- Academic Editor
To explore the sex-specific risk factors of associated with arterial stiffness.
A total of 28,291 participants from the Kailuan study cohort were enrolled in this study. A multivariate linear regression analysis and a multivariate logistic regression model were used to analyze the influencing factors of arteriosclerosis (indexed using the brachial–ankle pulse wave velocity, baPWV) between different sexes.
The incidence of arteriosclerosis (baPWV greater than or equal to 1400 cm/s) was 54.70%. The incidence of arteriosclerosis in males (62.13%) was higher than in females (37.41%) (p < 0.01). According to age stratification (5 years difference for each group), the baPWV values of males in all age groups <70 years were higher than in females (p < 0.01). The increase in baPWV values was higher in females over 45 years than in males and correlated with males in the 70–75 age group. The multivariate linear regression model showed that for every 5-year increase in age, the baPWV increased by 62.55 cm/s in males and 71.86 cm/s in females. Furthermore, for every 10 mmHg increase in systolic blood pressure (SBP), the baPWV increased by 61.01 cm/s in males and 51.86 cm/s in females. Regular physical exercise reduced the baPWV in males, but there was no statistical correlation in females. The waist-to-hip ratio (WHR) increased the baPWV in females yet was not statistically significant in males. Multivariate logistic regression analysis showed that after adjusting for confounding factors (age, WHR, SBP, heart rate, triglyceride, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), high-sensitivity C-reactive protein (hs-CRP), estimated glomerular filtration rate (eGFR), diabetes, higher education, higher income, smoking, drinking, and physical exercise), males were 1.89 times more likely than females to develop arteriosclerosis (p < 0.05). A stratified analysis of males and females showed that the risk of arteriosclerosis was higher in females than in males in the 45–60 and over 60 age groups compared with those in the under 44 age group (p < 0.01). Diabetes, LDL-C, and hs-CRP were more likely to be correlated with arteriosclerosis in females than in males (odds ratio (OR): 2.32, 1.26, 1.08 vs. 1.83, 1.17, 1.02, respectively, p < 0.05). Higher education levels reduced the risk of arteriosclerosis in males and females, with OR values of 0.64 and 0.84, respectively (p < 0.05).
The arteriosclerosis detection rate in males was higher than in females. Conversely, the increase in baPWV in females older than 45 years was higher than in males. Meanwhile, WHR, diabetes, LDL-C, and hs-CRP were more likely to be correlated with arteriosclerosis in females.
Chinese Clinical Trail Registry, URL: https://www.chictr.org.cn/showproj.html?proj=8050. Unique identifier: ChiCTR-TNRC-11001489 .
Arteriosclerosis is a process whereby the arterial wall thickens, resulting in decreased wall compliance, loss of elasticity, and narrowing of the lumen [1]. Increased arterial stiffness increases the risk of hypertension [2], coronary heart disease [3], stroke [4], renal dysfunction [5], cognitive dysfunction [6], and peripheral vascular disease [7]. Therefore, early prevention of increased arterial stiffness significantly reduces the risk of cardiovascular and cerebrovascular diseases. However, the compliance of the aorta decreases, and the stiffness increases with age [8]. The Framingham study found that females, hypertension, increased body mass index, and diabetes were also risk factors for arteriosclerosis [9]. Other studies found that uric acid excretion fraction and estimated glomerular filtration rate were inversely proportional to the brachial–ankle pulse wave velocity (baPWV) [10, 11]. However, regular aerobic exercise can reduce the degree of arteriosclerosis [12].
Carotid–femoral pulse wave velocity (cfPWV) is the gold standard for predicting
arteriosclerosis [7, 13], yet measuring the cfPWV is relatively complex. Notably,
the baPWV and cfPWV exhibit a good correlation. The baPWV method is simple and
reproducible and is a sensitive index to evaluate arteriosclerosis [14]. The
guidelines and consensus of China on the Prevention and Treatment of
Cardiovascular Disease published in 2008 consider a baPWV
The baPWV differs among different sex groups. Wang X et al. [16] found
that in patients under 50 years, the baPWV was higher in males than in females,
and when the age was over 50, there was no statistical difference between baPWV
in males vs. females. Benetos et al. [17] found the same
phenomenon in patients
Data were derived from the Kailuan study. Briefly, the Kailuan study is a prospective, community-based cohort study that aimed to investigate the epidemiology of cardiovascular diseases in Chinese adults. All participants underwent assessments via questionnaires, clinical examinations, and laboratory tests upon enrollment and were followed up every two years. Details regarding data collection were described previously [22, 23, 24, 25, 26].
The study enrolled participants older than 18 years who underwent baPWV measurements from 2010 to 2017. Exclusion criteria included a history of myocardial infarction, stroke, cancer, or peripheral vascular disease before the baPWV measurement. The ethics committees at Kailuan General Hospital approved the study, following the guidelines outlined by the Helsinki Declaration. Written informed consent was obtained from all participants. Baseline data on demographics and cardiovascular risk factors were collected when the baPWV measurements were performed.
We collected baPWV values using a BP-203 RPE III networked arteriosclerosis detection device produced by Omron Health Medical (Liaoning, China) Co., Ltd. Participants underwent baPWV measurements after at least 5 minutes of rest in the supine position. Cuffs were wrapped on both arms and ankles. The lower edge of the arm cuff was positioned 2–3 cm above the cubital fossa transverse striation, while the lower edge of the ankle cuff was positioned 1–2 cm above the medial malleolus. The heartbeat monitor was placed on the left edge of the sternum, and electrocardiogram electrodes were placed on both wrists. The resulting baPWV value could be directly read using the network connection. The methodology for the baPWV measurement remained constant for all participants.
The definition for arteriosclerosis was derived from the American Heart
Association Medical/Scientific Report (1993) criteria: baPWV
We presented continuous variables as the mean
We examined cross-sectional correlates of arteriosclerosis (baPWV
Two-sided p-values
From January 1, 2010, to December 31, 2017, 30,148 participants in the Kailuan study underwent baPWV measurements. After excluding participants with a history of myocardial infarction, stroke, cancer, or peripheral vascular disease, 28,291 participants were included in the current analysis (Fig. 1).
 Fig. 1.
Fig. 1.
Flow diagram of the patient selection in the current analysis. baPWV, brachial–ankle pulse wave velocity.
The average age among the 28,291 participants was 48.12
| Variable | Males (n = 19,783) | Females (n = 8508) | t/ |
p |
| Age (y) | 48.49 |
47.25 |
7.94 | |
| baPWV (cm/s) | 1550.37 |
1387.85 |
37.90 | |
| BMI (kg/m2) | 25.18 |
24.26 |
19.61 | |
| WHR | 0.92 |
0.87 |
35.16 | |
| SBP (mmHg) | 133.06 |
122.25 |
43.91 | |
| DBP (mmHg) | 83.84 |
77.90 |
43.44 | |
| Uric acid (mmol/L) | 330.42 |
262.43 |
64.32 | |
| Heart rate (bpm) | 74.55 |
72.99 |
10.46 | 0.01 |
| FBG (mmol/L) | 5.85 |
5.46 |
16.05 | |
| Triglyceride (mmol/L) | 1.86 |
1.42 |
14.29 | |
| LDL-C (mmol/L) | 2.77 |
2.56 |
16.45 | |
| HDL-C (mmol/L) | 1.43 |
1.53 |
–11.44 | |
| hs-CRP (mg/L) | 2.01 |
1.88 |
2.61 | |
| eGFR (mL/min/1.73 m2) | 103.13 |
98.43 |
15.22 | |
| Hypertension | 8451 (42.66) | 2140 (25.23) | 770.58 | |
| Diabetes | 2834 (14.31) | 795 (9.37) | 129.29 | |
| Dyslipidemia | 12,363 (62.41) | 4281 (50.47) | 349.55 | |
| Antihypertensive | 2765 (15.20) | 1034 (12.56) | 32.00 | |
| Antidiabetic | 906 (4.97) | 386 (4.69) | 1.00 | 0.32 |
| Lipid-lowering | 174 (1.31) | 85 (1.61) | 2.38 | 0.12 |
| High school or above | 4330 (32.73) | 3297 (50.46) | 579.84 | |
| Monthly income USD |
1012 (7.90) | 421 (6.91) | 5.78 | 0.02 |
| Current smoker | 9542 (50.72) | 146 (1.85) | 5730.73 | |
| Current alcohol drinker | 1664 (9.17) | 16 (0.19) | 767.38 | |
| Physical exercise | 2164 (11.86) | 1010 (12.23) | 0.75 | 0.39 |
Continuous variables are presented as the mean
baPWV, brachial–ankle pulse wave velocity; BMI, body mass index; WHR, waist-to-hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
The detection rate of arteriosclerosis in males (62.13%) was higher than in
females (37.41%) (p
| Age (y) | Males | Ratio (%) | Females | Ratio (%) | p |
| baPWV |
12,292 (62.13) | 69.93 | 3183 (37.41) | 30.07 | |
| 1310.24 |
8.71 | 1120.81 |
4.68 | ||
| 30–35 | 1361.09 |
7.58 | 1168.13 |
10.41 | |
| 35–40 | 1408.89 |
9.14 | 1202.75 |
10.28 | |
| 40–45 | 1454.68 |
13.78 | 1254.82 |
17.06 | |
| 45–50 | 1518.98 |
17.40 | 1335.88 |
22.54 | |
| 50–55 | 1570.81 |
17.20 | 1430.11 |
9.83 | |
| 55–60 | 1667.35 |
7.07 | 1563.88 |
8.49 | |
| 60–65 | 1738.61 |
6.88 | 1684.94 |
7.65 | |
| 65–70 | 1839.59 |
4.96 | 1818.30 |
4.23 | 0.50 |
| 70–75 | 1918.36 |
3.42 | 1997.92 |
2.57 | 0.05 |
| 75–80 | 2077.59 |
1.95 | 2108.83 |
1.532 | 0.66 |
| 2120.16 |
1.91 | 1992.39 |
0.72 | 0.62 | |
| p-trend | p |
– | p |
– | – |
baPWV, brachial–ankle pulse wave velocity.
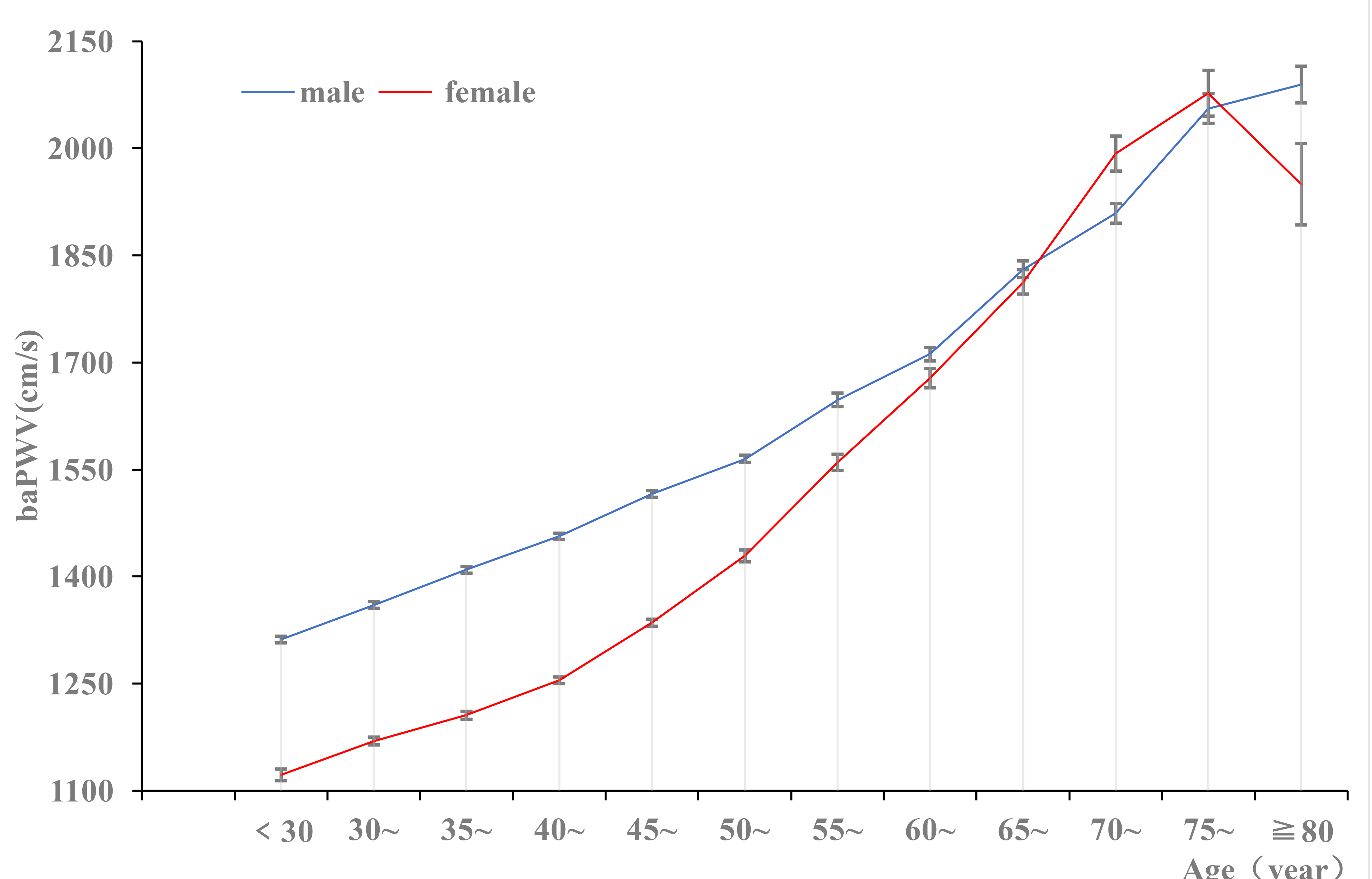 Fig. 2.
Fig. 2.
The prevalence of baPWV in different sex groups. baPWV, brachial–ankle pulse wave velocity.
Using baPWV as the dependent variable, the statistically significant correlated factors (Table 3) were entered into the multiple linear regression model; eight statistically significant factors were identified in the male and female populations. For every increase of 5 years in age, the baPWV in males increased by 62.55 cm/s and 71.86 cm/s in females. For every 10 mmHg increase in the SBP, the baPWV increased in males and females by 61.01 cm/s and 51.86 cm/s. Physical exercise significantly reduced the baPWV in males but was not statistically significant in females. Females with a higher WHR demonstrated a greater, although not significantly different, baPWV than males (Table 4).
| Variable | Total | Males | Females | |||
| r | p | r | p | r | p | |
| Sex | –0.223 | - | - | - | - | |
| Age (y) | 0.575 | 0.520 | 0.686 | |||
| WHR | 0.142 | 0.049 | 0.142 | |||
| SBP (+10 mmHg) | 0.546 | 0.476 | 0.599 | |||
| Heart rate (bpm) | 0.151 | 0.144 | 0.119 | |||
| Triglyceride (mmol/L) | 0.088 | 0.049 | 0.122 | |||
| LDL-C (mmol/L) | 0.084 | 0.015 | 0.15 | 0.165 | ||
| HDL-C (mmol/L) | 0.001 | 0.94 | 0.042 | –0.029 | 0.047 | |
| hs-CRP (mg/L) | 0.08 | 0.067 | 0.123 | |||
| Uric acid (mmol/L) | 0.122 | 0.002 | 0.86 | 0.190 | ||
| eGFR (mL/min/1.73 m2) | –0.160 | –0.186 | –0.21 | |||
| Diabetes | 0.257 | 0.227 | 0.305 | |||
| High school or above | –0.208 | –0.137 | –0.258 | |||
| Monthly income USD |
–0.018 | 0.03 | –0.029 | 0.004 | –0.003 | 0.86 |
| Salt bias | 0.017 | 0.04 | –0.012 | 0.25 | 0.025 | 0.08 |
| Current smoker | 0.065 | –0.066 | 0.018 | 0.21 | ||
| Current alcohol drinker | 0.057 | 0.02 | 0.047 | 0.01 | 0.51 | |
| Physical exercise | 0.105 | 0.096 | 0.143 | |||
baPWV, brachial–ankle pulse wave velocity; WHR, waist-to-hip ratio; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
| Sex | Variable | SE | SD | t | p | |
| Males | Age (+5 y) | 62.55 | 1.58 | 0.41 | 39.54 | |
| SBP (+10 mmHg) | 61.01 | 2.08 | 0.31 | 29.36 | ||
| Heart rate (bpm) | 4.81 | 0.32 | 0.15 | 15.11 | ||
| Physical exercise | –25.21 | 9.37 | –0.03 | –2.69 | 0.01 | |
| Dyslipidemia | 15.40 | 7.2 | 0.02 | 2.14 | 0.03 | |
| Diabetes | 71.48 | 9.75 | 0.07 | 7.33 | ||
| Triglyceride (mmol/L) | 3.43 | 1.41 | 0.02 | 2.43 | 0.02 | |
| Females | Age (+5 y) | 71.86 | 2.29 | 0.46 | 31.41 | |
| SBP (+10 mmHg) | 51.86 | 2.85 | 0.29 | 18.22 | ||
| Heart rate (bpm) | 4.75 | 0.49 | 0.12 | 9.75 | ||
| WHR | 179.45 | 68.43 | 0.03 | 2.62 | ||
| Diabetes | 67.63 | 14.87 | 0.06 | 4.55 | ||
| HDL-C (mmol/L) | –27.13 | 10.19 | –0.03 | –2.66 |
Using a multivariable logistic regression analysis, we found that the risk of
arteriosclerosis in males was 1.89 times higher than in females (p
| Variable | Wald |
OR | 95% CI | p | |
| Sex (males) | 0.61 | 146.02 | 1.89 | 1.73–2.06 | |
| Age (45–59 y) | 0.76 | 297.47 | 2.11 | 1.93–2.30 | |
| Age ( |
2.38 | 991.78 | 10.63 | 9.11–12.40 | |
| WHR | 0.01 | 0.10 | 1.01 | 0.93–1.11 | 0.75 |
| SBP (+10 mmHg) | 0.55 | 1353.32 | 1.74 | 1.69–1.79 | |
| Heart rate (bpm) | 0.01 | 73.45 | 1.01 | 1.01–1.02 | |
| Triglyceride (mmol/L) | 0.06 | 23.00 | 1.06 | 1.04–1.09 | |
| LDL-C (mmol/L) | 0.13 | 26.09 | 1.13 | 1.08–1.19 | |
| HDL-C (mmol/L) | –0.04 | 0.66 | 0.97 | 0.89–1.05 | 0.42 |
| hs-CRP (mg/L) | 0.02 | 8.33 | 1.02 | 1.01–1.03 | |
| eGFR (mL/min/1.73 m2) | –0.09 | 0.87 | 0.91 | 0.75–1.11 | 0.35 |
| Diabetes | 0.84 | 116.03 | 2.26 | 1.93–2.65 | |
| High school or above | –0.30 | 49.74 | 0.74 | 0.68–0.80 | |
| Monthly income USD |
–0.01 | 0.02 | 0.99 | 0.86–1.14 | 0.88 |
| Salt bias | 0.03 | 0.23 | 1.03 | 0.91–1.17 | 0.64 |
| Current smoker | 0.07 | 1.78 | 1.07 | 0.97–1.17 | 0.18 |
| Current alcohol drinker | 0.05 | 0.34 | 1.05 | 0.89–1.23 | 0.56 |
| Physical exercise | 0.08 | 1.76 | 1.11 | 0.98–1.26 | 0.09 |
| Variable | Sex | Wald |
OR | 95% CI | p | |
| Males | - | 0.50 | 68.97 | 1.65 | 1.46–1.85 | |
| Age* (y) | ||||||
| 45–59 | Males | 0.59 | 71.8 | 1.80 | 1.57–2.07 | |
| Females | 1.25 | 93.64 | 3.49 | 2.71–4.49 | ||
| Males | 2.38 | 378.42 | 10.81 | 8.50–13.74 | ||
| Females | 3.19 | 293.26 | 24.19 | 16.80–34.83 | ||
| SBP (mmHg) | Males | 0.49 | 442.47 | 1.63 | 1.56–1.71 | |
| Females | 0.51 | 180.21 | 1.66 | 1.54–1.80 | ||
| Heart rate (bpm) | Males | 0.03 | 73.32 | 1.03 | 1.02–1.03 | |
| Females | 0.03 | 21.25 | 1.03 | 1.02–1.04 | ||
| Triglyceride (mmol/L) | Males | 0.04 | 5.19 | 1.04 | 1.01–1.08 | 0.02 |
| Females | 0.00 | 0.07 | 1.01 | 0.97–1.01 | 0.80 | |
| LDL-C (mmol/L) | Males | 0.15 | 13.56 | 1.16 | 1.07–1.26 | |
| Females | 0.24 | 11.03 | 1.27 | 1.10–1.46 | ||
| HDL-C* (mmol/L) | Males | –0.01 | 0.01 | 0.99 | 0.89–1.11 | 0.91 |
| Females | –0.31 | 6.69 | 0.73 | 0.58–0.93 | 0.01 | |
| hs-CRP (mg/L) | Males | 0.02 | 5.47 | 1.02 | 1.01–1.04 | 0.02 |
| Females | 0.08 | 14.59 | 1.08 | 1.04–1.12 | ||
| Diabetes* | Males | 0.60 | 22.76 | 1.82 | 1.43–2.34 | |
| Females | 0.84 | 17.64 | 2.32 | 1.57–3.43 | ||
| Education* | Males | –0.18 | 7.92 | 0.83 | 0.74–0.95 | |
| Females | –0.46 | 17.07 | 0.64 | 0.51–0.79 |
* indicates variable by sex in interaction.
Our study found that the prevalence of arteriosclerosis in males was higher than
in females and that the baPWV in both males and females showed a trend of
increasing with age. In all age groups
In addition to age factors, this study found that SBP, heart rate, uric acid, diabetes mellitus, triglyceride, and LDL-C levels were positively correlated with the baPWV in males and females, and for every 10 mmHg increase in SBP, the baPWV in males and females increased by 61.01 cm/s and 51.86 cm/s, respectively. The BLSA study found that elevated SBP was significantly associated with elevated baPWV and exhibited a stronger correlation in the male population [20]. Physical exercise reduced baPWV values in males, which was not statistically different from females, while high-density lipoprotein cholesterol (HDL-C) was the opposite. Heart rate and diabetes promoted similar changes in male and female baPWV values.
After adjusting for confounding factors, the risk of arteriosclerosis in males was 1.89 times higher than in females (95% CI: 1.73–2.06). Moreover, the risk of arteriosclerosis increased by 9.63 times in the group aged over 60 years. The risk of arteriosclerosis increases by 0.74 times for every 10 mmHg increase in SBP. The risk of arteriosclerosis in the diabetic population is 2.26 times greater than in the non-diabetic population. In addition, heart rate, triglyceride, and LDL-C are also risk factors for arteriosclerosis. This shows that being male is an independent risk factor for arteriosclerosis, and age is the most significant factor in increased arterial stiffness. Blood pressure, diabetes, heart rate, and blood lipid levels also increase arterial stiffness to varying degrees. Conversely, having a higher education was a protective factor against arteriosclerosis (OR: 0.74, 95% CI: 0.68–0.80).
By analyzing the influencing factors of arterial stiffness by sex, it was found
that age, SBP, heart rate, LDL-C, hs-CRP, and diabetes are the risk factors for
increased baPWV values in males and females.Compared with people aged
Our study found significant differences in arterial stiffness between different sexes. Age, blood pressure, diabetes, LDL-C, heart rate, and hs-CRP are arteriosclerosis risk factors. Age and diabetes are more related to arterial stiffness in females than males, and blood pressure pertains more to arterial stiffness in males than females. Similarly, aging and blood pressure also increase the risk of arteriosclerosis in males and females, respectively. Therefore, actively monitoring and controlling blood pressure, blood sugar, and blood lipids to maintain them at relatively normal and stable levels can reduce the occurrence and development of subclinical arteriosclerosis.
Although our study found that the influencing factors of arterial stiffness are different between sexes, this study has some limitations: (1) cfPWV, the gold standard of predicting arteriosclerosis, was not used as the detection method. However, baPWV not only presents a good correlation with cfPWV but was also included in the recommended standard of arteriosclerosis evaluation by the American Heart Association [7]. (2) There is a lack of data on whether the female subjects were or were not menopausal, and the reasons for the accelerated growth of baPWV after age 45 are uncertain. (3) Furthermore, this study did not exclude the influence of patients taking antihypertensive, hypoglycemic, and lipid-lowering drugs from the results, meaning that it cannot be ruled out that such people were not included in the sensitivity analysis. (4) When analyzing the differences in influencing factors of arterial stiffness between different sexes, although possible confounding factors were corrected for as much as possible, other confounding factors exist, such as environmental changes and heritage factors, which were not corrected.
The detection rate of arteriosclerosis in males was higher than in females. The baPWV value in males under 70 years was higher than in females. However, after 45 years, the increase in the baPWV rate in females according to age was higher than in males. The WHR, SBP, diabetes, LDL-C, and hs-CRP measurements correlated more with female arteriosclerosis. Higher education and physical exercise levels reduced the risk of arteriosclerosis in males and females.
The data are obtainable on request from the corresponding author in this study. They are not publicly available due to privacy issues.
JY, TF and SW designed the research study. CW, CZ, YZ and ML performed the research and collected the data. JY and HL analyzed the data. JY and CW drafted the manuscript. JY, ML, CW, SW and TF reviewed and modified the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was carried out in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Kailuan General Hospital (Protocol No. 2006-05). Written informed consent was obtained from all participants.
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group; and all those who helped us during the writing of this manuscript. We would like to thank all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
