- Academic Editor
Statin therapy is associated with an increased risk of new-onset diabetes (NOD), possibly due to a reduction in coenzyme Q10 (CoQ10) levels as a result of statin use. This study aimed to investigate the relationship between exogenous CoQ10 supplementation and the development of NOD.
This study included 4394 participants from the National Health and Nutrition Examination Survey (NHANES). Baseline characteristics were compared between those with and without NOD and between those with and without CoQ10. Univariate logistic regression was performed to identify factors associated with NOD. Two models were used for confounding factors, including demographics and various covariates. Multifactor logistic regression further assessed the association between CoQ10 supplementation and NOD. Additionally, restricted cubic spline (RCS) analysis was conducted to evaluate the potential nonlinear relationship between daily CoQ10 dose and NOD.
Univariate logistic regression showed an association between CoQ10 supplementation and a reduced risk of NOD (odds ratio [OR] = 0.323, 95% confidence interval [CI] 0.157–0.668, p = 0.003), which remained significant after adjustments in model 1 (OR = 0.344, 95% CI 0.160–0.737, p = 0.006) and model 2 (OR = 0.232, 95% CI 0.057–0.942, p = 0.041). There was no evidence of a linear association between daily CoQ10 dose and NOD in logistic regression analysis (OR = 0.999, 95% CI 0.994–1.004, p = 0.720), and no evidence of a nonlinear correlation in the RCS analysis (p > 0.05).
CoQ10 supplementation in individuals taking statins was associated with a reduced risk of NOD, and this association was independent of the CoQ10 dose.
Cardiovascular Disease (CVD) is one of the leading causes of death and disability among adults worldwide, with hyperlipidemia playing a significant role in its development [1]. Statin therapy offers clear benefits, including reducing lipid levels, slowing the progression of atherosclerosis, decreasing cardiovascular events, and is generally regarded as safe and well-tolerated [2]. However, it is essential to recognize that numerous studies and meta-analyses have confirmed the potential risk of statin use in increasing the incidence of new-onset diabetes (NOD) [3, 4, 5, 6, 7]. While the adverse effects of statin-mediated NOD are outweighed by the benefits of statin medications, NOD may negatively impact medication adherence among statin users. The mechanism underlying this population may be related to coenzyme Q10 (CoQ10) [8].
Coenzyme Q10, also known as ubiquinone, is a lipophilic compound that functions similarly to a vitamin and is synthesized endogenously from tyrosine in the human body [9]. CoQ10 acts as a lipophilic antioxidant by neutralizing free radicals, inhibiting lipid peroxidation of biomembranes, and protecting mitochondrial proteins and DNA from oxidative damage [10, 11]. Additionally, CoQ10 binds to the inner mitochondrial membrane, participating in the electron transport chain and oxidative phosphorylation, playing a crucial role in cellular energy synthesis by generating adenosine triphosphate (ATP) [12].
However, factors such as genetics, aging, and statin therapy can decrease its physiological concentration [9, 13]. Notably, low-density lipoprotein (LDL) serves as the primary carrier of CoQ10 in circulation, and is reduced by statin therapy, which may lead to decreased circulating levels of CoQ10 [13]. Furthermore, statins inhibit the synthesis of CoQ10. Insufficient CoQ10 can induce mitochondrial oxidative stress, leading to beta-cell apoptosis, reduced insulin sensitivity, and ultimately manifesting as NOD or poor blood glucose control [9, 14, 15]. Therefore, we hypothesize that appropriate CoQ10 supplementation may help reduce the risk of NOD in individuals taking statin medications.
Previous studies have confirmed that CoQ10 is associated with a reduction of NOD in mice [16, 17], but relevant studies in human population are limited [18, 19]. This study aimed to investigate whether CoQ10 supplementation has a protective effect against new-onset diabetes in a statin-taking population and to explore the dose-response relationship. This will directly address the current research gap and provide insights for future studies targeting NOD.
We used data from participants in the National Health and Nutrition Examination Survey (NHANES), a well-established series of repeated cross-sectional surveys conducted in the United States. These surveys employ multistage probabilistic sampling strategies to select participants, including, where appropriate, oversampling of specific population segments [20]. The underlying protocol was approved by the research ethics review board of the National Center for Health Statistics (NCHS) [20]. The NHANES database is a publicly accessible resource, and all participants signed informed consent forms. The project underwent an ethical review, as detailed in the supplementary materials. For this study, we utilized NHANES data spanning from 1999 to 2018, selecting individuals who were taking statins, those without diabetes, and individuals with a confirmed diagnosis of diabetes and documented time of statin initiation, while excluding participants with a confirmed diagnosis of cancer. We identified the diagnosis of diabetes occurring after statin use as NOD.
We included indicators potentially related to NOD, encompassing participants’ demographic information such as age, sex, race, poverty income ratio (PIR), educational attainment (EDU), physical measurements (body mass index [BMI], waist circumference, blood pressure, physical activity, smoking status, alcohol consumption), medical history (hypertension, cardiovascular disease, heart failure, liver or kidney dysfunction), and laboratory parameters: triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), plasma glucose, hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR). Regarding CoQ10, we incorporated the following variables: CoQ10 (CoQ10 administration over the past 30 days), CoQ10 daily dosage (average daily intake of CoQ10 over the past 30 days, in mg), CoQ10 daily dosage per kilogram of body weight (average daily intake of CoQ10 per kilogram of body weight over the past 30 days, in mg/kg), CoQ10 daily dosage per body surface area (average daily intake of CoQ10 per body surface area over the past 30 days, in mg/m2). The instruments and methods used for the laboratory tests and the rules for calculating the indicators used in this document are described in the supplementary documents.
A total of 4394 collected samples were included in the study for analysis. As
NHANES employs a probability-based sampling approach, intricate adjustments like
sample weights, strata, and primary sampling units were used to handle the
complex survey design, including oversampling, non-response, and
post-stratification. All statistical analyses considered the NHANES database’s
intricate sampling design. Non-normally distributed continuous variables are
presented as median (Q25, Q75). The Wilcoxon rank-sum test was used to contrast
two data sets while the Kruskal-Wallis test was used to compare multiple sets.
Categorical variables are shown as absolute values (percentages). Pearson’s
chi-squared test examines non-ordered variables, the Wilcoxon rank-sum test
compares ordinal variables, and the Kruskal-Wallis test compares multiple groups
of ordinal variables. Analyses were conducted using R version 4.3.0 (R Foundation
for Statistical Computing, Vienna, Austria), with significance set at p
This study ultimately included 4394 participants from NHANES 1999–2018 who met the inclusion and exclusion criteria. The median follow-up duration was 86.00 (46.00, 135.00) months. The specific screening process is outlined in Fig. 1.
 Fig. 1.
Fig. 1.
Screening and selection process for study participants from NHANES. NHANES, National Health and Nutrition Examination Survey.
The baseline characteristics of participants with and without NOD are summarized
in Table 1. The mean age of the study population was 62.00 (54.00, 70.00) years,
with females comprising 47% of the total sample. Among individuals on statin
therapy, 276 (6.6%) developed NOD. This subgroup was characterized by older age
(p = 0.010), a higher proportion of males (p = 0.007), and a
greater prevalence of pre-existing conditions such as hypertension (p
| Characteristic | Overall | Non-diabetes after statin | Diabetes after statin | p value | |
| Individuals n (N) | 4291 (15,582,680) | 4015 (14,558,118) | 276 (1,024,562) | - | |
| Age (years) | 62.00 (54.00, 70.00) | 62.00 (54.00, 70.00) | 64.00 (56.00, 72.00) | 0.010 | |
| Sex (female) | 2038 (47%) | 1927 (48%) | 111 (40%) | 0.007 | |
| Race | 0.233 | ||||
| Non-hispanic white | 1931 (45%) | 1802 (45%) | 129 (47%) | ||
| Non-hispanic black | 954 (22%) | 895 (22%) | 59 (21%) | ||
| Mexican american | 611 (14%) | 584 (15%) | 27 (9.8%) | ||
| Other/multiracial | 422 (9.8%) | 382 (9.5%) | 40 (14%) | ||
| Other hispanic | 373 (8.7%) | 352 (8.8%) | 21 (7.6%) | ||
| PIR | 3.07 (1.61, 5.00) | 3.05 (1.60, 5.00) | 3.43 (1.69, 5.00) | 0.276 | |
| Educational attainment | 0.345 | ||||
| Below high school | 700 (16%) | 659 (16%) | 41 (15%) | ||
| High school | 1671 (39%) | 1562 (39%) | 109 (39%) | ||
| Above high school | 1918 (45%) | 1792 (45%) | 126 (46%) | ||
| Hypertension | 3248 (77%) | 3011 (76%) | 237 (86%) | ||
| CVD | 705 (17%) | 626 (16%) | 79 (29%) | ||
| Heart failure | 471 (11%) | 427 (11%) | 44 (16%) | 0.001 | |
| Smoking status | 0.269 | ||||
| Never smoker | 2057 (48%) | 1935 (48%) | 122 (44%) | ||
| Former smoker | 1598 (37%) | 1472 (37%) | 126 (46%) | ||
| Current smoker | 636 (15%) | 608 (15%) | 28 (10%) | ||
| Alcohol intake | |||||
| Non-drinker | 556 (19%) | 532 (19%) | 24 (13%) | ||
| Former drinker | 222 (7.5%) | 200 (7.2%) | 22 (12%) | ||
| Drinker | 2189 (74%) | 2045 (74%) | 144 (76%) | ||
| Physical activity | 78,240.00 (0.00, 283,518.15) | 78,240.00 (0.00, 284,207.72) | 76,047.74 (0.00, 273,798.43) | 0.578 | |
| BMI (kg/m2) | 29.78 (26.20, 34.50) | 29.70 (26.14, 34.45) | 31.07 (26.98, 35.10) | 0.160 | |
| Waist circumference (cm) | 105.20 (95.20, 116.00) | 104.83 (95.00, 115.70) | 109.20 (99.02, 118.17) | 0.004 | |
| HbA1c (%) | 5.80 (5.50, 6.30) | 5.70 (5.40, 6.20) | 6.70 (6.30, 7.10) | ||
| TG (mmol/L) | 1.39 (0.96, 1.98) | 1.38 (0.96, 1.96) | 1.59 (1.14, 2.07) | 0.042 | |
| TC (mmol/L) | 4.55 (3.93, 5.22) | 4.60 (3.98, 5.22) | 3.97 (3.59, 4.94) | ||
| LDL-C (mmol/L) | 2.48 (1.97, 3.00) | 2.51 (1.99, 3.00) | 2.07 (1.63, 2.54) | ||
| HDL-C (mmol/L) | 1.32 (1.09, 1.58) | 1.32 (1.09, 1.60) | 1.16 (1.06, 1.29) | ||
| eGFR (mL/min) | 172.90 (141.00, 202.82) | 172.96 (141.29, 202.96) | 168.35 (135.37, 194.61) | 0.228 | |
| Age when first taking statin | 58.00 (50.00, 66.00) | 58.00 (50.00, 66.00) | 53.00 (49.00, 61.00) | ||
| CoQ10 | 127 (3.0%) | 122 (3.0%) | 5 (1.8%) | 0.001 | |
| CoQ10 daily dosage (mg) | 50.00 (21.00, 100.00) | 50.00 (21.72, 100.00) | 45.19 (7.45, 101.75) | 0.604 | |
| CoQ10 daily dosage per kilogram of body weight (mg/kg) | 17.00 (6.48, 41.70) | 16.88 (6.50, 41.63) | 13.06 (2.15, 28.95) | 0.889 | |
| CoQ10 daily dosage per body surface area (mg/m2) | 723.02 (289.45, 1613.47) | 721.79 (313.34, 1613.12) | 588.69 (98.49, 1301.59) | ||
| All-cause mortality | 1089 (25%) | 1014 (25%) | 75 (27%) | 0.766 | |
| Cardiovascular mortality | 342 (8.0%) | 312 (7.8%) | 30 (11%) | 0.202 | |
| Cancer-related mortality | 166 (3.9%) | 157 (3.9%) | 9 (3.3%) | ||
| Diabetes-related mortality | 228 (5.3%) | 203 (5.1%) | 25 (9.1%) | 0.006 | |
| Survival length (months) | 86.00 (46.00, 135.00) | 89.00 (47.00, 138.00) | 62.00 (36.00, 100.00) | ||
BMI, body mass index; CoQ10, coenzyme Q10; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PIR, poverty income ratio; TC, total cholesterol; TG, triglycerides; NOD, new-onset diabetes. n represents the number of participants in the study sample, while N represents the weighted number of participants based on NHANES sampling principles. Continuous variables are presented as median (Q25, Q75). Categorical variables are presented as numbers (percentages).
Table 2 presents a comparison of baseline characteristics between individuals
who took CoQ10 and those who did not. In this study, 127 participants (3.8%)
reported CoQ10 supplementation. This subgroup was distinguished by significantly
higher socioeconomic status (p
| Characteristic | Non-CoQ10 | Taking CoQ10 | p value | |
| Individuals n (N) | 4164 (14,990,114) | 127 (592,567) | - | |
| Age (years) | 62.00 (54.00, 70.00) | 65.41 (57.00, 71.00) | 0.075 | |
| Sex (female) | 1972 (47%) | 66 (52%) | 0.2 | |
| PIR | 3.04 (1.58, 5.00) | 4.30 (2.50, 5.00) | ||
| Educational attainment | ||||
| Below high school | 695 (17%) | 5 (3.9%) | ||
| High school | 1627 (39%) | 44 (35%) | ||
| Above high school | 1840 (44%) | 78 (61%) | ||
| Diabetes | 2404 (58%) | 57 (45%) | 0.003 | |
| HbA1c (%) | 5.80 (5.50, 6.30) | 5.70 (5.43, 5.90) | 0.032 | |
| Age when first diagnosed with diabetes | 50.00 (40.00, 59.00) | 55.01 (50.00, 60.00) | 0.010 | |
| New onset diabetes after statin use | 271 (6.5%) | 5 (3.9%) | 0.001 | |
n represents the number of participants in the study sample, while N represents the weighted number of participants based on NHANES sampling principles. Continuous variables are presented as median (Q25, Q75). Categorical variables are presented as numbers (percentages).
We conducted one-way logistic regression to identify the factors associated with NOD. The statistically significant indicators, along with their corresponding odds ratios (OR) and 95% confidence intervals (CI) are presented in Table 3. The complete results of the one-way logistic regression can be found in Supplementary Table 2. Notably, CoQ10 use (p = 0.003) was significantly associated with NOD. However, the specific dosage of CoQ10 did not demonstrate a linear correlation with the incidence of NOD.
| Characteristic | OR | 95% CI | p value | |
|---|---|---|---|---|
| Age (years) | 1.019 | 1.006, 1.031 | 0.003 | |
| Sex (male) | 1.657 | 1.147, 2.394 | 0.007 | |
| Race | ||||
| Mexican american | — | — | ||
| Other hispanic | 1.899 | 0.837, 4.310 | 0.124 | |
| Non-hispanic white | 2.033 | 1.199, 3.449 | 0.009 | |
| Non-hispanic black | 1.832 | 1.050, 3.196 | 0.033 | |
| Other/multiracial | 2.537 | 1.459, 4.412 | 0.001 | |
| PIR | 1.053 | 0.948, 1.170 | 0.331 | |
| Educational attainment | ||||
| Below high school | — | — | ||
| High school | 1.150 | 0.730, 1.810 | 0.545 | |
| Above high school | 1.287 | 0.829, 1.998 | 0.259 | |
| Hypertension | 2.842 | 1.554, 5.197 | ||
| CVD | 2.344 | 1.443, 3.809 | ||
| Heart failure | 2.124 | 1.343, 3.361 | 0.001 | |
| Waist circumference (cm) | 1.015 | 1.006, 1.025 | 0.002 | |
| TG (mmol/L) | 1.054 | 0.985, 1.128 | 0.124 | |
| TC (mmol/L) | 0.621 | 0.502, 0.767 | ||
| LDL-C (mmol/L) | 0.591 | 0.433, 0.807 | 0.001 | |
| HDL-C (mmol/L) | 0.179 | 0.084, 0.383 | ||
| eGFR (mL/min) | 0.998 | 0.995, 1.001 | 0.207 | |
| Age when first taking statin | 0.971 | 0.959, 0.984 | ||
| CoQ10 | 0.323 | 0.157, 0.668 | 0.003 | |
| CoQ10 daily dosage (mg) | 0.999 | 0.994, 1.004 | 0.720 | |
| CoQ10 daily dosage per kilogram of body weight (mg/kg) | 0.991 | 0.973, 1.010 | 0.341 | |
| CoQ10 daily dosage per body surface area (mg/m2) | 1.000 | 0.999, 1.000 | 0.456 | |
Based on the aforementioned results, we developed two models to correct for confounding factors and conducted multifactor logistic regression. The specific regression values are shown in Supplementary Table 3. To illustrate the role of CoQ10 more clearly, we included it in a forest plot (Fig. 2). A post hoc power analysis of the weighted statistics yielded a power value of 1.0. We also employed the variance inflation factor to assess multicollinearity among the covariates in the logistic regression, with the results detailed in Supplementary Table 4.
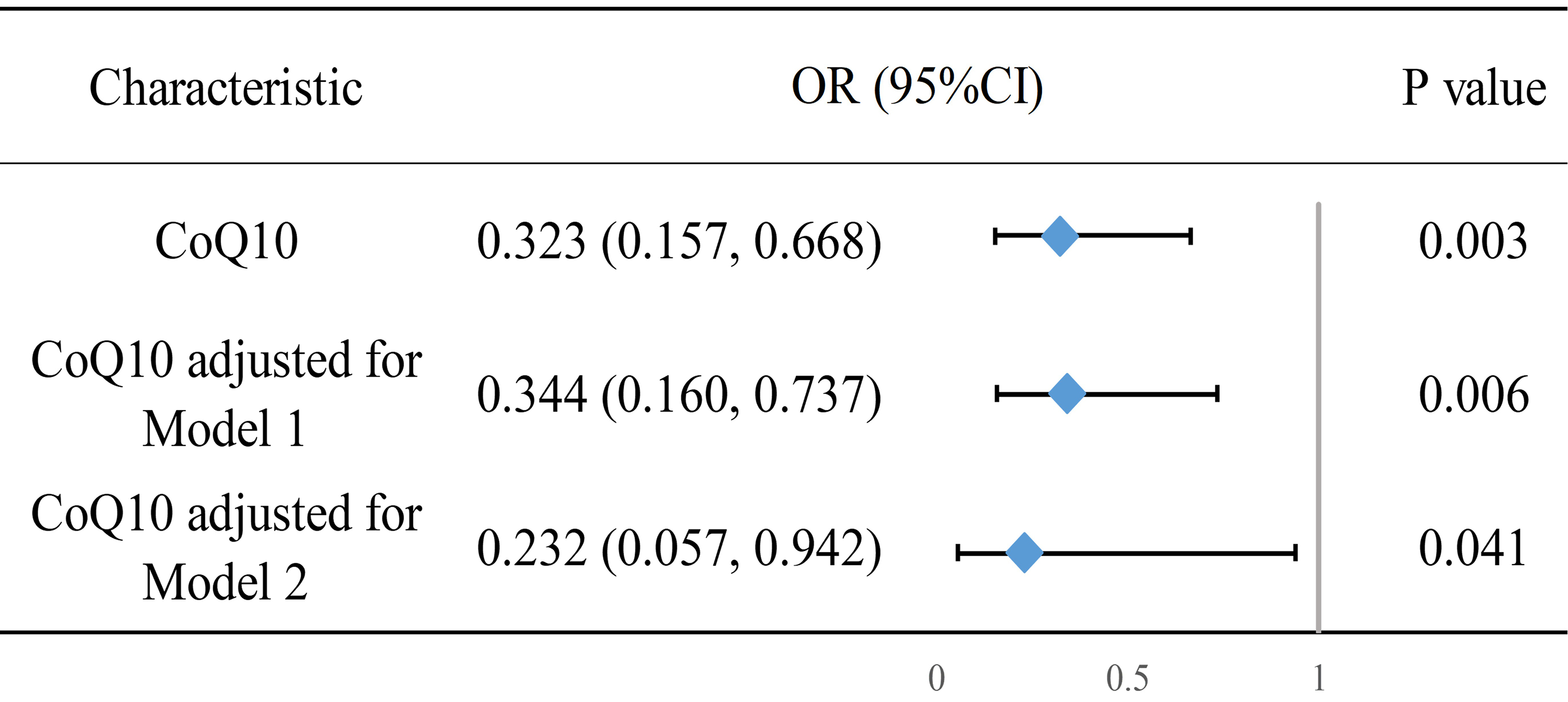 Fig. 2.
Fig. 2.
Forest plot of multifactorial logistic regression results of CoQ10 and NOD. Model 1 adjusts for age, sex, race, PIR, and educational attainment. Model 2 further adjusts for hypertension, CVD, heart failure, waist circumference, LDL-C, and the age at which statin therapy was initiated; OR, odds ratio.
The RCS analysis (Fig. 3) did not find a nonlinear correlation between CoQ10
daily dose and NOD. This lack of correlation persisted even after multiple
modeling adjustments. Further investigated the relationship between daily CoQ10
dose per unit of body weight and per unit of body surface area with NOD was
conducted using RCS (Supplementary Fig. 1). These analyses also
demonstrated no statistically significant nonlinear correlation (p
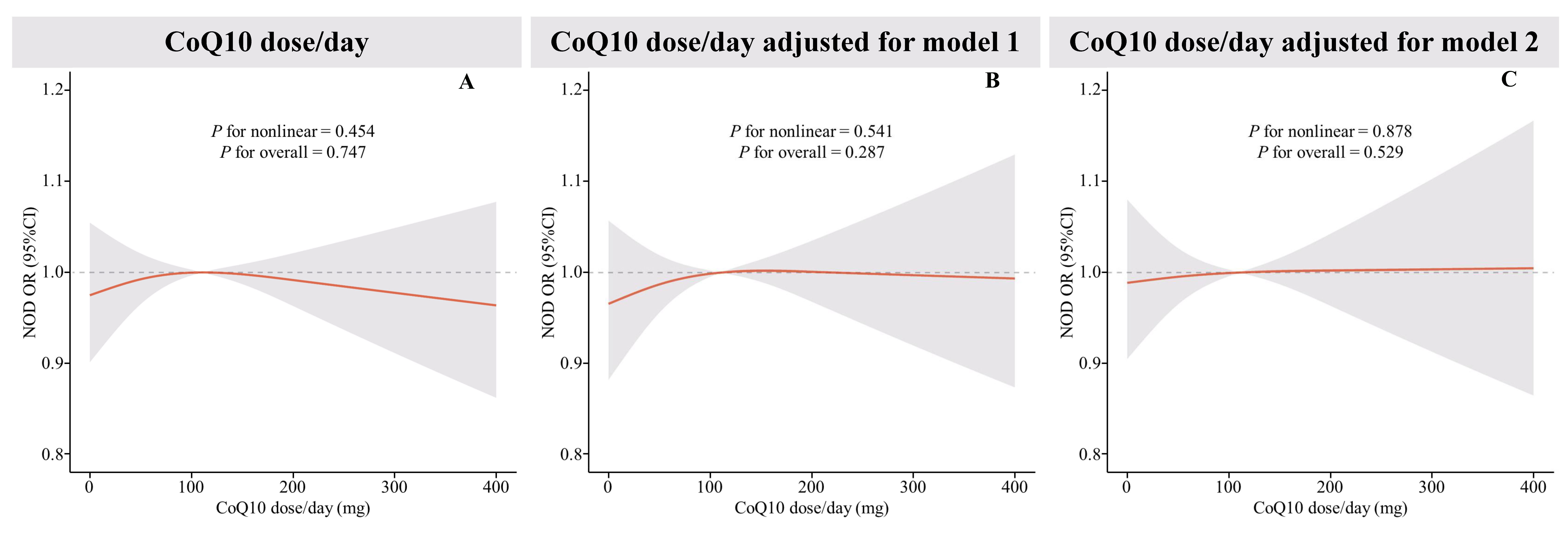 Fig. 3.
Fig. 3.
Restricted cubic spline (RCS) plot of the relationship between CoQ10 daily dose and new-onset diabetes. (A) RCS curves of the correlation between CoQ10 daily dose and new-onset diabetes were shown, (B) RCS curves of the correlation between CoQ10 daily dose and new-onset diabetes under Model 1 adjustment, and (C) RCS curves of the correlation between CoQ10 daily dose and new-onset diabetes under Model 2 adjustment. The red lines represent odds ratios and shading indicates 95% confidence intervals. New onset diabetes was derived via logistics regression using RCS, with adjustments made in model 1 and model 2. Model 1 accounts for age, sex, race, PIR, and educational attainment. Model 2 further adjusts for hypertension, CVD, heart failure, waist circumference, LDL-C, and the age at which statin therapy was initiated.
In our further analysis, we explored the interaction between CoQ10 and various factors, including age, BMI, CVD, waist circumference, hypertension, heart failure, and LDL-C. The results are presented in Supplementary Table 5. Notably, the data suggested an interaction between CoQ10 and the incidence of NOD, specifically with heart failure. However, due to the limited number of participants, subgroup analyses were not feasible. To overcome this limitation, we implemented propensity score matching based on demographic characteristics, including age, sex, race, PIR, and educational attainment. After matching, CoQ10’s impact on NOD was reevaluated using multifactorial logistic regression. A 1:1 matching for CoQ10 users versus non-users yielded an OR of 0.061 (95% CI: 0.006–0.336, p = 0.006), indicating a significant protective effect of CoQ10. A similar protective effect was observed in the 1:2 matching, which yielded an OR of 0.197 (95% CI: 0.051–0.610, p = 0.010). Both sensitivity test results were consistent with the conclusions of this study.
This study retrospectively included a total of 4394 patients from the NHANES
database spanning the years 1999 to 2018, with a median follow-up duration of
86.00 (46.00, 135.00) months. The key finding is that among patients taking
statin medications, the simultaneous co-administration of CoQ10 is associated
with a reduction in NOD. This correlation remained significant even after
adjusting for various confounding factors (p
While statin therapies are widely utilized in clinical practice, multiple studies have confirmed their association with NOD risk [3, 4, 5, 21, 22, 23], which may be related to CoQ10 deficiency. Research has demonstrated that statin drugs can decrease CoQ10 levels in the blood by 16% to 54% [24]. Two possible mechanisms contribute to the statin-induced reduction in CoQ10. Firstly, statin treatment inhibits the production of mevalonate, an intermediate in CoQ10 synthesis. Secondly, statin drugs lower LDL-C levels, potentially impacting CoQ10 transport. External supplementation of CoQ10 has been shown to elevate the levels of CoQ10 in the blood of patients undergoing statin therapy [24, 25]. Additionally, CoQ10 deficiencies can lead to impaired mitochondrial function and reduced antioxidant capacity, further decreasing insulin secretion and sensitivity, which may contribute to NOD development [25, 26]. Exogenous supplementation with CoQ10 may ameliorate this process [16]. In addition, studies have confirmed that supplementation with CoQ10 improves mitochondrial metabolism, which plays a crucial role in the pathogenesis of diabetes mellitus [19, 27].
Therefore, we hypothesized that exogenous supplementation with CoQ10 might help
reduce the occurrence of NOD in those taking statins [28]. In previous animal
experiments, Lorza-Gil et al. [16] found that pravastatin-treated
hypercholesterolemic LDL receptor knockout mice exhibited decreased insulin
secretion, increased islet cell death, and increased oxidative stress. Initiating
dietary supplementation of CoQ10 in these mice reversed fasting hyperglycemia,
improved glucose tolerance (by 20%), enhanced insulin sensitivity (by
Given the limited number of studies investigating CoQ10 supplementation in the context of NOD among statin-using populations, we must refer to related studies for insights into CoQ10 dosage. In statin-related studies, Fedacko et al. [33] found significant improvement in statin-related myopathy with a CoQ10 dosage of 200 mg/day over three months. Similarly, another study demonstrated that CoQ10 supplementation (240 mg/d for 22 months) markedly reduced the incidence of statin-related fatigue (from 84% to 16%) and myalgia (from 58% to 6%) [34]. Additionally, several studies have shown that CoQ10 supplementation at a dose of 150 mg/day or 200 mg/day for three months significantly improves fasting blood glucose and HbA1c levels [30, 31, 32]. This study found no statistically significant correlation between CoQ10 dosage and the incidence of NOD. We attribute this finding to several factors: (1) The retrospective design of this study posed several limitations, particularly with the small number of participants taking CoQ10 and the broad range of CoQ10 dosages, which may have obscured statistical differences. (2) The study relied on the average CoQ10 dose taken in the past month, which may not accurately reflect the total CoQ10 exposure over the entire period of statin therapy. We recommend future well-designed randomized controlled trials to explore the dose-response relationship between CoQ10 and NOD in statin users. Identifying the optimal dosage would provide a practical and effective means to prevent NOD in this population. A key strength of this study is that this is the first to leverage the NHANES database to investigate the association between exogenous CoQ10 supplementation and NOD in a statin-using population. The NHANES database’s robust sampling methodology, combined with the appropriate use of statistical weighting, allows the findings of this study to extend beyond the sampled cohort, and to be generalized to the entire U.S. population using statins. This broad generalizability enhances the sensitivity and reliability of the study’s conclusions, making its findings particularly valuable for public health applications.
Despite the aforementioned strengths, this study does have notable limitation. As a retrospective study, it is inherently constrained by the reliance on observational data, making it difficult to establish causality between CoQ10 supplementation and NOD—we can only infer a correlation. Furthermore, the small sample size of participants taking CoQ10, compared to those who were not, may have increased the margin of error in the statistical analysis of CoQ10’s effects. Additionally, several continuous variables in the analysis do not follow a normal distribution. This deviation necessitated the use of non-parametric tests, reducing the statistical power of some comparisons. Another limitation is the lack of data on the total duration of CoQ10 administration and serum CoQ10 concentrations, which could have provided more precise insights into CoQ10’s role. These limitations are common in retrospective analyses, and we hope that well-designed randomized controlled trials in the future will further explore the qualitative and quantitative relationships between CoQ10 supplementation and NOD risk in statin users.
The opportunity presented by this study is its suggestion of a potentially effective approach for preventing NOD in individuals who require statin therapy, but are concerned about the risk of developing NOD. This could enable more patients to benefit from the cardiovascular protective effects of statins without fear of adverse metabolic effects. Additionally, this research sets a foundation for future randomized controlled trials to further investigate the dose-response relationship between CoQ10 supplementation and NOD, providing a clear direction for subsequent studies.
In individuals taking statins, CoQ10 supplementation is associated with a
reduced risk of developing NOD. This relationship remains statistically
significant even after adjusting for confounding factors (p
ATP, adenosine triphosphate; BMI, body mass index; CoQ10, coenzyme Q10; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NCHS, National Center for Health Statistics; NHANES, National Health and Nutrition Examination Survey; NOD, new-onset diabetes; PIR, poverty income ratio; RCS, restricted cubic spline; TG, triglyceride; TC, total cholesterol.
Data described in the manuscript comes from the official NHANES website at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx. If needed, the corresponding author can provide the relevant R code upon request. This study is an observational retrospective study, and according to the Clinical Trial Registration Statement from the International Committee of Medical Journal Editors (ICMJE), registration is not required for purely observational studies.
XRH designed the study, analyzed the data, and drafted the manuscript. JXL and YZG contributed to the data analysis. YFL and WZ assisted in the interpretation of the data. NQL and AMD contributed to the conception and design of the study, critically reviewed the manuscript for important intellectual content, and approved the final version for publication. All authors contributed to the editorial revisions of the manuscript. All authors have read and approved the final manuscript. All authors have made substantial contributions to the work and agree to be accountable for all aspects of the research.
We use NHANES database, which is a well-established public database, participants signed informed consent forms, and the project was approved for ethical review. The data used in this article involves multiple ethics approval numbers, as detailed in the supplementary materials.
Not applicable.
This work was supported by grants from the National Key R&D Program of China [grant number 2022YFC3602400 and 2022YFC3602405] and the Key Projects of the National Health Commission [grant number 2020-ZD13].
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM26437.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
