- Academic Editor
†These authors contributed equally.
The clinical prognosis of ST-elevation myocardial infarction (STEMI) patients with mural thrombus in left ventricular aneurysm (MTLVA) remains poor; moreover, the risk factors associated with the non-resolution (persistent or recurrent) of MTLVA are not well understood. This study aimed to identify independent risk factors for MTLVA non-resolution.
A total of 133 STEMI patients (mean age 62 ± 11 years, 80.5% male) with MTLVA, admitted to our department between 2014 and 2022, were included in this retrospective analysis. Patients were categorized into two groups: resolution (n = 59) and non-resolution [persistent (n = 72) or recurrent (n = 2) MTLVA; n = 74]. The median follow-up duration was 25 months, during which adverse events were monitored, including stroke, re-revascularization, major bleeding, systemic embolism, and cardiac death.
The prevalence of non-resolution was 55.6%. Non-resolution was significantly associated with elevated lipoprotein (a) [Lp(a)] levels (>270 mg/L, hazard ratios (HR) 2.270, p = 0.003), larger left ventricular aneurysm (LVA) area (>4.5 cm2, HR 4.038, p < 0.001), and greater mural thrombus (MT) area (>2.2 cm2, HR 2.40, p = 0.002), independent of other risk factors, such as hypercholesterolemia and left circumflex artery (LCX)-related STEMI. Baseline left ventricular ejection fraction (LVEF) was lower in the non-resolution group (41.7% vs. 45.7%, p = 0.008). During follow-up, the LVEF remained lower in the non-resolution group and increased in the resolution group. The composite of adverse events was significantly higher in the non-resolution group (28.4% vs. 8.5%, p = 0.003), including stroke (p = 0.025) and systemic embolism (p = 0.034).
Independent risk factors for thrombus non-resolution in STEMI patients with MTLVA include elevated Lp(a), larger LVA and MT areas. These factors contribute to thrombus persistence and are associated with worse clinical outcomes. However, further studies are needed to assess targeted management strategies for high-risk patients.
Current revascularization strategies and modern antithrombotic therapy have markedly reduced the incidence of left ventricular thrombus (LVT) over the last decades [1]. Contemporary data showed that the incidence of LVT might still be as high as 15% to 25% in patients with ST-elevation myocardial infarction (STEMI) post percutaneous coronary intervention (PCI) and modern antithrombotic therapy [2, 3]. Nowadays, mural thrombus (MT) in left ventricular aneurysm (LVA) post STEMI remains a clinical challenge associated with poor outcome [4, 5, 6].
Guidelines recommend antithrombotic drug treatment for least 3 months and up to 6 months depending on follow-up imaging results for MT in LVA (MTLVA) patients [7, 8]. Persistence or recurrence MTLVA remained as difficult clinical scenario despite modern antithrombotic therapy [5, 9, 10, 11]. Observational research suggests that persistence of LVT is not uncommon [12]. Lattuca et al. [5] found that total regression was only achieved in 62.3% patients with LVT within a median time of 103 days. Recently, Zhou et al. [4] demonstrated similar LVT resolution rate (63.7%) in another LVT patient cohort, during a median follow-up of 1.2 years after LVT resolution, LVT recurrence rate was 24.3% (n = 28) in this patient cohort. As expected, persistent or recurrent LVT were associated with even higher risk of major adverse cardiovascular events (MACE), embolic, or major bleeding complications, as well as mortality as compared to patients with complete LVT resolution [2, 4, 6, 7].
Identifying independent risk factors for non-resolution (persistent or recurrence) MTLVA is of clinical importance for risk stratification and decision-making for therapeutic and monitoring strategies. In this retrospective study, we explored the independent determinants of MTLVA non-resolution and compared the clinical outcomes in MTLVA patients with or without complete resolution.
We screened 303 consecutive STEMI patients with LVA who were admitted to our department between March 2014 and June 2022. Of these, transthoracic echocardiography (TTE) identified MT in 133 patients, leading to their classification as MTLVA cases. Two independent experts confirmed the diagnosis of MT through imaging review. These 133 patients were included in this retrospective clinical cohort study for further analysis. All patients received standard antithrombotic therapy in accordance with the relevant guidelines issued by the European Society of Cardiology (post-LVA) [8, 13]. Smoking status was defined as former or current smoking based on self-reported history. Alcohol intake was defined as an average daily intake exceeding 40 g for men or 20 g for women, sustained for at least five years. Patients were followed for a median duration of 25 months, during which clinical visit and echocardiographic examinations were conducted at each follow-up visit.
Detection of MT and LVA was accomplished using TTE (Philips EPIQ-7C, Philips Healthcare, Amsterdam, The Netherlands). MT appeared as an echodense mass adjacent to the left ventricular (LV) wall, with confirmation of LVA achieved through akinetic or dyskinetic LV wall motion, which bulged outward during systole. To differentiate MT from the underlying myocardium, a distinct thrombus-blood interface was necessary, and MT had to be observable on at least two views throughout the cardiac cycle. Quantitative evaluation of LVA and MT characteristics was performed offline, involving measurements of the maximal long and short diameters of LVA (LVA_LD and LVA_SD), the area of LVA (LVA_area), the maximal long and short diameters of MT (MT_LD and MT_SD), and the area of MT (MT_area). All measurements were based on the mean of three assessments.
Patients were divided into two groups based on the resolution status of MTLVA. The MTLVA resolution group was characterized by the complete disappearance of MTLVA following antithrombotic treatment, as confirmed by echocardiographic examinations during follow-up. Conversely, the MTLVA non-resolution group comprised patients in whom MTLVA persisted or recurred despite antithrombotic treatment, as observed through echocardiographic assessment during follow-up.
The primary endpoint was defined as the occurrence of adverse events during follow-up, encompassing stroke, re-revascularization, major bleeding, systemic embolism, or cardiac death.
Continuous variables are expressed as mean
All patients underwent serial echocardiography follow-up for MTLVA assessment, with a median of 4 (3–5) evaluations. Over a median follow-up of 25 (14–39) months, MTLVA resolution was observed in 44.4% (59 out of 133) of patients with STEMI, while 55.6% (74 out of 133) exhibited non-resolution including two cases of MTLVA recurrence. Specifically, MTLVA disappeared in 42 patients at the second echocardiography [median period of 46 (21–98) days], in 52 patients at the third echocardiography [median period of 6.5 (2.8–18.2) months], and in 59 patients at the fourth echocardiography [median period of 12.0 (8.5–23.5) months].
Table 1 details the baseline characteristics of STEMI patients with MTLVA
resolution and non-resolution. The mean age was 62
| Total | MTLVA resolution | MTLVA non-resolution | p value | |||
| No. [n (%)] | 133 (100) | 59 (44.4) | 74 (55.6) | |||
| Age (years) | 62 |
63 |
62 |
0.811 | ||
| Male [n (%)] | 107 (80.5) | 45 (76.3) | 62 (83.8) | 0.278 | ||
| BMI (kg/m²) | 24.7 |
24.6 |
24.8 |
0.330 | ||
| Hypertension [n (%)] | 70 (52.6) | 31 (52.5) | 39 (52.7) | 0.985 | ||
| Diabetes mellitus [n (%)] | 37 (27.8) | 16 (27.1) | 21 (28.4) | 0.872 | ||
| Hypercholesterolemia [n (%)] | 50 (37.6) | 18 (30.5) | 32 (43.2) | 0.132 | ||
| Smoking [n (%)] | 66 (49.6) | 24 (40.7) | 42 (56.8) | 0.065 | ||
| Alcohol intake [n (%)] | 26 (19.5) | 7 (11.9) | 19 (25.7) | 0.046 | ||
| Laboratory data | ||||||
| WBC (109/L) | 7.48 (5.99–8.94) | 7.58 (6.11–9.11) | 7.29 (5.84–8.63) | 0.375 | ||
| Hb (g/L) | 140 (126–150) | 137 (128–155) | 141 (125–148) | 0.747 | ||
| PLT (109/L) | 215 (182–267) | 211 (174–260) | 215 (184–273) | 0.873 | ||
| D-Dimer (ng/mL) | 530 (349–1007) | 435 (297–687) | 720 (400–1297) | 0.002 | ||
| NT-proBNP (pg/mL) | 890 (323–2680) | 1077 (430–3094) | 800 (300–2702) | 0.346 | ||
| AST (U/L) | 23.0 (15.1–44.0) | 23.5 (17.0–53.5) | 20.5 (16.0–35.0) | 0.253 | ||
| ALT (U/L) | 25.0 (19.0–40.0) | 26.0 (15.3–41.0) | 24.0 (15.0–49.0) | 0.934 | ||
| Cr (mg/dL) | 82.0 (64.0–99.0) | 80.0 (63.0–95.7) | 86.0 (64.0–103.0) | 0.300 | ||
| TG (mmol/L) | 1.24 (0.92–1.75) | 1.28 (0.96–1.70) | 1.17 (0.86–1.86) | 0.803 | ||
| TC (mmol/L) | 4.20 (3.41–4.85) | 4.24 (3.45–4.95) | 4.03 (3.39–4.84) | 0.433 | ||
| LDL (mmol/L) | 2.43 (1.88–2.97) | 2.49 (1.90–3.00) | 2.40 (1.80–2.91) | 0.600 | ||
| Uric acid (µmol/L) | 364 (290–427) | 354 (285–408) | 379 (293–442) | 0.389 | ||
| Lp(a) (mg/L) | 182 (111–359) | 164 (79–269) | 204 (118–409) | 0.093 | ||
| STEMI treatment [n (%)] | 0.376 | |||||
| PCI only | 98 (73.7) | 47 (79.7) | 51 (68.9) | |||
| CABG only or PCI+CABG | 26 (19.5) | 9 (15.3) | 17 (23.0) | |||
| Thrombolytic administration only | 9 (6.8) | 3 (5.1) | 6 (8.1) | |||
| Involved coronary vessel | ||||||
| LAD | 118 (88.7) | 51 (86.4) | 67 (90.5) | 0.458 | ||
| LCX | 50 (37.6) | 16 (27.1) | 34 (45.9) | 0.026 | ||
| RCA | 39 (29.3) | 16 (27.1) | 23 (31.1) | 0.618 | ||
| Antithrombotic Strategy Following MTLVA [n (%)] | ||||||
| Aspirin | 103 (77.4) | 45 (76.3) | 58 (78.4) | 0.773 | ||
| Clopidogrel | 67 (50.4) | 30 (50.8) | 37 (50.0) | 0.923 | ||
| Ticagrelor | 23 (17.3) | 11 (18.6) | 12 (16.2) | 0.713 | ||
| Indobufen | 4 (3.0) | 4 (6.8) | 0 (0.0) | 0.023 | ||
| VKAs | 76 (57.1) | 39 (66.1) | 37 (50.0) | 0.062 | ||
| NOACs | 54 (40.6) | 17 (28.8) | 37 (50.0) | 0.013 | ||
| Rivaroxaban | 44 (33.1) | 14 (23.7) | 30 (40.5) | 0.041 | ||
| Dabigatran | 10 (7.5) | 3 (5.1) | 7 (9.5) | 0.342 | ||
| Anticoagulation only | 17 (12.8) | 6 (10.2) | 11 (14.9) | 0.420 | ||
| Anticoagulation + antiplatelet therapy | 35 (26.2) | 16 (27.1) | 19 (25.7) | 0.851 | ||
| Anticoagulation + dual antiplatelet therapy | 81 (60.9) | 37 (62.7) | 44 (59.5) | 0.703 | ||
| Cardiac related medications [n (%)] | ||||||
| ß-blockers | 112 (84.2) | 52 (88.1) | 60 (8.1) | 0.268 | ||
| ACEIs/ARBs | 46 (34.6) | 18 (30.5) | 28 (37.8) | 0.377 | ||
| ARNIs | 35 (26.3) | 21 (35.6) | 14 (18.9) | 0.030 | ||
| MRAs | 86 (64.7) | 37 (62.7) | 49 (66.2) | 0.674 | ||
| SGLT2i | 8 (6.0) | 2 (3.4) | 6 (8.1) | 0.300 | ||
| Loop diuretic | 124 (93.2) | 51 (86.4) | 73 (98.6) | 0.011 | ||
| CCB | 33 (24.8) | 19 (32.2) | 14 (18.9) | 0.078 | ||
| Nitrates | 133 (100) | 59 (100) | 74 (100) | - | ||
| Statins | 133 (100) | 59 (100) | 74 (100) | - | ||
| Echocardiography | ||||||
| LVEF (%) | 43.5 |
45.7 |
41.7 |
0.008 | ||
| LVA_LD (cm) | 3.68 |
2.78 |
4.40 |
|||
| LVA_SD (cm) | 2.31 |
1.83 |
2.70 |
|||
| LVA_area (cm²) | 5.65 (3.53–10.15) | 3.53 (2.83–4.95) | 8.89 (5.99–12.80) | |||
| MT_LD (cm) | 2.44 |
1.86 |
2.91 |
|||
| MT_SD (cm) | 1.29 |
0.99 |
1.53 |
|||
| MT_area (cm²) | 2.16 (1.15–3.94) | 1.34 (0.82–2.12) | 3.56 (1.79–4.91) | |||
| Outcomes [n (%)] | ||||||
| FUP duration (months) | 20 (12–33) | 25 (15–40) | 16 (10–27) | |||
| Surgery for LVA | 5 (3.8) | 2 (3.4) | 3 (4.1) | 1.000 | ||
| CV death | 7 (5.3) | 1 (1.7) | 6 (8.1) | 0.132 | ||
| Major bleeding | 7 (5.3) | 1 (1.7) | 6 (8.1) | 0.132 | ||
| rPCI | 1 (0.8) | 1 (1.7) | 0 (0.0) | 0.444 | ||
| Stroke | 13 (9.8) | 2 (3.4) | 11 (14.9) | 0.027 | ||
| Systemic embolism | 4 (3.0) | 0 (0.0) | 4 (5.4) | 0.129 | ||
| Adverse events | 26 (19.5) | 5 (8.5) | 21 (28.4) | 0.004 | ||
ACEIs, angiotensin-converting enzyme inhibitors; ALT, alanine aminotransferase; ARNIs, angiotensin receptor neprilysin inhibitors; ARBs, angiotensin II receptor antagonists; AST, aspartate aminotransferase; BMI, body mass index; CABG, coronary artery bypass graft; CCB, calcium antagonist; Cr, creatinine; FUP, follow-up; Hb, hemoglobin; LAD, left anterior artery; LCX, left circumflex artery; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); LVA, left ventricular aneurysm; LVA_LD, left ventricular aneurysm long diameter; LVA_SD, left ventricular aneurysm short diameter; LVEF, left ventricular ejection fraction; MRAs, mineralcorticoid recept antagonist; MT_LD, mural thrombus long diameter; MTLVA, mural thrombus in left ventricular aneurysm; MT_SD, mural thrombus short diameter; NOACs, non-vitamin K oral anticoagulants; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCI, percutaneous coronary intervention; PLT, platelet; RCA, right coronary artery; SGLT2i, sodium-dependent glucose transporters 2 inhibitors; STEMI, ST-segment elevation myocardial infarction; TC, total cholesterol; TG, triglyceride; VKAs, vitamin K antagonists; WBC, white blood cell; CV, cardiovascular; rPCI, repeat percutaneous coronary intervention.
All patients received low molecular weight heparin at admission. Post-discharge, 57.1% (n = 76) were on vitamin K antagonists (VKAs), 33.1% (n = 44) on rivaroxaban, and 7.5% (n = 10) on dabigatran. Anticoagulation was not applied to three patients due to high bleeding risk. Concomitant antiplatelet therapy was common (87.2%, n = 116), with 26.2% (n = 35) on anticoagulation + single antiplatelet and 60.9% (n = 81) on anticoagulation + dual antiplatelet therapy (Table 1). 12.8% patients (n = 17) received anticoagulation alone due to high bleeding risk. Anticoagulant and antiplatelet medication usage showed no significant difference between MTLVA resolution and non-resolution groups (Table 1).
Among the patients, 73.7% (n = 98) underwent PCI, 19.5% (n = 26) underwent coronary artery bypass grafting (CABG) or a combination of PCI and CABG, and 6.8% (n = 9) received conservative treatment. The rates of coronary revascularization did not differ significantly between the resolution and non-resolution groups (Table 1). However, STEMI involving the left circumflex artery (LCX) was more common in the non-resolution group compared to the resolution group (45.9% vs. 27.1%, p = 0.026).
LVEF was significantly lower in the MTLVA non-resolution group compared to the
MTLVA resolution group (mean 41.7% vs. 45.7%, p = 0.008).
Additionally, the size of the LVA and MT was significantly larger in the MTLVA
non-resolution group than in the MTLVA resolution group (all p
Larger LVA_area and MT_area demonstrated excellent diagnostic performance for
predicting MTLVA non-resolution, with an AUC of 0.875 (95% CI 0.817–0.933,
p
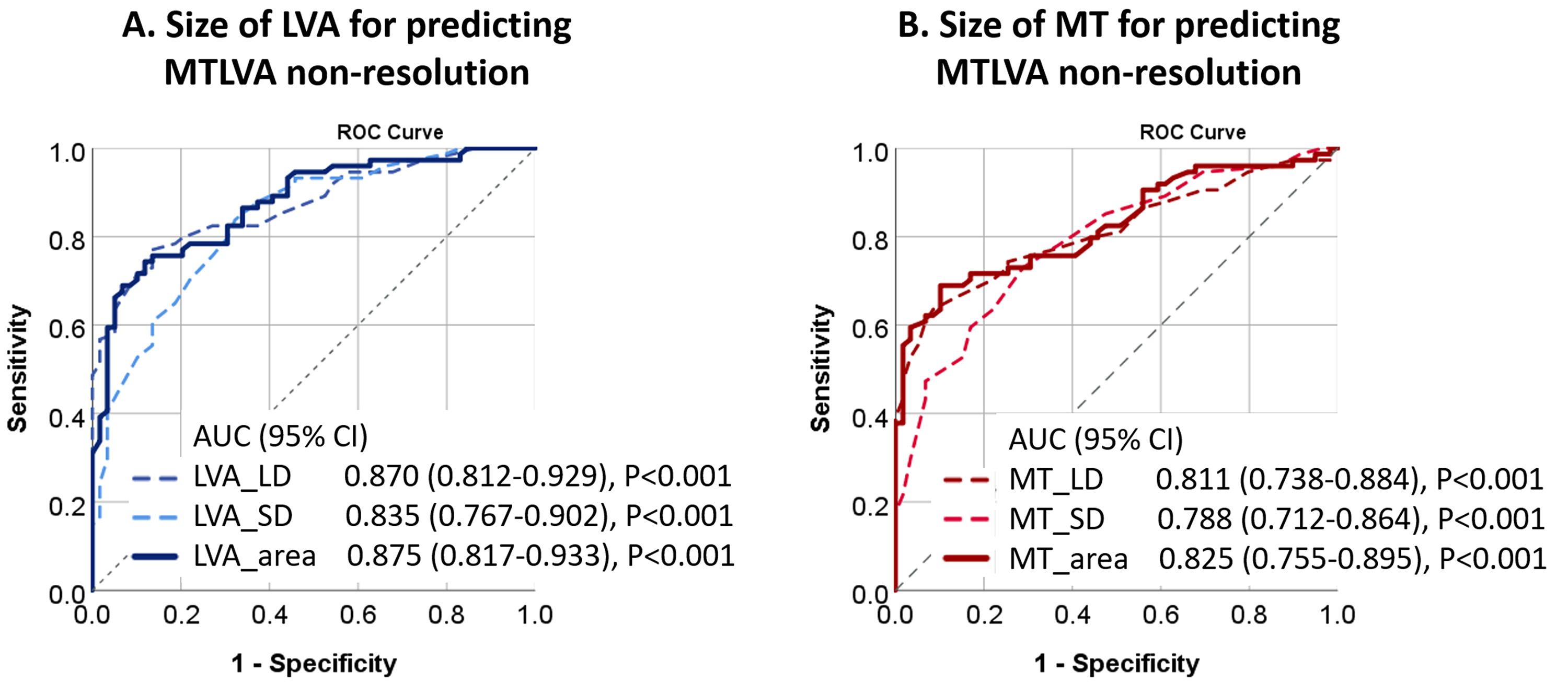 Fig. 1.
Fig. 1.
Diagnostic performance of LVA and MT in predicting MTLVA non-resolution. (A) ROC curve showing the diagnostic performance of LVA_area for predicting MTLVA non-resolution, with an AUC of 0.875. (B) ROC curve demonstrating the diagnostic ability of MT_area, with an AUC of 0.825, both indicating strong predictive value. CI, confidence interval; MT, mural thrombus; ROC, receiver operating characteristic; AUC, areas under curves.
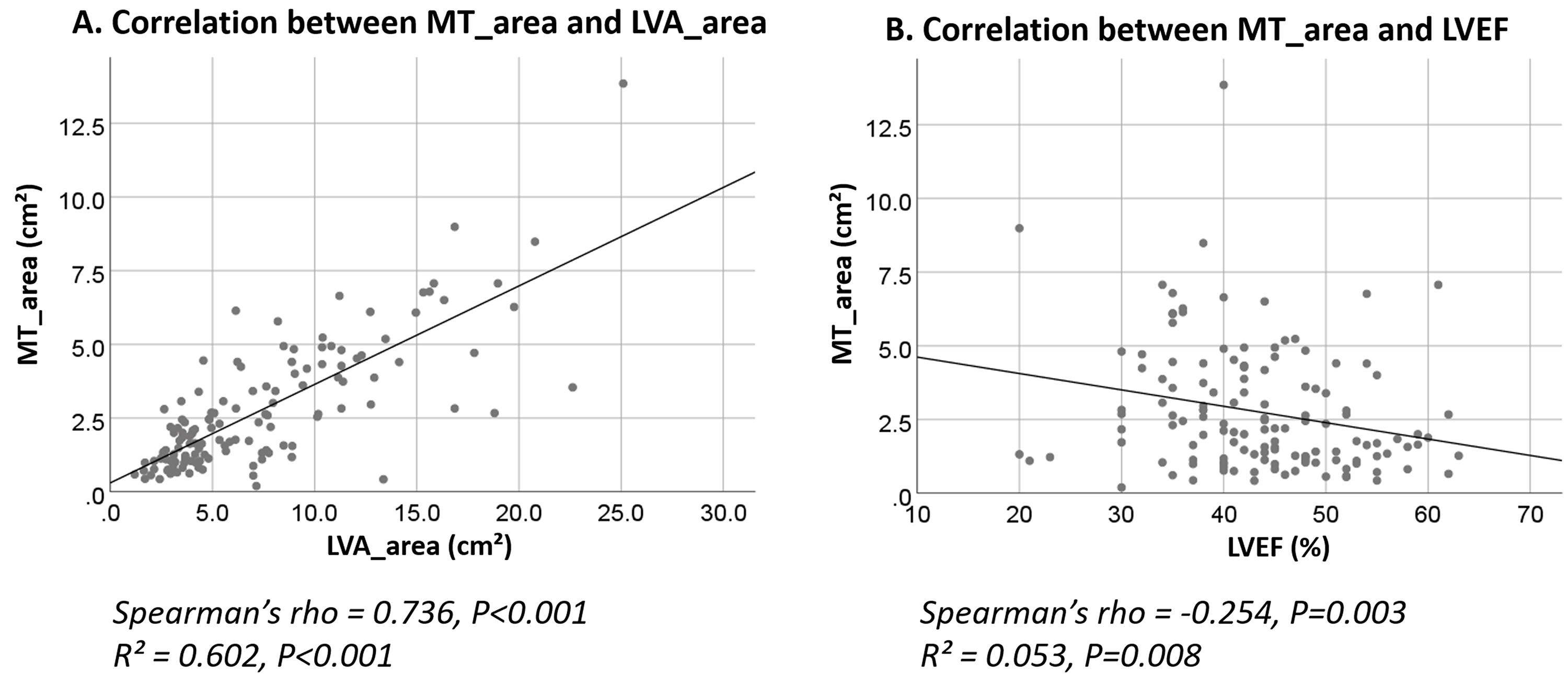 Fig. 2.
Fig. 2.
Correlation analysis of LVA_area, MT_area, and LVEF in relation to MTLVA non-resolution. (A) Scatter plot showing a strong positive correlation between MT_area and LVA_area, suggesting that larger thrombus and aneurysm areas are linked to MTLVA non-resolution. (B) Scatter plot showing a negative correlation between LVEF and MT_area, indicating that lower ejection fraction is associated with larger mural thrombus areas.
Univariable Cox regression analysis identified several potential risk factors
for non-resolution of MTLVA, including hypercholesterolemia, LCX-related STEMI,
D-Dimer
| Univariable Cox model | Univariable HR (95% CI) | p value | |
| Age | 0.990 (0.990–1.011) | 0.338 | |
| Male vs. female | 1.327 (0.712–2.471) | 0.373 | |
| Hypercholesterolemia | 1.624 (1.018–2.590) | 0.042 | |
| LCX-related STEMI | 1.871 (1.175–2.978) | 0.008 | |
| D-Dimer |
1.628 (1.001–2.648) | 0.050 | |
| Lp(a) |
1.889 (1.130–3.159) | 0.015 | |
| Baseline LVEF |
1.681 (1.061–2.664) | 0.027 | |
| LVA_area |
3.544 (1.944–6.461) | ||
| MT_area |
2.952 (1.779–4.900) | ||
| Model A | a Multivariable HR (95% CI) | p value | |
| Hypercholesterolemia | 2.135 (1.243–3.667) | 0.006 | |
| LCX-related STEMI | 2.228 (1.281–3.875) | 0.005 | |
| Lp(a) |
2.270 (1.315–3.918) | 0.003 | |
| LVA_area |
4.038 (2.083–7.829) | ||
| Model B | a Multivariable HR (95% CI) | p value | |
| Hypercholesterolemia | 1.715 (1.017–2.893) | 0.043 | |
| LCX related STEMI | 1.875 (1.099–3.196) | 0.021 | |
| Lp(a) |
2.264 (1.323–3.876) | 0.003 | |
| MT_area |
2.398 (1.377–4.178) | 0.002 | |
a Multivariable Cox regression with “backward stepwise” method.
Variables in the Model A included age, sex, hypercholesterolemia, LCX-related
STEMI, D-Dimer
Variables in the Model B included age, sex, hypercholesterolemia, LCX related
STEMI, D-Dimer
HR, hazard ratio.
Given the significant collinearity between LVA_area and MT_area (Spearman’s
rho = 0.736, p
In Model A, which adjusted for age, sex, hypercholesterolemia, LCX-related
STEMI, D-Dimer
As depicted in Fig. 3, throughout the follow-up periods, the mean LVEF was
consistently lower in the non-resolution group, while in the resolution group,
LVEF demonstrated a trend of increase over time (p = 0.006,
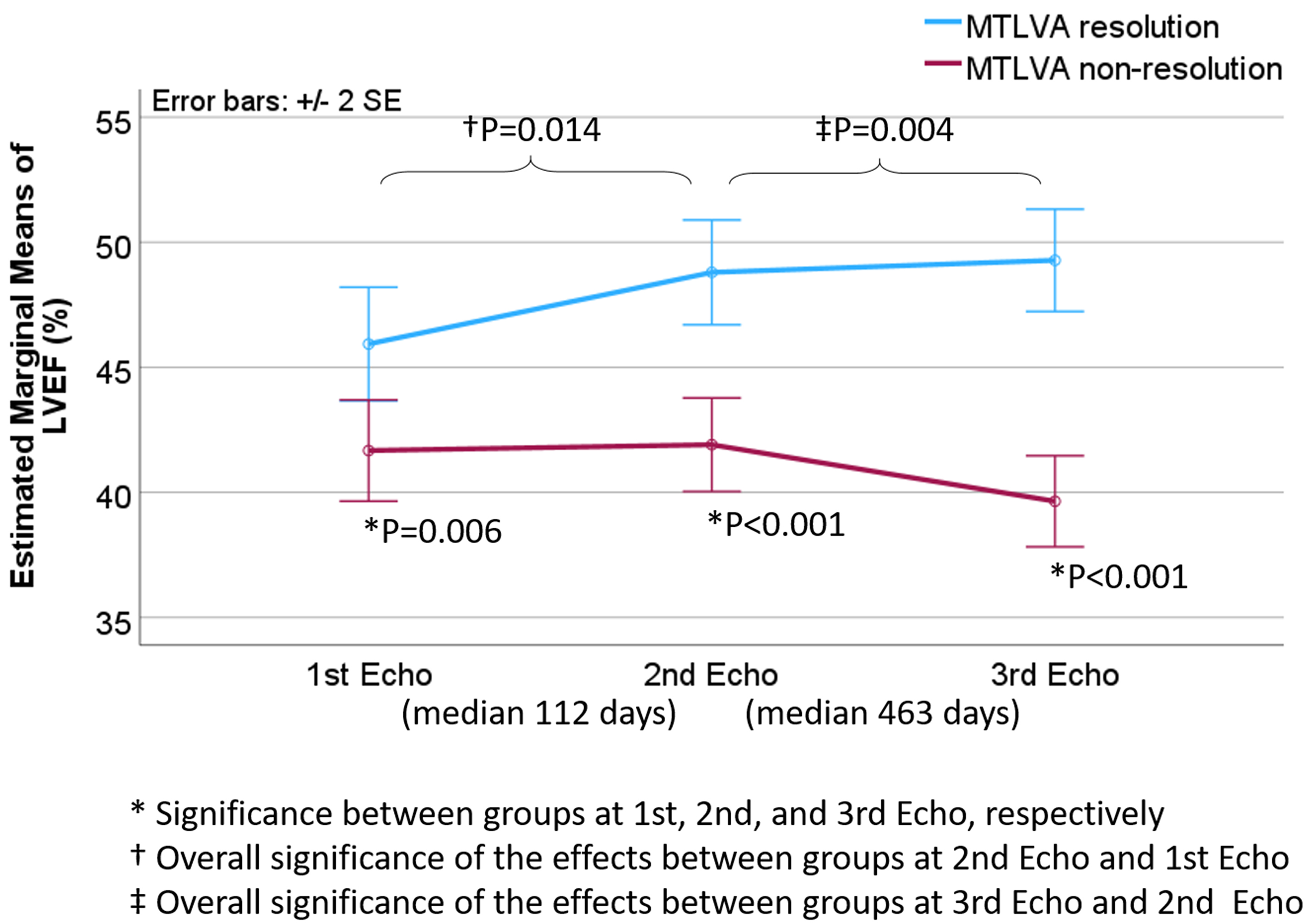 Fig. 3.
Fig. 3.
Dynamic changes in LVEF associated with non-resolution of MTLVA. The plot shows that, throughout the follow-up period, the mean LVEF remained
significantly lower in the non-resolution group compared to the resolution group.
In contrast, the resolution group exhibited a consistent increase in LVEF over
time. The differences between the two groups were statistically significant at
the 1st, 2nd, and 3rd echocardiographic evaluations (p = 0.006,
Non-resolution of MTLVA was associated with significantly worse outcomes compared to resolution (Table 1 and Fig. 4). Cardiovascular mortality (Fig. 4A) was higher in the non-resolution group (8.1% vs. 1.7%), though not statistically significant (p = 0.066). Major bleeding events (Fig. 4B) showed a similar trend, occurring more frequently in the non-resolution group (8.1% vs. 1.7%, p = 0.059). Stroke (Fig. 4C) was significantly more common in the non-resolution group (14.9% vs. 3.4%, p = 0.025). Systemic embolism (Fig. 4D) occurred in 5.4% of the non-resolution group but was absent in the resolution group (p = 0.034). The composite of adverse events (Fig. 4E), including stroke, re-revascularization, major bleeding, systemic embolism, and cardiac death, was significantly higher in the non-resolution group than in the resolution group (28.4% vs. 8.5%, p = 0.003).
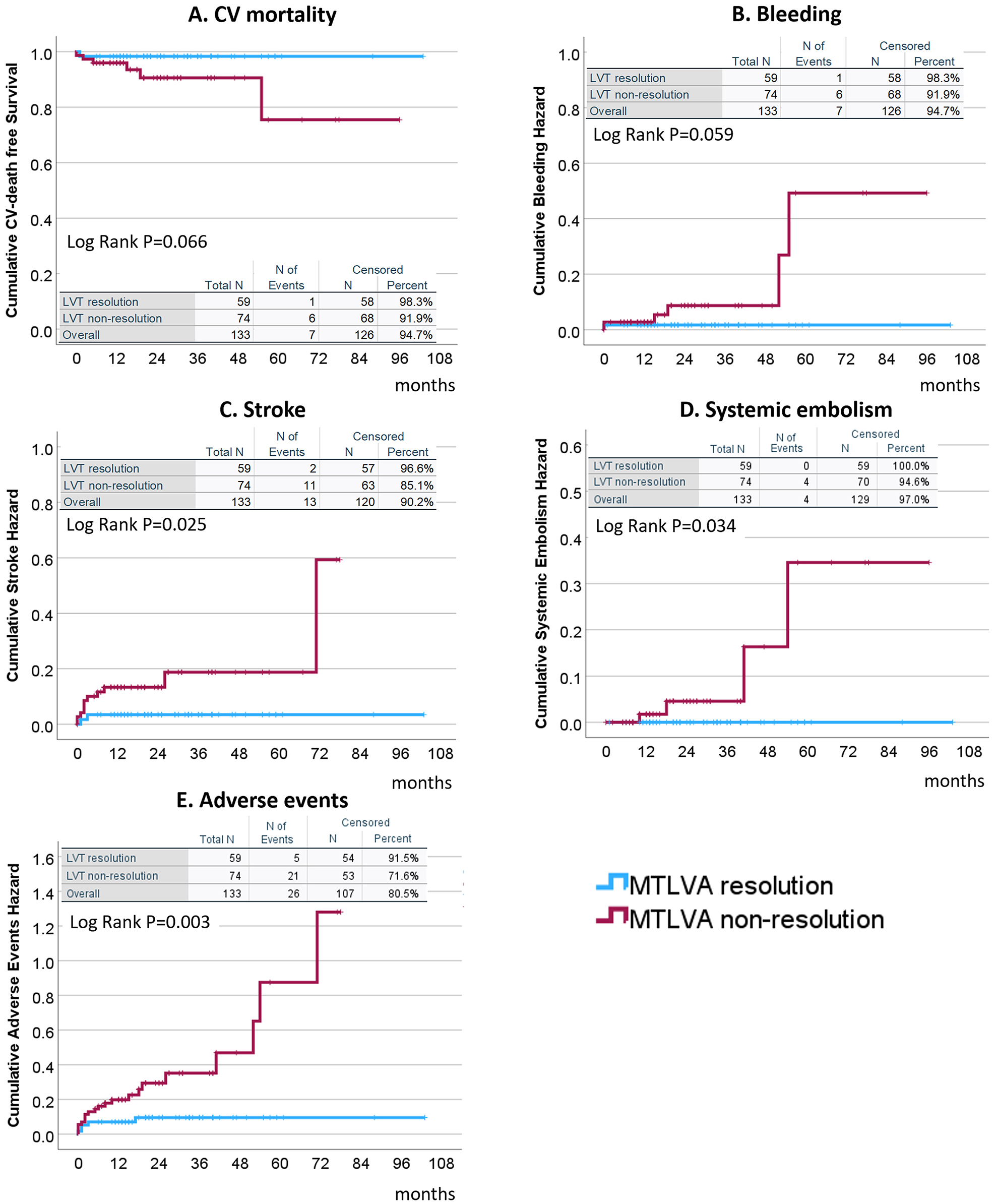 Fig. 4.
Fig. 4.
Clinical outcomes associated with non-resolution of MTLVA. Non-resolution of MTLVA was associated with worse clinical outcomes. Cardiovascular mortality (A) and major bleeding events (B) showed higher rates in the non-resolution group (p = 0.066 and p = 0.059, respectively). Stroke (C) was significantly more frequent in the non-resolution group (14.9% vs. 3.4%, p = 0.025). Systemic embolism (D) occurred in 5.4% of the non-resolution group but was absent in the resolution group (p = 0.034). The composite adverse event rate (E), including cardiovascular death, major bleeding, stroke, and systemic embolism, was significantly higher in the non-resolution group (28.4% vs. 8.5%, p = 0.003). LVT, left ventricular thrombus.
Present study identified 6 potential prognostic markers associated with the occurrence of adverse events in this cohort, including N-terminal pro-brain natriuretic peptide (NT-proBNP), serum creatinine, total cholesterol (TC), LVEF, LVA_area, and MTLVA non-resolution (Table 3). Multivariable Cox regression analysis revealed that NT-proBNP, TC, and MTLVA non-resolution remained independently associated with adverse events, while the correlation with adverse events for serum creatinine level, LVEF, and LVA_area was no longer significant after multivariable adjustment (Table 3).
| Univariable Cox model | Univariable HR (95% CI) | p value |
| Age (years) | 0.989 (0.954–1.026) | 0.559 |
| Male vs. female | 5.559 (0.746–41.409) | 0.094 |
| Ln (NT-proBNP) | 1.528 (1.145–2.039) | 0.004 |
| Cr (mg/dL) | 1.008 (1.001–1.015) | 0.029 |
| TC (mmol/L) | 0.585 (0.377–0.909) | 0.017 |
| Baseline LVEF (%) | 0.936 (0.892–0.983) | 0.008 |
| LVA_area (cm2) | 1.072 (0.995–1.172) | 0.066 |
| MTLVA non-resolution | 3.314 (1.226–8.959) | 0.018 |
| Multivariable Cox model | a Multivariable HR (95% CI) | p value |
| Ln (NT-proBNP) | 1.460 (1.103–1.933) | 0.008 |
| TC (mmol/L) | 0.648 (0.408–1.029) | 0.066 |
| MTLVA non-resolution | 3.270 (1.077–9.930) | 0.037 |
a Multivariable Cox regression with “backward stepwise” method (Likelihood ratio).
Variables in the multivariable model included age, sex, Ln (NT-proBNP), Cr, TC, baseline LVEF, and MTLVA non-resolution.
This retrospective study aimed to identify independent risk factors for the
non-resolution of MT in patients with LVA following STEMI. Our analysis revealed
that a significant proportion of patients with MTLVA (55.6%) did not experience
thrombus resolution. Key independent clinical predictors of non-resolution
included elevated lipoprotein (a) [Lp(a)] levels (
Our study highlights the ongoing challenge of MTLVA non-resolution despite contemporary antithrombotic therapies. In our cohort, resolution occurred in only 45.4% of STEMI patients with MTLVA over a median follow-up of 25 months. Similarly, Lattuca et al. [5] reported a resolution rate of 62.3% within 103 days, while Zhou et al. [4] observed non-resolution in 32.6% of patients during a median follow-up of 1.2 years, with 24.3% experiencing recurrence.
Importantly, our study identified independent predictors of MTLVA
non-resolution, including elevated Lp(a) levels (
We observed that a higher proportion of patients in the resolution group were on VKAs compared to the non-resolution group (66.1% vs. 50.0%, p = 0.062). In contrast, the non-resolution group had a greater proportion of patients on non-vitamin K oral anticoagulants (NOACs, 50.0% vs. 28.8%, p = 0.013). This suggests that VKAs may be more effective in facilitating thrombus resolution compared to NOACs in this patient cohort with MT in the LVA. However, previous studies, although based on small sample sizes, have generally reported similar LV thrombus resolution rates between VKAs and NOACs [14]. Therefore, the comparative efficacy of VKAs versus NOACs in thrombus resolution should be explored in larger, prospective studies to better understand their roles in this clinical context.
Our analysis revealed that a baseline LVEF
Lp(a) is a genetically determined lipoprotein with pro-thrombotic and
anti-fibrinolytic properties [17], recognized as a causal risk factor for
atherosclerotic cardiovascular disease and associated with vascular inflammation
and thrombus burden [18, 19, 20, 21, 22, 23]. While Celik et al. [24] reported no
association between Lp(a) and LVT risk in acute myocardial infarction, our
findings suggest that Lp(a)
D-dimer, a marker of coagulation and fibrinolysis activation in response to the
body’s hypercoagulable state [25, 26], has been associated with thrombus
formation in conditions like dilated cardiomyopathy and post-myocardial infarction (MI) LV dysfunction
[27, 28]. Although we observed a potential link between elevated D-dimer levels
(
Patients with an LVA_area
The association between larger LVA_area and MT_area with thrombus non-resolution remains unclear. Several factors may contribute to this relationship: (1) In larger LVA areas, blood flow could be slower, potentially resulting in a more stable thrombus that is less susceptible to resolution; (2) Larger thrombus areas might be more resistant to effective resolution by antithrombotic therapy, possibly due to insufficient drug penetration or thrombus composition. Given these considerations, it may be reasonable to extend the duration of antithrombotic therapy in patients with larger LVA_area and MT_area to improve the chances of thrombus resolution.
Severe myocardial injury, especially in large infarctions, creates a
pro-thrombotic environment by promoting persistent endothelial dysfunction, local
inflammation, and microvascular damage [29]. These pathological processes
facilitate MT formation and hinder its resolution. In our study, patients with
larger LVA areas (LVA_area
Reperfusion injury adds another layer of complexity. The restoration of blood flow to ischemic myocardium, while critical for salvaging viable tissue, can intensify damage through microvascular obstruction, oxidative stress, and inflammation. This cascade is often accompanied by intramyocardial hemorrhage (IMH), a result of microvascular rupture during reperfusion [30, 31]. IMH introduces blood products into the myocardium, forming a nidus for thrombus development and further complicating its resolution. The recent Canadian Society of Cardiology definition of tissue injury severity emphasizes the impact of extensive myocardial injury, including IMH, on adverse clinical outcomes [31], and highlights the interplay between severe myocardial injury and reperfusion injury in driving thrombus persistence. Together, these mechanisms suggest that even under standard antithrombotic therapy, severe tissue damage and associated complications like IMH play a pivotal role in the non-resolution of MT. Future studies utilizing advanced imaging modalities, such as cardiac magnetic resonance imaging, could provide a deeper understanding of these mechanisms. Such insights may help identify high-risk patients and inform tailored therapeutic strategies to improve thrombus resolution and clinical outcomes.
Our results confirm that MTLVA non-resolution is associated with worse clinical outcomes. Non-resolution patients in our study had a higher incidence of adverse events compared to resolution patients. Consistent with Zhou et al. [4], who reported higher rates of MACE, embolic events, and stroke among patients with LVT recurrence, these findings underscore the importance of achieving complete thrombus resolution to reduce adverse cardiovascular and cerebrovascular outcomes.
Defining independent risk factors of non-resolution of MTLVA might add the risk stratification of patients with MTLVA. Patients with higher risk of non-resolution MTLVA might benefit from more guideline-adherent antithrombotic treatment.
This study has several limitations. First, its retrospective design and single-center setting introduce the possibility of selection bias, which may limit the generalizability of our findings. Larger and prospective studies are needed to confirm these results and provide more robust evidence. Second, the diagnosis of MTLVA was primarily based on transthoracic echocardiography, which, while a standard and widely accessible imaging modality, may lack the sensitivity and specificity of advanced techniques such as contrast-enhanced ultrasound or cardiac magnetic resonance imaging. Future studies incorporating these modalities could enhance diagnostic accuracy and provide additional insights. Third, we did not capture reperfusion metrics such as symptom-to-balloon or door-to-balloon times, which are critical determinants of STEMI outcomes and may have influenced our findings. Finally, the relatively small sample size, although representative of this patient population, may have reduced the statistical power to detect certain associations. Thus, our findings should be interpreted as exploratory and hypothesis-generating, warranting validation in larger, multicenter cohorts.
In STEMI patients with MTLVA, non-resolution of mural thrombus is associated with a significantly higher risk of adverse events, including stroke and systemic embolism, compared to those with thrombus resolution. Independent risk factors for non-resolution include elevated Lp(a) levels, larger LVA and MT areas. These factors underscore the complexity of thrombus resolution and highlight the need for personalized management strategies. Prolonged or intensified antithrombotic therapy may benefit patients with non-resolution MTLVA. An individualized risk stratification approach, considering patient characteristics, disease vessel features, and thrombus evolution, should guide clinical decision-making and improve outcomes in this patient population.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CI, confidence interval; CRP, C-reactive protein; Cr, creatinine; DAPT, dual antiplatelet therapy; HR, hazard ratio; IMH, intramyocardial hemorrhage; LAD, left anterior artery; LCX, left circumflex artery; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); LVA, left ventricular aneurysm; LVA_LD, left ventricular aneurysm long diameter; LVA_SD, left ventricular aneurysm short diameter; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MT, mural thrombus; MT_LD, mural thrombus long diameter; MT_SD, mural thrombus short diameter; MTLVA, mural thrombus in left ventricular aneurysm; NOACs, non-vitamin K oral anticoagulants; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCI, percutaneous coronary intervention; RCA, right coronary artery; STEMI, ST-segment elevation myocardial infarction; TC, total cholesterol; TTE, transthoracic echocardiography; TG, triglyceride; UA, uric acid; VKAs, vitamin K antagonists.
Data are available on reasonable request (contact the corresponding author Dr. Junhua Ge).
JG and SM designed the research study. MW, ML, WL and JL performed the research and involved in drafting the manuscript; ZW, DC and QG made substantial contributions to acquisition of data, or analysis and interpretation of data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript to be published. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the ethical standards of the responsible committee on human experimentation declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University research (approval number: QYFY WZLL 28368), and informed consent was obtained from patients or their legal guardians.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
