- Academic Editor
†These authors contributed equally.
Aortic diseases, such as aortic dissection and aortic rupture, often lead to catastrophic complications, significantly increasing morbidity and mortality. Population-based screening for early detection in asymptomatic individuals is not feasible due to high costs and practical challenges. However, recent advancements in four dimensions (4D) Flow magnetic resonance imaging (MRI) offer a comprehensive tool for evaluating hemodynamic changes within the aortic lumen. This technology allows for the quantification and visualization of flow patterns and the calculation of advanced hemodynamic parameters, such as wall shear stress (WSS). WSS is crucial in the development, risk stratification, and surgical outcomes of aortic diseases and their complications, enabling noninvasive and quantitative screening of high-risk populations. This review explores the current status and limitations of 4D flow MRI-derived WSS imaging for aortic disease.
Complications from aortic disease, such as aortic dissection and aortic rupture, are often catastrophic, resulting in increased morbidity and mortality [1]. The development of aortic disease is closely related to the luminal hemodynamic environment, with wall shear stress (WSS) playing an important role. Under physiological conditions, vascular endothelial cells possess mechanosensors that detect the magnitude and direction of WSS, including ion channels, cell-cell junctions, G protein-coupled receptors, integrins, and glycocalyx [2, 3, 4]. These mechanosensors regulate the expression of relevant genes and their proteins by activating signal transduction pathways [5]. Normal WSS maintains vascular homeostasis and exerts anti-proliferative, anti-apoptotic, anti-inflammatory and anti-thrombotic effects. Conversely, abnormal WSS can lead to vascular dysfunction, inflammation and thrombosis [6, 7]. This underscores the importance of WSS in the physiological stages and progression of vascular diseases.
The aorta is mainly evaluated by Color Doppler ultrasound, computed tomography angiography (CTA), digital subtraction angiography (DSA) and other techniques. But these techniques have some defects, for example, ultrasound is susceptible to gas and the quality and accuracy of its images are related to the level of the imaging physician; CTA and DSA require the introduction of contrast agents, which can only obtain information on the morphology of the aorta. They need to be combined with hydrodynamic post-processing software in order to provide hemodynamics. Computational fluid dynamics (CFD) is a common hemodynamic measurement that provides high spatial and temporal resolution [8], but it simulates the real blood flow information by computer modeling, and the data obtained are to some extent virtual. Four dimensions (4D) flow magnetic resonance imaging (MRI) is a new non-invasive, contrast-free imaging technique that can truly reflect the status of intravascular blood flow and retrospectively analyze the aortic hemodynamic information to dynamically evaluate the abnormal blood flow status in the aorta for early intervention. This article summarizes the current status and limitations of 4D flow MRI-derived WSS in aortic disease, aiming to provide new insights into the clinical management of aortic disease and its complications.
Currently, Siemens, Philips and GE MRI vendors are able to perform 4D Flow MRI acquisitions, and the WSS obtained from two consecutive 4D Flow scans on the same magnetic resonance (MR) scanner has good consistency and reproducibility [9, 10, 11, 12]. However, the quantitative values of WSS obtained by different MR vendors have not been standardized [13]. In addition, there was variability in WSS values between 1.5 T and 3.0 T from the same MR supplier [14, 15].
Efficient post-processing software is essential for the quantification and visualization of hemodynamic parameters in both scientific research and clinical applications. The example of 4D flow MRI–based visualization of aortic hemodynamics is shown in Fig. 1. Current post-processing software includes CAAS 5.1 (Pie Medical Imaging, Maastricht, Limburg, the Netherlands), CVI42 6.0.2 (Circle Cardiovascular Imaging, Calgary, Alberta, Canada), GT Flow 3.1.14 (GyroTools, Zurich, Switzerland), iT Flow 1.9 (Cardio Flow Design Inc., Chiyoda-ku, Tokyo, Japan) and MEVISFlow 10.3 (Fraunhofer MEVIS, Bremen, Germany). In addition, some researchers have developed advanced flow analysis parameters using MATLAB R2022b (MathWorks Inc., Natick, MA, USA) or other programming languages to visualize the advanced flow data using post-processing tools in the field of fluid dynamics, such as Ensight and Paraview. It is important to note that there are significant differences in WSS quantitative reference values derived from different post-processing software [16]. These discrepancies may be related to variations in background phase offset corrections, contour splitting, and software algorithms.
 Fig. 1.
Fig. 1.
4D flow magnetic resonance imaging (MRI)–based visualization of aortic hemodynamics in a healthy volunteer. (A) 4D-flow-Image streamlines for the aorta. (B) 4D-flow-Image wall shear stress for the aorta. The color gradient change reflects the magnitude of velocity and wall shear stress (WSS). Red and blue are the maximum and minimum values, respectively. 4D, four dimensions.
Limitations: The lack of uniformity in WSS measurement limits its clinical application. However, it has been suggested that aortic remodeling can be predicted by differentiating between areas of high and low WSS [17]. In the future, standardization of scanning protocols and uniformity of post-processing methods are needed before 4D Flow can be introduced into routine clinical applications.
In recent years, 4D Flow MRI-derived WSS is a hemodynamic parameter highly relevant to aortic disease. It plays a significant role in the occurrence, development, risk stratification, and surgical evaluation of aortic diseases such as aortic aneurysm, aortic dissection, aortic atherosclerosis, bicuspid aortic valve, and Marfan syndrome (Table 1, Ref. [12, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59]).
| Type of disease | Author | Date published | Research | Research Finding |
| Aneurysm | Natsume et al. [26] | 2017 | 16 aortic arch aneurysms, 8 young healthy volunteers and age-matched control subjects | Aneurysm geometry affects WSS distribution |
| Jamaleddin Mousavi et al. [21] | 2021 | 2 ATAA, 2 healthy subjects | Computational modeling coupling hemodynamics with mechanobiology as a promising approach for exploring aneurysm progression | |
| Ramaekers et al. [20] | 2021 | 25 TAA, 22 controls | Asymmetrically distributed peak WSS values in TAA | |
| Salmasi et al. [24] | 2021 | 10 ATAA | Elevated WSS values associated with aortic wall degradation in ATAA disease | |
| Trenti et al. [18] | 2022 | 18 AAA, 22 age-matched controls, 23 young subjects | RRT as a marker for abnormal AAA hemodynamics | |
| Aalbregt et al. [12] | 2024 | 22 asymptomatic AAA | A 4D flow MRI is robust for assessing the hemodynamics within AAAs | |
| Bouaou et al. [19] | 2024 | 17 ATAA, 17 healthy controls, 13 younger healthy subjects | Flow and pressure indices associated with WSS | |
| Zeng et al. [25] | 2024 | 11 TAAA, 19 AAA, 21 controls | Lower WSS in TAAA | |
| Dissection | Veger et al. [28] | 2021 | A porcine aorta dissection model | Strict heart rate control is of major importance in reducing the mean and peak WSS in uncomplicated acute TBAD |
| Ruiz-Muñoz et al. [27] | 2022 | 54 Patients with chronic AD | WSS positively correlated with aortic growth rate | |
| Stokes et al. [29] | 2023 | A 56-year-old male patient with chronic TBAD | Oscillatory shear is highly sensitive to inlet velocity distribution and flow volume | |
| Atherosclerosis | Harloff et al. [30] | 2010 | 62 aortic atherosclerosis, 31 healthy volunteers | 4D flow MRI was successfully used to analyze multiplanar WSS distribution |
| Markl et al. [33] | 2013 | 70 patients with complex plaques, 12 healthy volunteers | Decreased WSS and increased OSI in patients with atherosclerosis | |
| Winter et al. [31] | 2021 | 5 wildtype and 5 ApoE mice | A new post processing method with 4D flow MRI | |
| Andelovic et al. [32] | 2021 | 6 healthy wildtype and 6 atherosclerotic ApoE mice | WSScirc as potential marker of plaque size and composition in advanced atherosclerosis | |
| Bicuspid aortic valve | Barker et al. [43] | 2012 | 15 BAV, 45 controls | Increased and asymmetric WSS according to BAV fusion pattern |
| Meierhofer et al. [36] | 2013 | 18 BAV, 18 healthy individuals | Increased WSS in healthy BAV individuals | |
| van Ooij et al. [35] | 2015 | 13 BAV, 10 controls | Elevated WSS correlated with peak systolic velocity | |
| Guzzardi et al. [22] | 2015 | 20 BAV | Regions of increased WSS show greater medial elastin degradation | |
| Shan et al. [41] | 2017 | 50 BAV, 15 TAV | Severe aortic insufficiency or stenosis resulted in further elevated WSS | |
| van Ooij et al. [45] | 2017 | 270 BAV, 245 TAV with aortic dilatation, 56 healthy subjects | Increased WSS in BAV and TAV with AS | |
| Rodríguez-Palomares et al. [44] | 2018 | 101 BAV, 20 healthy subjects | Different BAV-phenotypes present different flow patterns | |
| Farag et al. [40] | 2018 | 48 BAV, 25 healthy individuals | Increased WSS in BAV patients with AS | |
| Bollache et al. [37] | 2018 | 27 BAV | Increased aortic valve-mediated WSS associated with elastic fiber thinning | |
| Soulat et al. [38] | 2022 | 72 BAV, 136 controls | WSS correlated with AAO dilation | |
| Guala et al. [23] | 2022 | 47 BAV | Circumferential WSS predict progressive dilation of the ascending aorta in patients with BAV | |
| Minderhoud et al. [39] | 2022 | 32 BAV, 28 healthy controls | WSS angle associated with aortic growth | |
| Trenti et al. [42] | 2024 | 42 BAV, 22 normal controls | Elevated OSI in BAV with aortic regurgitation | |
| Maroun et al. [34] | 2024 | 20 BAV, 125 controls | The long-term stability of 4D flow MRI-derived WSS and WSS-derived heatmaps | |
| Aortic valve replacement | von Knobelsdorff-Brenkenhoff et al. [49] | 2014 | 38 AVR, 9 healthy controls | AVR types affect WSS |
| Trauzeddel et al. [53] | 2016 | 17 TAVI, 12 AVR, 9 healthy controls | Increased WSS after TAVI and AVR compared to healthy controls | |
| van Kesteren et al. [50] | 2018 | 14 stented and 14 stentless bioprosthesis | Lower WSS for stentless prosthesis | |
| Bissell et al. [51] | 2018 | 30 AVR, 30 patients with a native aortic valve, 30 healthy subjects. | Decreased WSS after mechanical AVR or Ross procedures | |
| Bollache et al. [47] | 2018 | 33 patients received operation, 20 control patients did not | Proximal aortic WSS decreased after AVR | |
| Farag et al. [52] | 2019 | 14 post-TAVR patients and 10 healthy controls | Increased WSS in the ascending aorta after TAVR | |
| Komoriyama et al. [48] | 2021 | 32 Pre-TAVR and post-TAVR patients | TAVR improves blood flow dynamics | |
| Wiesemann et al. [46] | 2023 | 7 patients received an AVR, 13 control patients did not | Decreased WSS compared to patients who were not operated on | |
| Marfan syndrome | Geiger et al. [54] | 2013 | 24 MFS, 12 older healthy volunteers | Higher WSS at the inner curvature in the proximal AAO and at the anterior part in the more distal AAO |
| Wang et al. [57] | 2016 | 20 MFS and 12 age-matched normal subjects | Lower WSSaxial in the aortic root and the WSScirc in the arch | |
| van der Palen et al. [58] | 2017 | 25 MFS, 21 healthy controls | Lower WSS in pediatric MFS patients | |
| Geiger et al. [55] | 2017 | 19 adolescent MFS, 10 healthy volunteers | Lower WSS in the inner proximal DAO in a 3-year follow-up | |
| Guala et al. [59] | 2019 | 75 Marfan, 48 healthy subjects | Abnormal circumferential WSS in Marfan patients | |
| van Andel et al. [56] | 2022 | 55 MFS, 25 healthy subjects | Deviant directed WSS in the DAO was more frequently seen in male patients and in patients with a HI mutation type |
ATAA, ascending thoracic aortic aneurysm; TAA, thoracic aortic aneurysm; AAA, abdominal aortic aneurysm; RRT, relative residence time; TAAA, thoracoabdominal aortic aneurysm; TBAD, type B aortic dissection; AD, aortic dissection; OSI, oscillatory shear stress; ApoE, apolipoprotein E-deficient; WSScirc, circumferential WSS; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve; AS, aortic valve stenosis; AAO, ascending aorta; AVR, aortic valve replacement; TAVR, transcatheter aortic valve replacement; MFS, Marfan syndrome; WSSaxial, axial WSS; DAO, descending aorta; HI, haploinsufficient.
Additionally, some scholars have derived the oscillatory shear index (OSI) based on WSS [60, 61], which indicates the degree of directional change of WSS during a cardiac cycle [62, 63]. A high OSI implies significant directional changes in WSS, reflecting large shear stress fluctuation. Another study introduced relative residence time (RRT), a parameter based on the values of WSS and OSI [18]. RRT can identify areas with low WSS and high OSI, which are prone to aortic diseases such as plaque formation. Therefore, high RRT can be used to locate high-risk areas [18, 64].
Risk stratification of aortic aneurysms is a prominent and challenging research topic, with increasing attention being paid to the influence of hemodynamics on aneurysms [19, 20, 21]. WSS is closely related to aneurysm formation, growth, and rupture, and it changes continuously during aneurysm progression.
A series of histopathological studies have revealed that high WSS leads to dysregulation of the extracellular matrix and degeneration of elastic fibers, resulting in thinning of the arterial wall, promoting aneurysm formation [22, 65, 66]. A study utilizing 4D Flow MRI has reached similar conclusions, showing that elevated WSS is strongly associated with an increased rate of aortic diameter growth. In particular, high circumferential WSS may be an independent predictor of aneurysm formation [23].
During aneurysm evolution, there are morphological and hemodynamic differences between aneurysms with different WSS, and both high and low WSS potentially contributing to aneurysm growth and rupture [67, 68]. Salmasi et al. [24] assessed the relationship between preoperative 4D Flow MRI images and postoperative tissue specimen characteristics in patients with ascending aortic aneurysms, finding that areas of high WSS were associated with aortic wall thinning, elastin abundance, and decreased smooth muscle cell counts. This suggests that degradation and thinning of the aneurysm wall are associated with hemodynamic impairment and high WSS (Fig. 2). Conversely, low WSS leads to inflammatory cell-mediated endothelial injury and apoptosis, with low and oscillating WSS areas being prone to plaque formation. This results in large, thick-walled aneurysms due to the combination of the inflammatory response and plaque buildup [69].
 Fig. 2.
Fig. 2.
A patient with descending aortic aneurysm. (A) 4D-flow-Image with streamlines demonstrates low velocity inside the thoracic aortic aneurysm (blue arrow) and high velocity around the aneurysm (red arrow). (B) 4D-flow-Image demonstrates high WSS inside the thoracic aortic aneurysm (blue arrow).
Aortic aneurysms often exhibit vortex or helical flow, with lesion areas showing low WSS and high OSI [25, 26, 60, 61]. These abnormal hemodynamics tend to promote aortic atherosclerosis, which in turn leads to progressive aortic dilatation and increases the risk of aneurysm rupture. Additionally, it has been found that RRT seems to be a more powerful predictor of hemodynamic changes in aortic aneurysms than the commonly used OSI [18], as it accounts for both the magnitude and direction of WSS.
Aortic dissection is one of the common types of acute aortic syndrome (AAS) with rapid onset and high mortality. Early screening for the potential risk of aortic dissection in high-risk groups would facilitate clinical preventive measures and reduce patient mortality. Fig. 3 shows aortic dissection evaluated by imaging modalities. Research has found that the area of highest WSS is highly coincident with the location of the tear in stanford type A aortic dissection [70]. This suggests that increased WSS may be an important factor in endovascular injury. Patients with stanford type B aortic dissection can be treated medically or surgically based on clinical assessment, and real-time monitoring of the dynamic evolution of the aortic dissection is mandatory. Increased aortic diameter and partial thrombosis of the false lumen are associated with late adverse events in type B aortic dissection [71, 72]. WSS may be a good indicator for monitoring these changes. On the one hand, WSS is positively correlated with the aortic growth rate [27], which could be used as a predictor of the distant expansion of aortic dissection. On the other hand, low WSS, high RRT, flow patterns are correlated with thrombosis [73]. There is increasing evidence to indicate that 4D flow MRI-based hemodynamics plays an important role in aortic dissection management and prognosis [27, 28, 29, 74].
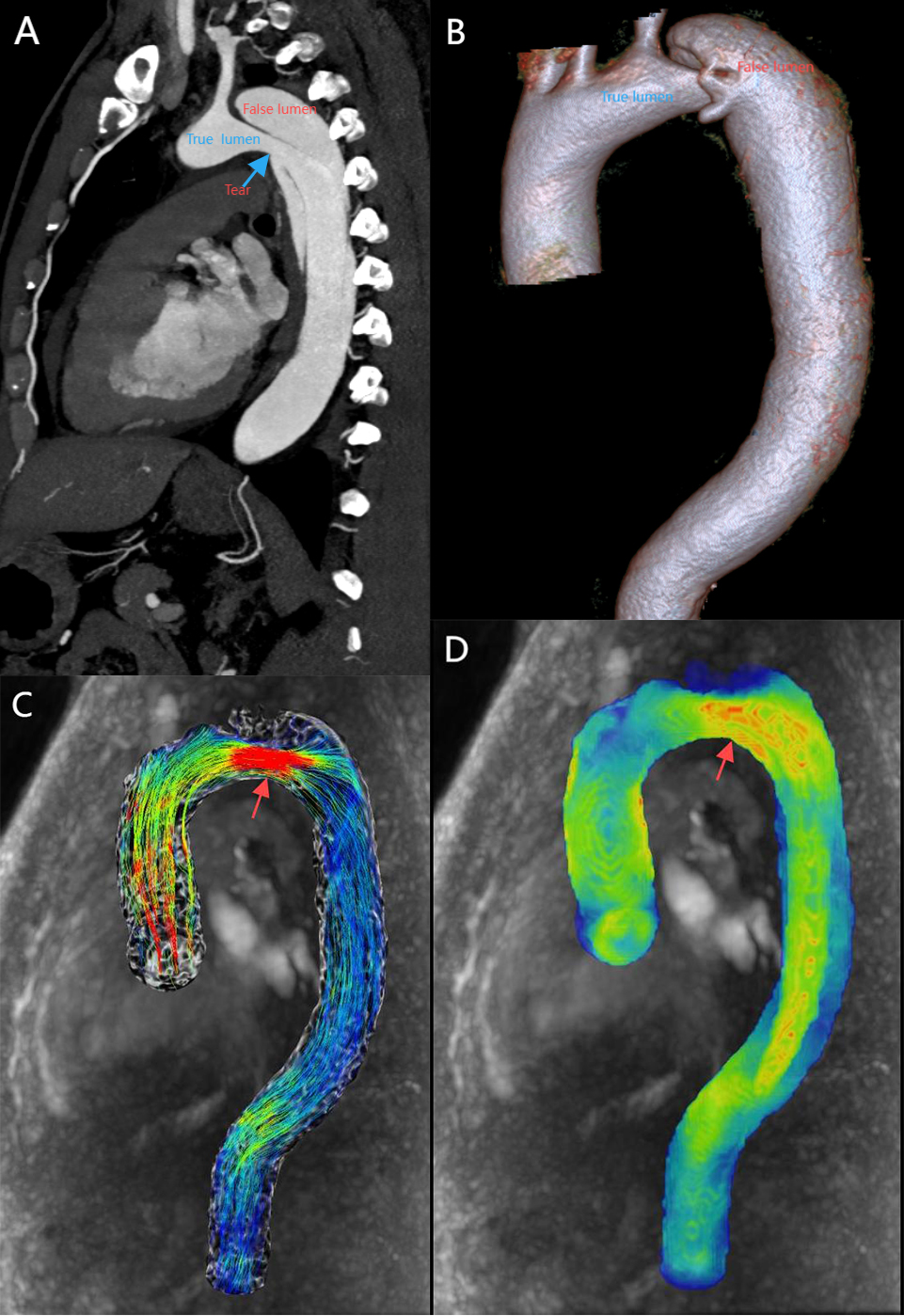 Fig. 3.
Fig. 3.
A patient with stanford type B aortic dissection. (A) 2D sagittal view, showing true and false lumen as well as entry tear (blue arrow). (B) 3D VR displays true lumen, false lumen. (C) 4D-flow-Image with streamlines demonstrates high velocity at the entry tear (red arrow). (D) 4D-flow-Image demonstrates high WSS at the entry tear (red arrow). 2D, two dimensions; 3D, three dimensions; VR, volume rendering.
WSS is one of the most important hemodynamic parameters in the formation and progression of atherosclerosis. Previous studies have demonstrated that persistent abnormal WSS can cause pathophysiological changes such as vascular remodeling, cell death, extracellular matrix degradation, and pro-inflammatory responses, which can promote plaque formation [75, 76, 77]. 4D Flow MRI provides a powerful tool for monitoring the distribution of aortic WSS in patients with atherosclerosis, allowing measurement of vessel wall parameters in the region of interest from any direction and angle [30, 31]. Circumferential WSS may be an important parameter in the noninvasive assessment of atherosclerotic plaque characteristics, correlating with plaque size, macrophage content, calcification, and necrotic core area [32], suggesting that abnormal circumferential WSS is critical for plaque growth and progression towards vulnerability. A study [33] analyzing 140 complex plaque locations using 4D Flow MRI found that aortic branches, bifurcations, or bends near the aorta are susceptible to disturbed and flocculated flow, generating areas of low and oscillating WSS, which are common areas of plaque formation. 4D Flow MRI-based study confirms that low WSS and high OSI promote the occurrence and development of atherosclerotic plaque [78, 79].
The rupture of atherosclerotic plaques can lead to vascular obstruction and trigger serious adverse consequences. High WSS increases metalloproteinase activity, accelerates angiogenesis and transformation, thereby enhancing plaque vulnerability and inducing plaque rupture [80, 81]. There are few studies applying 4D Flow to predict aortic plaque rupture. In a study of carotid plaques, high-risk plaques were found to have higher WSS, which proved that high WSS might be related to plaque rupture and more likely to cause cerebrovascular events [82].
The bicuspid aortic valve (BAV) is a common malformation of the aortic valve, occurring most often in males. 4D Flow MRI-derived WSS has demonstrated great potential for hemodynamic measurements in patients with BAV [34, 35]. Even in patients with normal BAV, the ascending aorta shows elevated WSS [36]. Areas with increased WSS are prone to aortic extracellular matrix dysregulation and elastic fiber thinning, which are associated with subsequent aortic disease [22, 37]. A prospective longitudinal study [23] found that elevated circumferential WSS predicted the growth rate of ascending aortic diameter in patients with BAV and may be a marker for the risk of ascending aortic dilatation. Other studies have confirmed the relationship between high WSS and aortic growth [38, 39]. BAV can be associated with valvular dysfunction, with elevated WSS observed in cases of aortic stenosis [40, 41] and elevated OSI observed in cases of aortic regurgitation [42].
Hemodynamic changes are also associated with the BAV valve fusion phenotype [43]. Right-left bicuspid aortic valve (RL-BAV) patients present a higher axial WSS at the aortic root while right-non coronary bicuspid aortic valve (RN-BAV) present a higher circumferential WSS in the mid and distal ascending aorta (AAO) [44]. However, moderate-to-severe aortic stenosis blurs the difference in WSS between these valve types, as the presence of aortic stenosis dominates ascending aortic hemodynamics irrespective of the valve fusion phenotype [41, 45].
Aortic valve replacement (AVR) significantly improves symptoms, quality of life, and prolongs survival in patients with aortic stenosis. A 4D Flow MRI-based study found improved aortic hemodynamics and reduced WSS after AVR compared with preoperative patients with aortic disease [46, 47, 48]. Different types of AVR present different hemodynamic changes post-surgery [49, 50]. Bissell et al. [51] compared the mechanical parameters of blood flow in 30 patients with BAV who underwent AVR using different surgical modalities and found that the aorta returned to a normal flow pattern in patients after mechanical valve replacement or the Ross procedure. However, higher WSS and abnormal spiral flow persisted in patients after bioprosthetic AVR. Transcatheter aortic valve replacement (TAVR) is an effective alternative to surgical aortic valve replacement (SAVR) for elderly and high-risk patients with aortic stenosis [83]. Ascending aortic WSS was increased and asymmetrically distributed after TAVR compared to healthy controls [52, 53].
Marfan syndrome (MFS) is a hereditary connective tissue disorder in which aortic complications are the main cause of death, including aortic dissection and aortic aneurysm rupture. Early prediction of the development of aortic complications in patients with Marfan syndrome by hemodynamic parameters is of great significance. Local helix flow patterns in the ascending aorta and proximal descending aorta were confirmed in a study of adolescent MFS patients using 4D Flow MRI [84]. This resulted in a heterogeneous regional WSS distribution, with elevated WSS in the proximal inner curvature of the ascending aorta [54] and decreased segmental WSS in the inner proximal descending aorta [55]. At follow-up after 3 years, WSS was reduced in the inner proximal descending aorta, which may be related to the risk of aortic dissection [55, 56]. Some scholars have disagreed, suggesting that proximal WSS in the ascending aorta is reduced, hypothesizing that the inconsistent results are due to the fact that young MFS patients exhibit higher WSS [57, 58]. In addition, both circumferential and axial WSS of the proximal descending aorta are independently correlated with local lumen diameter, and decreased circumferential WSS may be one of the early markers of descending aortic dilatation in patients with Marfan syndrome [59]. Patients with MFS need to be monitored for a long period of time, and in the future, 4D Flow MRI could be added as part of regular observations, which may help clinicians to predict the occurrence of MFS complications at an early stage.
Currently, 4D flow MRI technology still has many shortcomings and is mainly used for small-sample studies. First of all, 4D flow MRI scanning time is long, but the clinical environment still needs shorter imaging time, and image resolution is relatively low, which makes it difficult to accurately recognize aortic complex anatomies and cases, and at the same time affects the accuracy of WSS parameter values. In the future, we need to improve the K-space sampling, compression perception technology and deep learning to shorten the scanning time and enhance the spatial and temporal resolution. Second, the variability in parameters obtained from different post-processing vendors makes it difficult to establish a unified normal reference value for WSS. Therefore, developing and establishing a standardized workflow is crucial. Third, research based on 4D Flow MRI-derived WSS in aortic disease is limited. There is a need for more and larger prospective cohorts to explore how this technology could be integrated into existing clinical workflows and its expected impact on patient management and treatment outcomes.
In conclusion, 4D flow MRI-derived WSS is a promising tool for assessing the occurrence and development, risk stratification, and surgical efficacy of aortic disease and its complications.
YL and XLM contributed to the design and implementation of the article, data collection and article writing. YXW and ZX contributed to the literature search and figure production. YS led the research team, provided critical guidance on the study design and methodology, and ensured the scientific rigor of the work. YS was responsible for finalizing the manuscript, managing all aspects of the publication process. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
