- Academic Editor
This study aimed to investigate the correlation between calcific aortic valve disease (CAVD) and carotid artery elasticity using ultra-fast pulse wave velocity (UFPWV) technology. Early detection of alterations in carotid artery elasticity, coupled with the prompt implementation of intervention strategies, can effectively decrease the incidence of cardiovascular diseases.
Patients with CAVD were recruited from the University-Town Hospital of Chongqing Medical University and placed in the observation group. Meanwhile, an equivalent number of patients with non-calcified aortic valve disease were recruited as controls. All participants underwent comprehensive health assessments, including measurements of blood lipids, fasting blood sugar, and other biochemical indicators. Additionally, bilateral carotid intima-media thickness (CIMT) was measured, as well as pulse wave velocity (PWV) at the beginning of systole (PWV-BS) and the end of systole (PWV-ES). Differences in various indicators between the two groups were analyzed, and the factors associated with CAVD and carotid artery elasticity were investigated. The correlation between CAVD and carotid artery elasticity was also evaluated.
Patients with CAVD exhibited significantly higher CIMT, PWV-BS, and PWV-ES levels than those with non-calcified aortic valve disease (p < 0.01). PWV-BS and PWV-ES showed progressive increases according to the severity of calcification. Coronary atherosclerotic heart disease and PWV-BS were all identified as independent risk factors for CAVD. The risk factors associated with PWV-BS include hypertension, coronary atherosclerotic heart disease, total cholesterol, and homocysteine (p < 0.05 for all). The risk factors related to PWV-ES include hypertension, coronary atherosclerotic heart disease, total cholesterol, and glycated hemoglobin (p < 0.05 for all).
UFPWV technology is a novel method for the early diagnosis of carotid elasticity. Evaluating carotid artery atherosclerosis in patients with CAVD may lead to earlier detection and intervention and reduce the incidence of cardiovascular events.
The most common etiology of aortic stenosis (AS) is calcified aortic valve disease (CAVD) [1]. The calcification of cardiac valves and atherosclerosis show similar alterations in tissue pathology [2]. The number of CAVD cases reported among younger patients has recently increased [3]. Carotid atherosclerosis indicates the occurrence and progression of arterial atherosclerotic lesions throughout the body [4]. The 2018 guidelines for hypertension management by the European Society of Hypertension and the European Society of Cardiology highlight pulse wave velocity (PWV) as the gold standard for assessing arterial stiffness due to its direct correlation with Young’s modulus [5]. The significance of using ultra-fast pulse wave velocity (UFPWV) technology in assessing carotid artery elasticity has previously been well-established [6]. However, the application of changes in carotid artery elasticity in patients with CAVD is currently limited, and there is a paucity of research on this topic. Therefore, the present study utilized UFPWV technology to assess the elasticity of the carotid artery. We then investigated the association between CAVD and alterations in carotid artery elasticity. Identifying the risk factors for CAVD and carotid artery elasticity has significant clinical importance for risk stratification and managing cardiovascular diseases.
This single-center, retrospective study evaluated 105 patients diagnosed with CAVD who were admitted to the University-Town Hospital of Chongqing Medical University between September 2022 and February 2024. In addition, 105 healthy subjects were recruited from our hospital during the same period as controls.
Patients diagnosed with CAVD by echocardiography were informed about the study,
and those who agreed to participate signed an informed consent. Patients with a
diagnosis of carotid artery atherosclerotic plaques, as
characterized by an intima-media thickness (IMT) of
The ACUSON Redwood (Serial No. 561045. Healthineers SIEMENS, Seongnam-si, Gyeonggi-do, Korea) with a cardiac probe (2–4 MHz) was used in this study as the cardiovascular imaging ultrasonic diagnostic instrument.
Two experienced ultrasound doctors performed standardized echocardiograms in accordance with the 2022 British Society of Echocardiography (BSE) Practice Guidelines [8]. Enhanced echo and leaflet stiffness of the aortic valve and a valve thickness exceeding 3 mm were applied to indicate a diagnosis of CAVD. The extent of aortic valve calcification was assessed. Subsequently, the subjects were categorized into three groups based on the severity of calcification: Group One, mild calcification of the aortic valve, characterized by the presence of calcification but occupying no more than one-third of the leaflet area; Group Two, moderate calcification of the aortic valve, where the extent of calcification covered less than two-thirds of the valve surface area; Group Three, severe calcification involving more than two-thirds of the leaflet area.
The medical staff collected all clinical
data pertaining to the study population. This study encompassed
several fundamental health metrics, including body mass index (BMI) and
biochemical parameters such as blood lipids, and also documented the comorbidity
profiles of participants. The diagnostic criteria for
hypertension in patients were as follows: In the absence of antihypertensive
medication, a clinical blood pressure exceeding 140/90 mmHg; home blood pressure
exceeding 135/85 mmHg; or 24 h ambulatory blood pressure readings exceeding
130/80 mmHg, with daytime readings exceeding 135/85 mmHg and night-time readings
exceeding 120/70 mmHg. The diagnostic criteria for hypertension were derived from
the 2024 “Guidelines for the Management of Hypertension and Elevated Blood
Pressure” issued by the European Society of Cardiology (ESC)
[9]. The diagnostic criteria for diabetes were fasting blood
glucose
Two physicians with at least 10 years of experience conducted the carotid artery ultrasound examinations per the 2017 ESC “Guidelines for Diagnosing and Treating Peripheral Arterial Diseases” [12] and standardized carotid PWV measurements using ultrafast ultrasound imaging. After completing the pertinent training and subsequent evaluations, two physicians assessed the carotid arteries of patients without knowledge of their overall clinical indicators.
The participants underwent carotid ultrasound imaging in the supine position
using the Aixplorer ultrasound system (Serial No. IQ78PR61. SuperSonic lmagine, Les Jardins de la Duranne, BÃt E et F, 510, rue René Descartes, Aix-en-Provence, France), equipped with a linear array probe (4–15 MHz and utilizing the vascular PWV mode). The measurement technique for CIMT and
carotid artery PWV was described in an earlier multicenter study conducted by
Lixue Yin et al. [13]. The subject reclines and rests for 15 minutes
before fully exposing the neck. The probe was positioned along the outer border
of the sternocleidomastoid muscle to capture a longitudinal section of the
carotid artery spanning from the proximal to the distal end and ensuring
continuous display of the IMT. The IMT of the posterior
wall was measured 1 cm below the bifurcation point of the common carotid artery
during diastole. The patient was instructed to hold their breath and initiate PWV
once the image had stabilized. The Aixplorer
ultrasound system automatically measured the pulse wave velocity at the beginning of systole (PWV-BS) and pulse wave velocity at the end of systole (PWV-ES). The system
automatically computed the variance in the PWV measurements during the beginning of systole
(BS) and end of systole (ES), reported as
Measurement data were analyzed using the SPSS 27.0 statistical software package
(SPSS Software version 27, IBM Corp., Chicago, IL, USA).
Quantitative data were subjected to the PWV normal distribution
test. Qualitative data adopts the chi-square test. Data are
expressed as the mean
No statistically significant differences were observed between the observation
group and the Non-CAVD group regarding
gender, age, BMI, proportion of smokers, proportion of diabetes patients, and
fasting blood glucose levels (all
p
| Variables | Non-CAVD group | CAVD group | p-value | |
| (n = 105) | (n = 105) | |||
| Age (years) | 58.0 [54.0; 62.0] | 58.0 [56.0; 60.0] | 0.622 | |
| Gender | Female | 48 (45.7%) | 45 (42.9%) | 0.6771 |
| Male | 57 (54.3%) | 60 (57.1%) | ||
| Smoking | 25 (23.8%) | 38 (36.2%) | 0.050 | |
| BMI (kg/m2) | 24.16 |
24.77 |
0.319 | |
| Consolidate disease conditions | Hypertension | 41 (39.0%) | 65 (61.9%) | |
| Diabetes | 40 (38.1%) | 44 (41.9%) | 0.573 | |
| Coronary atherosclerotic heart disease | 8 (7.6%) | 24 (22.9%) | 0.002 | |
| Hyperlipidemia | 21 (20.0%) | 10 (9.5%) | 0.032 | |
| The laboratory examination | HbA1c | 5.20 [4.20; 6.70] | 5.45 [4.61; 6.87] | 0.133 |
| C-peptide | 2.45 [1.83; 3.08] | 2.89 [2.36; 3.25] | 0.006 | |
| FPG | 5.56 [4.79; 7.18] | 5.60 [4.75; 7.43] | 0.796 | |
| TC (mmol/L) | 3.2 [2.49; 4.23] | 4.04 [3.19; 4.65] | ||
| LDL-C (mmol/L) | 2.14 [1.66; 2.68] | 2.27 [1.71; 2.93] | 0.060 | |
| HDL-C (mmol/L) | 1.45 [1.25; 1.65] | 1.46 [1.25; 1.62] | 0.524 | |
| FFA (mmol/L) | 0.46 [0.33; 0.62] | 0.58 [0.46; 0.65] | ||
| TG (mmol/L) | 1.42 [1.25; 1.74] | 1.35 [1.02; 1.65] | 0.315 | |
| HCY | 8.33 [7.60; 9.21] | 8.65 [7.29; 10.24] | 0.082 | |
| UA | 346 [310; 385] | 346 [307; 379] | 0.915 | |
| TSH | 2.15 [1.00; 3.20] | 2.05 [1.07; 2.90] | 0.816 | |
| CYSC | 0.89 [0.75; 1.02] | 0.92 [0.81; 1.06] | 0.062 | |
Abbreviations: BMI, body mass index; FFA, free fatty acids; TC, total cholesterol; TG, triglycerides; HbA1c, glycated hemoglobin; FPG, fasting blood glucose; HCY, homocysteine; UA, uric acid; CYSC, cystatin C; CAVD, calcific aortic valve disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoproteincholesterol; TSH, thyroid stimulating hormone.
The observation group showed higher CIMT and PWV than the normal group
(p
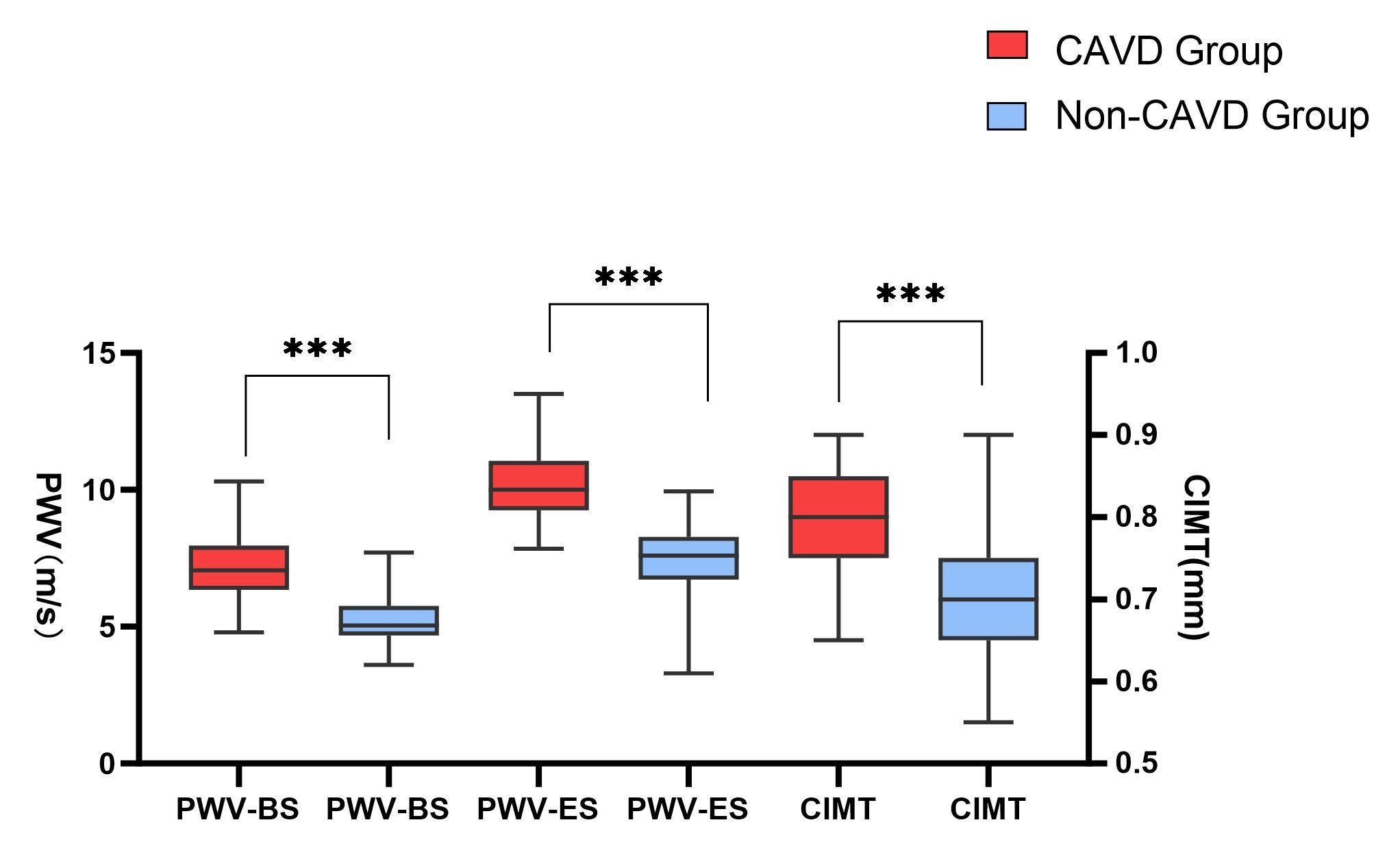 Fig. 1.
Fig. 1.
Boxplot of the carotid intima-media thickness (CIMT) and carotid
artery pulse wave velocity (PWV) in the observation and Non-CAVD groups. The
boxplots present interquartile ranges (in mm and m/s) (*** p
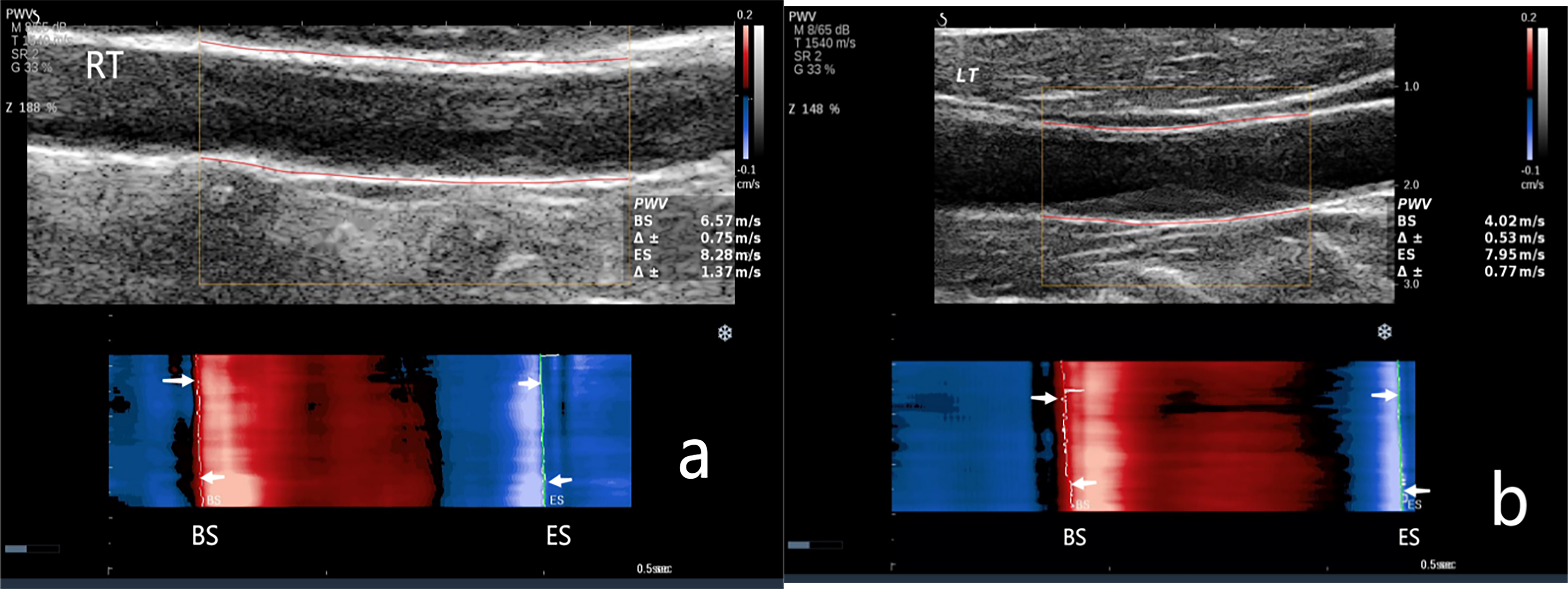 Fig. 2.
Fig. 2.
The carotid artery PWV in the Non-CAVD group. (a) PWV measurement of the right carotid artery. PWV-BS, 6.57 m/s; PWV-ES, 8.28 m/s. (b) PWV measurement of the left carotid artery. PWV-BS, 4.02 m/s; PWV-ES, 7.95 m/s. NOTE: PWV-BS corresponds to the slope of the most prominent line in the red band, whereas PWV-ES is associated with the slope of the most prominent line in the blue band. These features are highlighted in the figure by the white arrows. M, map; T, tissue tuner; SR, speckle reduction; G, gain; Z, zoom; dB, decibel; BS, beginning of systole; ES, end of systole.
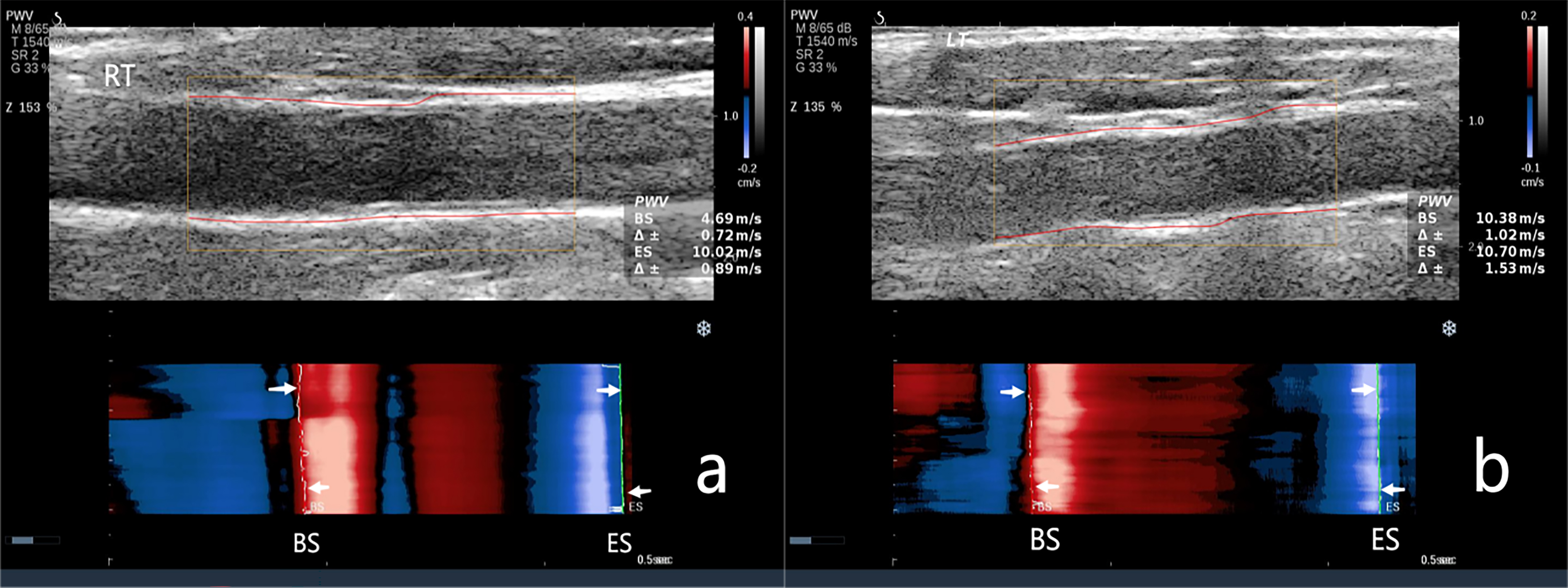 Fig. 3.
Fig. 3.
The carotid artery pulse wave velocity (PWV) in the CAVD group. (a) PWV measurement of the right carotid artery. PWV-BS, 4.69 m/s; PWV-ES, 10.02 m/s. (b) PWV measurement of the left carotid artery. PWV-BS, 10.38 m/s; PWV-ES, 10.07 m/s. NOTE: PWV-BS corresponds to the slope of the most prominent line in the red band, whereas PWVES is associated with the slope of the most prominent line in the blue band. These features are highlighted by the white arrows in the figure.
| Group | Case | IMT | PWV-BS | PWV-ES |
| Group One | 48 | 0.79 |
6.81 |
9.36 |
| Group Two | 35 | 0.80 |
7.32 |
10.93 |
| Group Three | 22 | 0.79 |
8.02 |
11.2 |
| F-value | 0.10 | 10.75 | 46.72 | |
| p-value | 0.904 |
Abbreviations: IMT, intima-media thickness. Different superscript letters (a, b) indicate that the differences between groups are statistically significant (p
Coronary atherosclerotic heart disease and PWV-BS were all
significant independent risk factors for CAVD (all p
| B-value | SE | χ2 value | p-value | OR value | 95% CI | |
| CHD | 1.91 | 0.5 | 14.64 | 6.74 | (2.54–17.92) | |
| PWV-BS | 1.20 | 0.2 | 34.44 | 3.31 | (2.42–4.93) |
Abbreviations: CHD, coronary atherosclerotic heart disease; OR, odds ratio.
| SE | t | p-value | |||
| Hypertension | 0.90 | 0.18 | 5.06 | (0.55–1.25) | |
| CHD | 0.70 | 0.24 | 2.89 | 0.004 | (0.22–1.18) |
| TC | 0.17 | 0.07 | 2.54 | 0.012 | (0.04–0.30) |
| HCY | 0.03 | 0.01 | 2.57 | 0.011 | (0.01–0.05) |
| SE | t | p-value | (95% CI) | ||
| Hypertension | 1.01 | 0.22 | 4.56 | (0.57–1.44) | |
| CHD | 1.43 | 0.30 | 4.77 | (0.84–2.02) | |
| HbA1c | 0.15 | 0.05 | 2.89 | 0.004 | (0.05–0.25) |
| TC | 0.25 | 0.1 | 2.56 | 0.011 | (0.06–0.44) |
CAVD is characterized by progressive fibrous calcification of the aortic valve
leaflets, leading to deformity and impaired valve opening and closing. This
eventually results in aortic stenosis and/or regurgitation, as well as
hemodynamic alterations [14]. In the initial stage, the pathogenesis resembles
atherosclerosis, with previous studies demonstrating a correlation between
endothelial dysfunction and the development of heart valve sclerosis [15]. An
inflammatory response, aberrant calcium and phosphorus metabolism, and oxidative
stress are pivotal in valve calcification progression. These mechanisms
facilitate calcium deposition within valve tissues and significantly influence
arterial elasticity. For example, the inflammatory response exacerbates valve
cell damage and fibrosis by activating immune cells and releasing proinflammatory
cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha
(TNF-
In the present study, CAVD was influenced by various factors, including C-peptide, free fatty acids and total cholesterol levels. These factors exhibited a positive correlation with CAVD risk, consistent with the findings of an analysis of factors influencing heart valve calcification by Small AM et al. [17]. This current study showed a higher prevalence of hypertension in patients from the observation group compared to those in the Non-CAVD group. Hypertension may contribute to increased pressure on the aortic valve and alterations in shear forces on blood vessels, which may be causally linked. Moreover, direct calcification of the aortic valve can increase the workload on the myocardium. Therefore, prolonged exposure of the aortic valve to elevated pressure can potentially increase calcification.
PWV-BS and PWV-ES were associated with
risk factors such as hypertension, coronary atherosclerotic heart disease and
total cholesterol (p
In this study, it is significant differences were observed between the
observation group and the Non-CAVD group regarding free fatty acids and total
cholesterol (p
The carotid artery IMT was within the normal range for all subjects in this
study. However, the CAVD group exhibited a significantly higher
IMT than the Non-CAVD group (p
Our study also revealed that PWV-BS is an independent risk factor for CAVD and exhibits a positive correlation. In clinical practice, PWV-BS is regarded as a superior approach for the early diagnosis and quantitative assessment of arterial stiffness. This aligns with the findings by Zhu et al. [21] on carotid stiffness and atherosclerotic risk.
In summary, for individuals in high-risk groups, the regular assessment of carotid PWV can serve as a vital preventive strategy. This diagnostic tool enables healthcare professionals to evaluate an individual’s cardiovascular status more precisely and promptly identify potential risk factors. Conditions such as hypertension, diabetes, and hypercholesterolemia are significant contributors to cardiovascular disease, and alterations in PWV often serve as early indicators of these conditions. Consequently, routine monitoring of carotid PWV facilitates early risk detection and familial predisposition and provides a scientific foundation for subsequent therapeutic interventions. Meanwhile, proactive management and mitigation of recognized risk factors are equally important in addition to regular screening. This includes maintaining a balanced diet, engaging in consistent physical activity, abstaining from smoking, moderating alcohol consumption, and adhering to prescribed medication regimens. A holistic approach to health management can markedly reduce the likelihood of cardiovascular incidents, enhancing overall quality of life.
In summary, adopting a proactive stance toward cardiovascular health issues is an important way to safeguard high-risk individuals. Finally, this study is not without limitations. Indeed, this study was conducted at a single center, and given the unique characteristics of the CAVD population and the limited sample size, some selection bias is inevitable. Therefore, future studies should be multi-center and aim to expand the sample size to overcome this limitation.
The UFPWV technique can detect early changes in arterial stiffness in patients with CAVD, thereby highlighting the initial development of arteriosclerosis. Based on these identified risk factors, future risk stratification may be performed for carotid artery stiffness patients with CAVD. This study has significant clinical implications for the early prevention, diagnosis, and treatment of arterial stiffness in patients with CAVD. Furthermore, the results significantly impact early diagnosis, control of calcification progression, and mitigation of vascular damage caused by arteriosclerosis to improve prognoses and slow the progression of cardiovascular disease.
Due to privacy considerations, the data for this study are not publicly available. However, the corresponding author can provide the data upon reasonable request.
Yan-Z: conception and design; drafting of the paper; HW, QH: conception and design; analysis and interpretation of the data; Yan-Z, QH, Yu-Z, WW, ZJZ: analysis and interpretation of the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University-Town Hospital of Chongqing Medical University (Protocol No. LL-202201). Before the examination, each patient signed a form providing informed consent.
The authors thank all volunteers who participated in the study.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
