- Academic Editor
Acute aortic dissection (AAD) is a rare but life-threatening disease, and its rapid and correct diagnosis is important. Heart rate (HR) is a risk factor for death in patients with AAD, but their relationship remains unknown. This meta-analysis aimed to evaluate whether there was a significant correlation between HR and AAD mortality risk.
By searching PubMed, Embase, and Web of Science databases, the studies reporting the correlation between HR and AAD were obtained, and their methodological quality was evaluated. Relative risk (RR) with 95% confidence interval (CI) was used as the effect size. Subgroup analysis, sensitivity analysis, and publication bias test (Egger’s test and funnel chart) were used to find the source of heterogeneity and evaluate the stability of the results.
Ten studies enrolling >4000 patients were included. Increased HR was positively correlated with increased AAD mortality risk (RR [95% CI] = 1.04 [1.01–1.07], p = 0.006). There was significant statistical heterogeneity among the included studies. The timing of HR monitoring, AAD type, and follow-up time were sources of heterogeneity. Sensitivity analysis showed that the combined results were stable. There was a significant publication bias in the included studies; however, the shear-fill method showed that the publication bias had little effect on the combined results (RR [95% CI] = 1.038 [1.010–1.066], p = 0.008).
There was a positive relationship between increased HR and increased AAD mortality.
Acute aortic dissection (AAD) is a rare but highly lethal disease, with severe chest, back pain and a tearing sensation as the primary clinical manifestation [1]. AAD is usually divided into two categories; Stanford type A and B. Stanford type A involves the ascending aorta, and its treatment is primarily by surgical repair (replacement of the ascending aorta). For type A aortic coarctation, treatment can be delayed with a semi-elective surgical approach unless cardiac tamponade or malperfusion syndrome is present. Timely diagnosis and surgical treatment are important to improve the survival rate [1]. Stanford type B is based on the fact that the ascending aorta proximal to the innominate artery is not involved in the process, and its treatment is mainly medical therapy, focusing on controlling blood pressure and heart rate (HR) [2, 3]. Over time, thoracic endovascular aortic repair (TEVAR) has been recommended for complex type B AAD. However, TEVAR carries certain perioperative complications, and the timing of the treatment must be determined based on the patient’s specific condition. This typically involves monitoring the aortic diameter and assessing any new complications [4]. The comprehensive treatment for AAD has improved over the past two decades, but the mortality rates of diagnosis and treatment in hospital remain relatively high [5, 6], reaching approximately 27.4% [7].
Several factors, such as advanced age, arterial hypertension, and aortic aneurysm, are associated with adverse outcomes of AAD [8, 9]. Although the mechanisms underlying its progression remain unclear, timely and accurate diagnosis is crucial because of the exceedingly high AAD mortality rate, with an hourly death rate of 0.5%, particularly within the first 48 h after symptom onset, in which the mortality rate reaches 23.7% [1, 5]. Biomarkers, such as D-dimer [10], tenascin-C [11], and smooth muscle myosin heavy chain [12], have good diagnostic value for AAD. However, the treatment time of AAD is urgent, and the reference range of biomarkers was not completely effective in diagnosing AAD [13]. Therefore, exploring risk factors and identifying promising biomarkers to immediately identify and diagnose high-risk patients are essential.
Increased resting HR is a major risk factor for cardiovascular disease and is associated with mortality [14, 15]. Variation of HR increased the mortality of coronary heart disease, stroke, heart failure, and other cardiovascular diseases [16]. Several studies have indicated that HR is a powerful predictor of long-term mortality and may be used as a significant risk marker to predict the prognosis of patients with AAD [17, 18]. For instance, Zhou et al. [17] proved that HR was an independent risk factor for patients with AAD and positively correlated with long-term mortality. However, the retrospective observation in this study may cause bias, and the influence of preoperative medication on the study was overlooked. Krenz et al. [19] found that controlling HR through esmolol treatment could achieve the treatment of patients with AAD and evaluate the safety of this treatment method; however, this study lacked a multicenter analysis. Considering the limitations of these studies and the potential benefits of focusing on the relationship between HR and cardiovascular health for patient care [18], the association between HR and AAD should be comprehensively assessed. Here, we conducted a meta-analysis to assess HR and AAD mortality risk.
The review team developed a literature search strategy in advance. Literature retrieval was performed in the PubMed, EmBase, and Web of Science databases. Search keywords included “aortic dissection”, “acute”, and “heart rate”. If the categories of keywords were similar or different, we used “OR” or “AND”, respectively, to combine them. Combined controlled vocabulary and free-text terminology was used to search the database. The specific retrieval steps of each electronic database are shown in Supplementary Tables 1–3. Moreover, the search language was not limited. Additionally, this study screened the relevant reviews and references of included studies to obtain more studies that could be used for meta-analysis. This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Two researchers conducted an independent evaluation of these references. Studies were included if they met the following Population Intervention Comparison Outcome (PICO) criteria; (1) the participants were patients with AAD, and treatment methods were not limited, (2) a prospective or retrospective cohort study, (3) the study reported the relationship between HR and risk of in-hospital death and death after follow-up, and (4) the correlation strength was expressed by odd ratio (OR), relative risk (RR), or hazard ratio and 95% CI, or could be calculated according to other data. Exclusion criteria included the following; (1) case reports, editorials, and review articles, (2) non-acute patients, and (3) if multiple articles were published or had the same data, only one article was kept, including the one with the most comprehensive research information, and the remaining were excluded. If there were any differences between the two researchers, a consensual process was conducted.
After the documents included in the analysis were determined, the data were extracted independently according to the pre-designed table. Each included study was carefully evaluated by two independent professionals, and the extracted data included the first author, basic characteristics of the research subjects (sample size, gender, and age), HR measurement time, categories of AAD, treatment methods, and research outcomes. After data extraction, the two authors exchanged audit extraction forms and discussed and solved any inconsistencies.
According to the Newcastle Ottawa Scale (NOS) evaluation scale, the
methodological quality of case-control and cohort studies was evaluated. The
evaluation content included research subject selection, comparability, and
exposure (eight scoring items, total score was 9) [20]. Scores of 7–9 were
classified as high-quality research, 4–6 as medium, and
For patients with AAD who reported death and survival, the study on the
difference in average HR was converted into OR (95% CI) by the Chinn method
[21]. In some studies, HR was used as a grouping variable, and the effect value
of high vs. low HR and 95% CI were used for meta-analysis. However, some studies
reported the change in risk of death for every one-beat increase in HR. Because
these studies reflected the correlation between HR increase and mortality
outcome, the effect values were combined. A p
RR (95% CI) was used as an effect size to evaluate the relationship between HR
and AAD mortality risk. Because of the high heterogeneity of the included
studies, meta-analysis was combined using the random effect model. Cochran’s Q
and I2 tests were used for heterogeneity testing [22]. If the Q statistic
p
This meta-analysis retrieved 509 articles in PubMed (88 articles), EmBase (346 articles), and Web of Science (75 articles). After eliminating 110 duplicate articles, 399 articles remained. After browsing the titles and abstracts, 383 articles that did not meet the inclusion criteria were excluded. Six of the 16 articles were eliminated after full-text reading, and the remaining 10 articles [17, 26, 27, 28, 29, 30, 31, 32, 33, 34] were included in the meta-analysis. The study retrieval results and screening process are shown in Fig. 1.
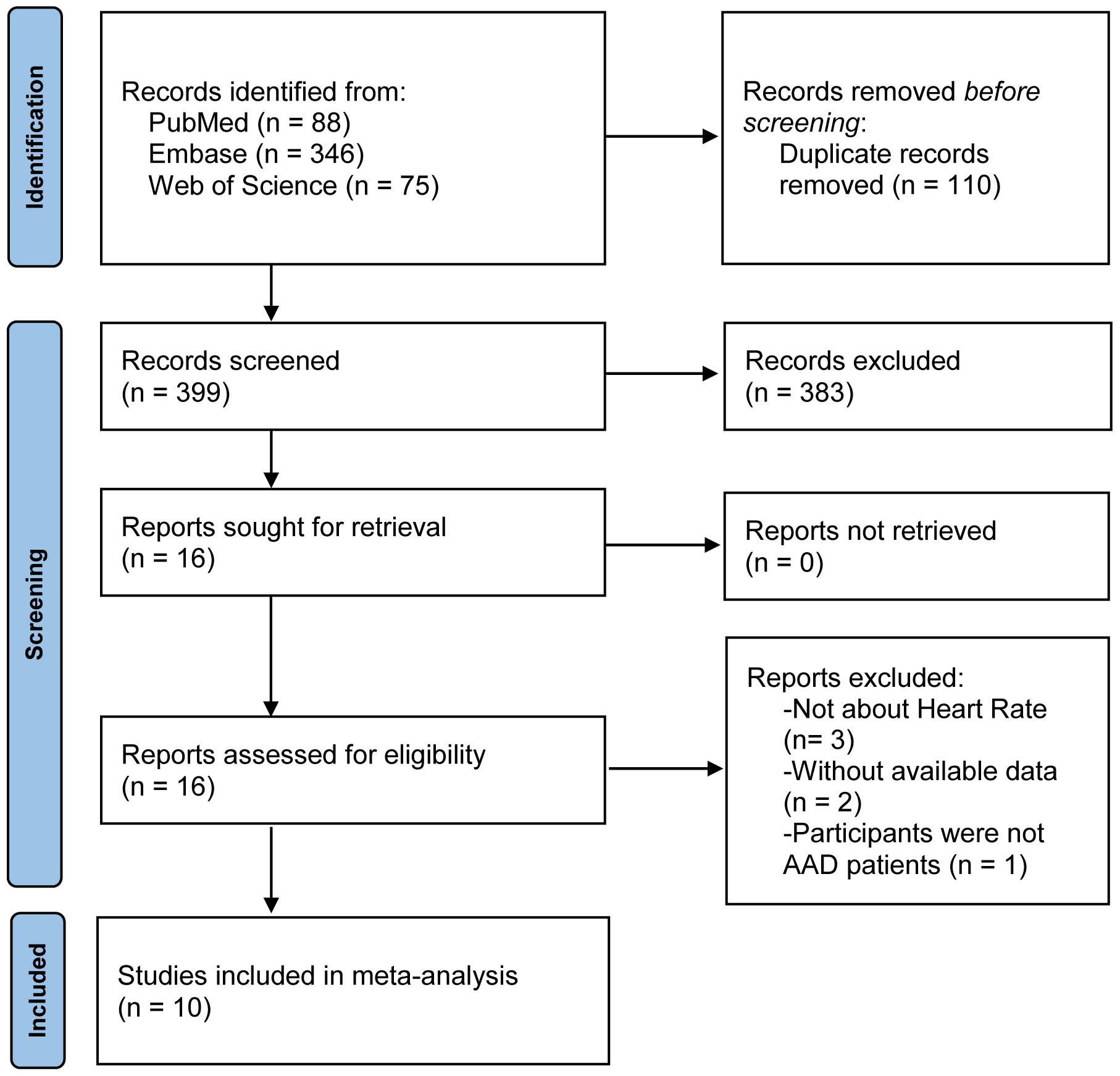 Fig. 1.
Fig. 1.
A flow chart of the study selection process. Abbreviations: AAD, acute aortic dissection.
The characteristics of the included studies were summarized in Table 1 (Ref. [17, 26, 27, 28, 29, 30, 31, 32, 33, 34]). However, the study by Jia et al. [30] was a prospective cohort studies (PCS), and the other articles were retrospective cohort studies (RCS). The included studies were published from 2015–2023, and study regions were mainly distributed in China, Japan, Iran, and Israel. Among these studies, Siti et al. [31] reported the results of Stanford type A and B AAD. The sample size was 155–721, with 4174 individuals (2942 males and 1232 females). The average age across the studies ranged from 46.6 years to 67.5 years.
| Study | Location | Design | Detected time of HR | n, M/F | Age, years | Stanford type, A/B | Treatment |
| Chen, Z et al., 2023 [28] | Israel | RCS | Pre-treatment | 374, 227/147 | 67.5 (55.3–77.4) | 240/134 | Medical therapy, Aortic surgery, TEVAR, ICU MV |
| Hagiya, K et al., 2021 [29] | Japan | RCS | Post-treatment | 721, 368/353 | 65.8 |
721/0 | Surgery |
| Jia, Y et al., 2023 [30] | China | PCS | Pre-treatment | 155, 125/30 | 55 (46–65) | 96/59 | Medical therapy, surgery |
| Ohnuma, T et al., 2015 [27] | Japan | RCS | Post-treatment | 434, 221/213 | 63.3 |
434/0 | Surgery |
| Rahmanian, M et al., 2023 [26] | Iran | RCS | Pre-treatment | 201, 143/58 | 59.9 |
201/0 | Surgery |
| Siti, D et al., 2018 [31] | China | RCS | Pre-treatment | 234, 187/47 | 50.6 |
88/0 | Medical therapy, surgery, TEVAR |
| 0/146 | |||||||
| Wang, MM et al., 2023 [32] | China | RCS | Pre-treatment | 715, 582/133 | 52.1 |
0/715 | NR |
| Xu, Y et al., 2023 [33] | China | RCS | Pre-treatment | 320, 273/47 | 51.8 |
103/217 | Medical therapy, surgery, TEVAR |
| Yuan, H et al., 2021 [34] | China | RCS | Pre-treatment | 313, 264/49 | 48 |
312/0 | Emergency surgery |
| Zhou, Y et al., 2021 [17] | China | RCS | Pre-treatment | 707, 552/155 | 46.6 |
707/0 | TAR+FET |
Abbreviations: M, male; F, female; NR, not reported; RCS, retrospective cohort study; PCS, prospective cohort study; TEVAR, thoracic endovascular aortic repair; ICU MV, intensive care unit mechanical ventilation; TAR+FET, total aortic arch replacement combined with the frozen elephant trunk.
The quality evaluation results are shown in Supplementary Table 4, and the NOS score included in the study was 5–8 (total score was 9). Seven articles [27, 28, 29, 30, 31, 32, 34] were rated as medium-quality and three [17, 26, 33] as high-quality research.
The forest diagram of the correlation analysis between AAD mortality risk and HR
was shown Fig. 2. The RR of the included studies was
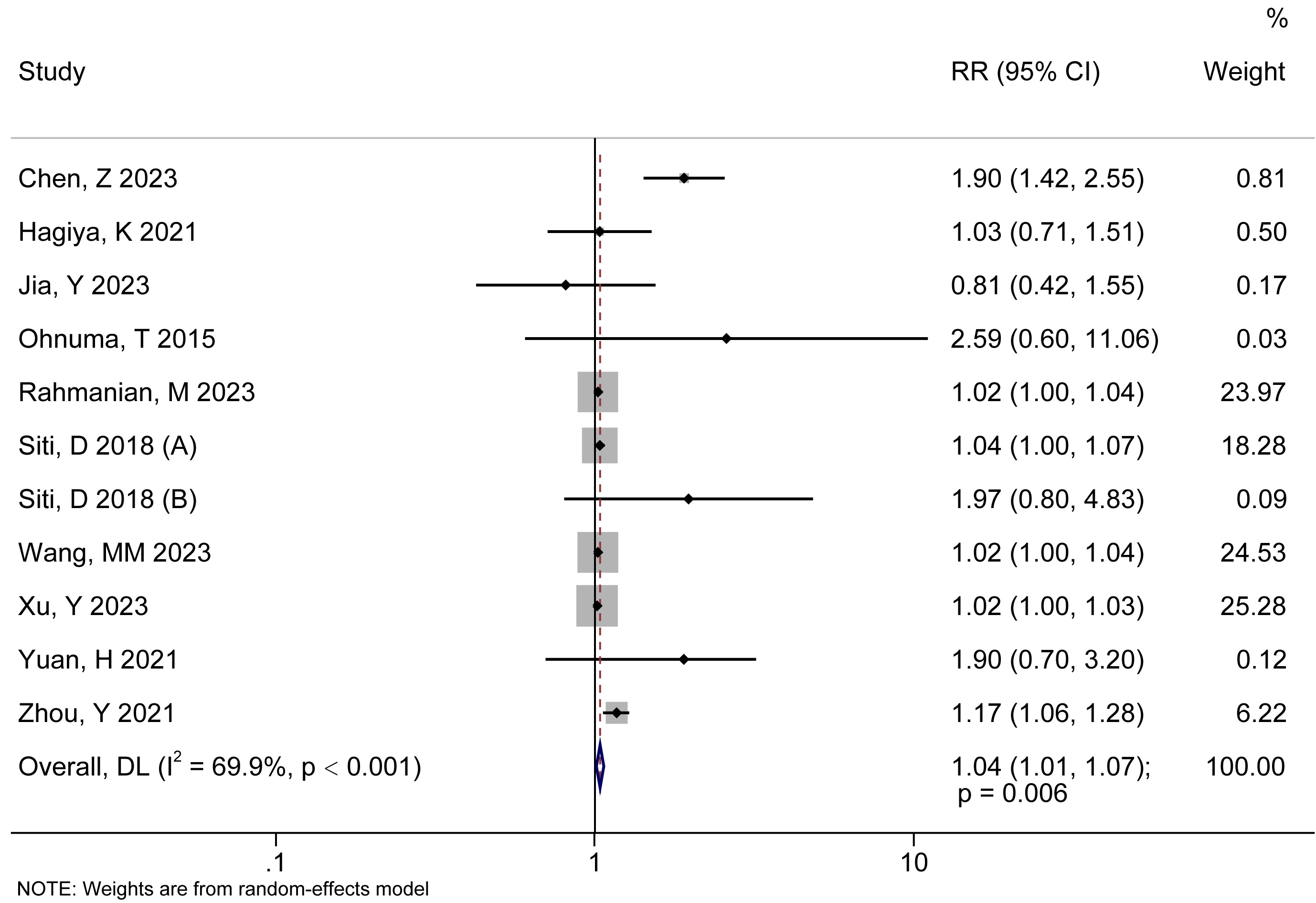 Fig. 2.
Fig. 2.
The forest diagram of the correlation analysis between AAD death risk and HR. RR, relative risk; DL, DerSimonian–Laird; 95% CI, 95% confidence interval.
Subgroup analysis was used to analyze the sources of heterogeneity. Fig. 3 and
Table 2 showed the results of subgroup analysis. Whether as a categorical or
continuous variable, the correlation between HR and AAD mortality risk was
statistically significant, and the combined results were RR (95% CI) = 1.41
(1.05, 1.89) (p = 0.023) and RR (95% CI) = 1.02 (1.01, 1.03)
(p
 Fig. 3.
Fig. 3.
Outcomes of subgroup analyses. Classification subgroup (A), regional subgroup (B), study type subgroup (C), disease types subgroup (D), detection time subgroup (E), whether there are correction types subgroup (F), death type subgroup (G), and research quality subgroup (H).
| Outcomes | No. of studies | RR (95% CI) | pA | Heterogeneity test | ||
| p | I2 (%) | |||||
| Total | 11 | 1.04 (1.01, 1.07) | 0.006 | 69.9 | ||
| Comparison | ||||||
| Categorical | 5 | 1.41 (1.05, 1.89) | 0.023 | 0.013 | 68.5 | |
| Continuous | 6 | 1.02 (1.01, 1.03) | 0.603 | 0.0 | ||
| Location | ||||||
| China | 7 | 1.03 (1.01, 1.06) | 0.014 | 0.027 | 57.9 | |
| Non-China | 4 | 1.31 (0.89, 1.93) | 0.175 | 84.1 | ||
| Design | ||||||
| RCS | 10 | 1.04 (1.01, 1.07) | 0.006 | 72.5 | ||
| PCS | 1 | 0.81 (0.42, 1.55) | 0.524 | NA | NA | |
| Stanford type | ||||||
| A | 6 | 1.05 (1.01, 1.11) | 0.029 | 0.037 | 57.7 | |
| B | 2 | 1.21 (0.69, 2.11) | 0.510 | 0.153 | 51.1 | |
| A or B | 3 | 1.20 (0.74, 1.95) | 0.453 | 89.0 | ||
| Detected time of HR | ||||||
| Pre-treatment | 9 | 1.04 (1.01, 1.07) | 0.007 | 74.7 | ||
| Post-treatment | 2 | 1.24 (0.61, 2.51) | 0.559 | 0.232 | 30.0 | |
| Adjusted | ||||||
| No | 7 | 1.32 (0.97, 1.80) | 0.078 | 0.001 | 75.0 | |
| Yes | 4 | 1.03 (1.01, 1.05) | 0.013 | 0.027 | 67.2 | |
| Mortality | ||||||
| Hospital | 7 | 1.02 (1.01, 1.03) | 0.295 | 17.7 | ||
| Non-Hospital | 4 | 1.24 (0.92, 1.66) | 0.160 | 0.008 | 74.7 | |
| Quality | ||||||
| Moderate | 8 | 1.08 (1.00, 1.17) | 0.055 | 0.001 | 71.3 | |
| High | 3 | 1.03 (1.00, 1.06) | 0.046 | 0.014 | 76.4 | |
Abbreviations: pA, p value for test of the association.
Sensitivity analysis showed that the range of the combined results had an RR
(95% CI) = 1.03 (1.00, 1.05) to 1.06 (1.01, 1.10). Excluding any study, the
combined results of the remaining studies remained statistically significant
(p
 Fig. 4.
Fig. 4.
The sensitivity analysis of the relationship between AAD death risk and HR.
The results of Egger’s test and funnel chart showed whether there was significant publication bias among studies. All major outcomes were significant by Egger test (p = 0.016) (Fig. 5A). The funnel chart revealed that the distribution symmetry of scatter plot was poor, suggesting an asymmetry in the studies reporting primary AAD results (Fig. 5B). Thus, shear-fill method was used to adjust the analysis. The combined result was RR (95% CI) = 1.038 (1.010, 1.066) (p = 0.008) after adding two filler studies (Fig. 5C), indicating that the combined result had little effect on publication bias.
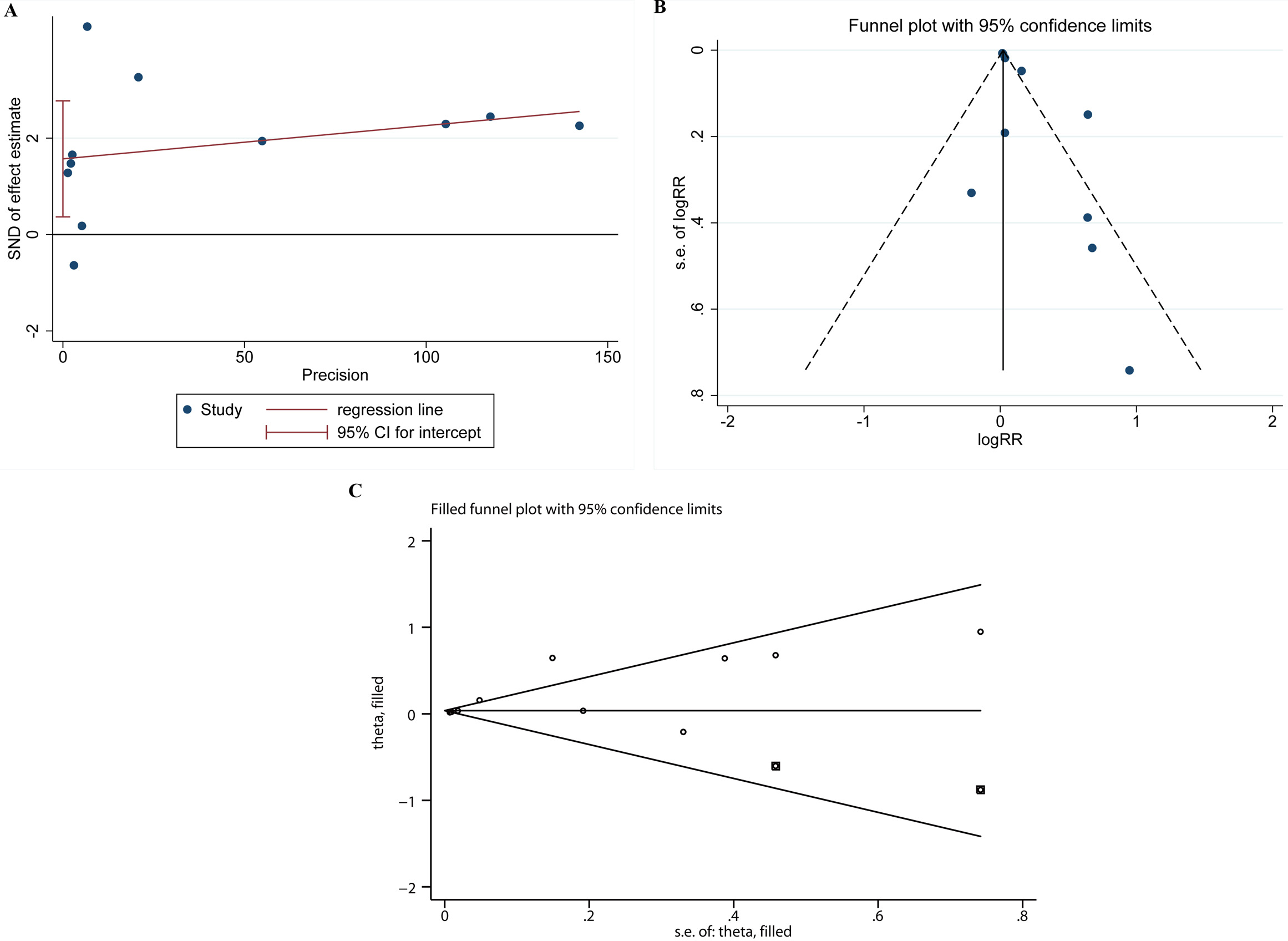 Fig. 5.
Fig. 5.
The publication bias test. Egger’s test (A), Egger’s funnel plot (B), and shear-fill method (C). SND, standard normal deviate.
Recently, meta-analyses have focused on the surgical treatment scheme and prognosis of AAD [13, 35, 36]. Accurate early diagnosis and effective treatment of AAD remains of paramount importance to improve the survival rate of patients. Missed diagnosis of patients with AAD carries a significant mortality risk [20]. Our study showed that increased HR was significantly correlated with increased AAD mortality risk. However, the heterogeneity of the selected studies was significant regarding timing of HR monitoring, AAD type, and follow-up, which may affect the correlation strength and significance between HR and AAD. It is suggested that the follow-up study should form a unified standard for HR measurement time, grouping threshold, and study outcome evaluation to evaluate the relationship between them more accurately.
Currently, computed tomography (CT), magnetic resonance imaging (MRI), and transesophageal echocardiography (TEE) are usually performed to identify or exclude the AAD of patients, which are regarded as a grade I recommendation (evidence grade B) [37]. Besides the concerns caused by transferring potentially critical patients to radiology, the disadvantages of TEE, CT, and MRI mainly include the risk of venography and ionizing radiation. Consultation is not widely provided in many emergency departments [38]. Aside from imaging, biomarkers, such as D-dimer, troponins, and serum calcium, have been used to aid in AAD diagnosis. The potential problem of applying D-dimer in clinical practice lies in its poor specificity and lack of prospective verification of its application in decision-making [20, 39]. Other biomarkers, such as troponins [40] and serum calcium [41], exhibited the potential of risk stratification in the diagnosis of patients with AAD. However, it is expensive and inefficient for doctors and patients to use imaging techniques and biomarker detection to diagnose AAD, and not all hospitals are equipped with them. Therefore, a rapid, economical, and convenient diagnosis and prediction method is needed.
Resting HR is the core of cardiac output and is influenced by the changes in
many diseases. Abnormal HR usually affects the amplitude and frequency of tensile
stress on arterial wall and local hemodynamic environment, resulting in changes
in endothelial cell structure and functions [42]. The imbalance of the autonomic
nervous system, increased sympathetic nerve activity, and/or decreased
parasympathetic nerve activity may be related to the pathogenesis of increased
HR, increased blood pressure, diabetes, and obesity in some patients [43]. In the
case of aortic coarctation, this autonomic imbalance may exacerbate tensile
stresses on the arterial wall and changes in the local hemodynamic environment,
thereby affecting endothelial cell structure and function. Current evidence shows
that HR is an important indicator of cardiovascular diseases, including heart
failure and AAD [44]. For example, Oliva et al. [45] expounded that HR
can be used as a prognostic biomarker and is strongly associated with the
prognosis of heart failure and acute heart failure. A retrospective study found
that patients with sinus rhythm could significantly benefit from reduced HR. A
large-scale meta-analysis (including 46 studies, including 1,246,203 patients)
showed that increased HR was positively correlated with all-cause mortality and
cardiovascular mortality, and the HR increased by 10 times/min. The overall
all-cause mortality RR was 1.09 and 95% CI was 1.07–1.12, which showed that the
mortality risk increased significantly. The RR of cardiovascular mortality was
1.08 and 95% CI was 1.06–1.10, indicating that the risk rate of cardiovascular
mortality is obviously increased [46]. This suggests that HR control is critical
for improving the prognosis of patients with cardiovascular diseases. Here, we
demonstrated that HR is an influential independent risk factor for AAD. Chen
et al. [28] found that HR (
Additionally, meta-subgroup analysis showed that China, RCS, Stanford type A,
the study of measuring HR before treatment, the study after multi-factor
correction, hospital mortality, and high-quality research had significant effects
on heterogeneity (p
Our study provided new insights into the risk management of AAD and helped to predict the diagnosis and progression in patients with AAD, thereby improving the survival rate of AAD. In 2010, the American Heart Association (AHA) guidelines [47] formally proposed that the initial treatment of Stanford type B AAD should reduce the stress of the aortic wall by controlling HR and blood pressure. However, further randomized controlled trials are needed to validate these results. This study laid a foundation for further research, particularly in determining that early detection and control of HR can help reduce AAD mortality. Our study was used to comprehensively evaluate the relationship between HR and AAD mortality risk, and subgroup analysis was used to evaluate the influence on the results. Although there was a significant publication bias, the clipping method results suggested that publication bias had little influence on the merger results. The one-by-one exclusion method demonstrated good stability of the meta-analysis.
This study has some limitations. The heterogeneity of included studies was significant, and subgroup analysis did not identify any significant influencing factors. Most of the included studies were retrospective, and there were many confounding factors, which may affect the authenticity of the results. Moreover, the included studies came from Asian countries, and more high-quality studies were needed to verify the extrapolation of the results. Therefore, more studies with a large sample, randomized and blind study design are required to explore the correlation between HR and AAD diagnosis to make the results more clinically significant.
This meta-analysis revealed a positive relationship between increased HR and increased mortality in patients with AAD. HR has convenient and excellent diagnostic performance in AAD diagnosis, and monitoring of HR may improve the prognosis of patients with AAD.
The datasets used in our study are available from the corresponding author on reasonable request.
Conception and design of the research: TYW and YSW. Acquisition of data: LS. Analysis and interpretation of the data: ZZN. Statistical analysis: JXW. Writing of the manuscript: TYW and LS. Critical revision of the manuscript for intellectual content: YSW, ZZN and JXW. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM27755.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
