- Academic Editor
-
-
-
†These authors contributed equally.
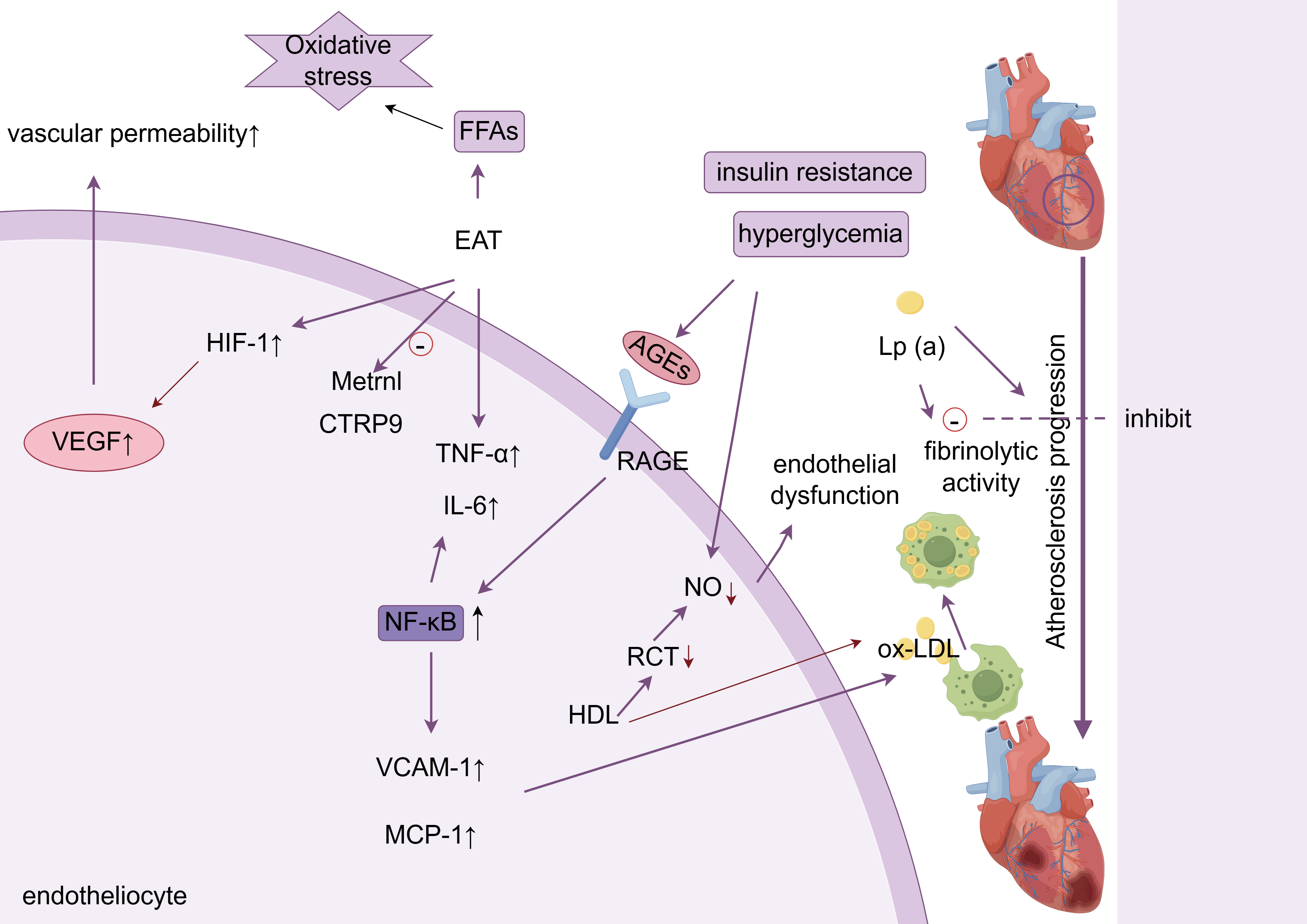
This article reviews the latest research progress (2018–2025) on the molecular mechanisms linking glucose and lipid metabolism disorders (GLMDs) to cardiovascular injury, specifically atherosclerotic cardiovascular disease (ASCVD), diabetic cardiomyopathy (DbCM), heart failure (HF), and cardiac autonomic neuropathy (CAN). This review employed a targeted analysis of key publications from the PubMed, Web of Science, and EMBASE databases, as well as citation tracking, prioritizing molecular pathways and interventions for these four complications. The key mechanisms include: metabolic inflammation: the advanced glycation end products (AGEs)–receptor of AGE (RAGE) axis activates NF-κB, promotes vascular cell adhesion molecule-1 (VCAM-1)/monocyte chemoattractant protein-1 (MCP-1) overexpression, and accelerates monocyte infiltration; myocardial lipotoxicity: CD36 mediates fatty acid overload → mitochondrial damage → cyclic guanosine monophosphate-adenylate synthetase (cGAS)-STING pathway activation → myocardial apoptosis; metabolic memory: hyperglycemia continuously releases small extracellular vesicle (sEV) miR-15-16 clusters through the O-GlcNAc–CaMKIIδ–STAT1 loop, mediating remote myocardial injury; gut–heart axis disorder: Trimethylamine N-Oxide (TMAO) promotes thrombosis and endothelial injury. Precision strategies based on the above mechanisms, such as SGLT2 inhibitors to improve myocardial energy metabolism, targeting acyl-coenzyme A binding protein (ACBP)/TGR5 to alleviate lipotoxicity, and microbiota regulation, have demonstrated potential in clinical research. Future focus should include (1) GLMD heterogeneity typing; (2) tissue-targeted delivery system; (3) multi-omics–AI dynamic risk modeling.