory factors [53].
Our study also confirmed the mediating role of heart failure and quantified its proportion in the causal relationship between ApoB and TB-BMD. Although lipoproteins are typically not viewed as the primary risk factor for heart failure, certain studies suggest an association with the occurrence of heart failure [54, 55]. A study in Sweden showed a positive link between ApoB/ApoA-1 levels and the onset of heart failure [56]. Furthermore, a recent MR analysis proposed that therapies aimed at lowering lipids related to ApoB might provide greater advantages in lowering the risk of heart failure [57]. There is considerable research supporting the association between heart failure and a heightened risk of low BMD [58]. Shared risk factors between the two include reduced exercise endurance, decreased levels of 25(OH)D, and the presence of diabetes mellitus. The reduced physical activity in heart failure patients may impact their vitamin D levels and subsequently affect bone metabolism [59]. Heart failure patients elevate their adiponectin levels internally to counteract metabolic damage caused by the disease, and adiponectin has been confirmed as one of the lipid factors most associated with decreased BMD [60]. Furthermore, they exhibit common pathogenic mechanisms, such as activation of the renin-angiotensin-aldosterone system, elevated levels of parathyroid hormone, and the response to oxidative stress [61]. In future mechanistic studies, we can further explore the mechanisms by which ApoB directly affects bone cells (osteoblasts/osteoclasts) through lipoprotein particles, for example, through oxidative stress or inflammatory signals. At the same time, we can also analyze the driving factors for the reduction of BMD in heart failure patients, such as insufficient bone perfusion due to decreased cardiac output or neurohormonal activation [62].
This study stands as the first comprehensive exploration delving into the relationship between serum ApoB and BMD using an epidemiological observational analysis with population-wide data and MR studies of large-scale genetic data. These findings support the theory that elevated serum levels of ApoB adversely affect bone health and suggests that improving heart function might be an important target for intervening in osteoporosis associated with elevated ApoB levels. These findings are significant for assessing the long-term effects of ApoB-lowering drugs on BMD and the bone protective effects in high-risk heart failure populations, and they provide important guidance on whether early intervention in heart failure is crucial in reversing the impact of elevated ApoB on BMD.
This study has several strengths. Firstly, in our observational study, we conducted adjustments for a multitude of confounding factors closely associated with BMD and had a relatively large sample size. Secondly, we established strict mediation screening criteria to ensure the credibility of the results. Additionally, consistent outcomes were observed across various sensitivity analyses, reflecting the robustness of the evidence and suggesting that confounding factors are unlikely to explain the observed correlations. Nevertheless, there are certain limitations present in this study. First, despite our use of various MR methods to mitigate pleiotropy-related confusion, it is important to acknowledge the presence of residual bias, a recognized limitation of MR studies. Second, MR analysis typically examines the lifelong influence of risk factors on outcomes, which can complicate the identification of causal effects at various stages of disease development. Third, our observational study and genetic data were obtained from different populations, with the cross-sectional study conducted among the American population and the MR study involving individuals of European ancestry, this may lead to results that cannot be generalized to other races or regions. Fourth, the age range of the study subjects is 20–59 years, which does not include the elderly population that is at high risk for osteoporosis, limiting the generalizability of the conclusions. At the same time, we also lack a discussion on the potential impact of different age stages on BMD, such as the fact that bone loss is often more common in postmenopausal women. In the future, we could additionally include different age groups to eliminate such confounding factors. Regarding data sources, there may be differences in measurement standards of different GWAS data, lifestyle habits of populations, and methods of covariate adjustment, which could affect the consistency of results. Additionally, the GWAS data for candidate mediating diseases may come from different sources, and their diagnostic criteria or phenotype definitions may not be consistent. Furthermore, in terms of statistical methods, although we tested for horizontal pleiotropy using methods like MR-Egger, we cannot completely rule out the potential bias of instrumental variables affecting the results through other unknown pathways. We also simplified the mediation analysis by only quantifying the mediating proportion of heart failure, without exploring the effects of other potential mediating factors such as inflammatory markers and vitamin D levels. Given these limitations, this finding needs to be validated in well-designed prospective cohort studies and cross-ethnic cohort studies, combined with metabolomics and proteomics data, to identify key biomarkers in the ApoB-BMD association and further investigate its potential mechanisms.
Through cross-sectional study and MR analysis, we elucidated the association between elevated serum ApoB levels and decreased TB-BMD and LS-BMD. Furthermore, we discovered that heart failure may mediate the causal relationship between ApoB and TB-BMD. The study findings indicated that reducing serum ApoB levels could improve BMD. In addition, in individuals with suboptimal ApoB control, preventing heart failure may have mitigated the detrimental effects of decreased BMD resulting from elevated ApoB levels.
Publicly available datasets are available online for this study. The NHANES repository/repositories name and accession numbers are available online at (https://www.cdc.gov/nchs/nhanes/index.html). The entirety of the data used in this MR study was sourced from the publicly accessible Open GWAS project database (https://gwas.mrcieu.ac.uk/), available for free to all interested parties. Parts of the graphical abstract were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/). All information used for this study is publicly available as deidentified GWAS summary statistics.
ACS, YDC, JCZ, and ZTL designed the research study. ACS performed the research. ACS, YW, and ZZL analyzed the data. ACS, YDC, YW, and ZZL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of National Center for Health Statistics (Protocol No. 2011-17). The patients/participants provided their written informed consent to participate in this study.
We gratefully thank Wentao Ni (Department of Pulmonary and Critical Care Medicine, Peking University People’s Hospital, Beijing, China) and Qiushi Shi (Huawei Technologies Co., Ltd., Shanghai, China) for their contribution to the statistical support and comments regarding the manuscript.
This research was funded by “Special Research Project of CACMS on Enhancing the Level of Clinical Evidence-Based Evidence for TCM, grant number XYZX020111”, “Special Research Project of CACMS on Enhancing the Level of Clinical Evidence-Based Evidence for TCM, grant number XYZX020121”.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM31395.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
