- Academic Editor
Limited evidence exists for an association between dilated cardiomyopathy (DCM) and the angiotensin-converting enzyme (ACE) gene with an insertion/deletion (I/D) angiotensinogen (AGT) M235T gene polymorphism. A systematic review and meta-analysis were conducted to elucidate the role of ACE I/D and AGT M235T in the morbidity of DCM. This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines for Abstracts.
The PubMed, Embase, and Cochrane Library databases, as well as the Chinese Biomedical Literature Database, were reviewed to identify and collect all relevant studies. The association between ACE I/D, AGT M235T gene polymorphism, and DCM was estimated by pooling the odds ratio (OR) using the RevMan5.4.1 and Stata12.0 software.
A total of 27 eligible studies that explored the ACE I/D gene polymorphism in a healthy control group and the DCM patients were included in the present meta-analysis. A recessive genetic model was presented in the ACE I/D genotype. The pooled OR (DD vs. DI+II) following recessive genetic modelling was 1.37 (95% confidence interval (CI): 1.13, 1.66; p < 0.01). DCM patients tend to carry the DD genotype, indicating that the ACE I/D gene polymorphism might be associated with DCM. Similarly, seven studies were analyzed that presented a correlation between AGT M235T polymorphism and DCM morbidity. The OR (MT + TT vs. MM) value, according to a dominant genetic model, was 1.83 (95% CI: 0.90, 3.73; p > 0.05).
The AGT M235T polymorphism was not significantly associated with DCM; however, the ACE I/D polymorphism was related to a risk of DCM.
Dilated cardiomyopathy (DCM) is a myocardial disease characterized by the dilatation of either the left or both ventricles with impaired systolic function [1]. Despite recent advances in medical and surgical therapies, it remains an important cause of mortality and is a leading indication for heart transplantation. The prevalence of idiopathic DCM is approximately 1 in 250 individuals [2]. However, the causes of dilated cardiomyopathy are heterogeneous and unclear [3]. Nonfamilial DCM may have multifactorial etiologies resulting from an interaction between genetic and environmental factors. Several modifier genes also influence the DCM phenotype. Polymorphisms in the genes involved in the renin-angiotensin system (RAS) are associated with a higher risk of DCM [4, 5, 6].
In the RAS, angiotensinogen (AGT) is synthesized primarily by the liver and released into the blood, where it is cleaved by renin to generate angiotensin I. The latter is subsequently converted to angiotensin II by the angiotensin-converting enzyme (ACE). Angiotensin II is involved in cellular hypertrophy and proliferation [7], and thus regulates cardiac function, blood pressure, and electrolyte homeostasis [8]. The ACE gene is located on chromosome 17q23. An insertion/deletion (I/D) polymorphism (a 287-base-pair Alu repeat sequence) is usually present within intron 16 [9]. The AGT gene, located on chromosome 1q4, has an M235T polymorphism [10]. The ACE I/D gene and/or AGT M235T polymorphism are involved in cardiomyopathies [4, 5, 6, 11, 12, 13, 14, 15, 16, 17]. However, some studies could not establish any correlation between ACE I/D or the AGT M235T genotype and DCM [18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]. Thus, the role of ACE I/D and AGT M235T genotype in the pathogenesis of nonfamilial DCM remains controversial. We decided to perform a meta-analysis to evaluate the effects of ACE I/D and AGT M235T gene polymorphism on the DCM phenotype. We reviewed case-control studies which explored the ACE I/D gene (OMIM number: 106180) and AGT M235T (OMIM number: *106150) gene polymorphism in healthy control and DCM patients, to determine the role of these two gene polymorphisms.
A comprehensive search for relevant studies was conducted in PubMed, Embase, Cochrane Library, and the China Biology Medicine disc till February 2025. The review was prepared on the basis of published protocols [37, 38]. To find studies exploring the relationship between ACE I/D with DCM, the search words used in the PubMed database were: ACE or “angiotensin converting enzyme”, polymorphism or mutation, and “dilated cardiomyopathy or dilated cardiomyopathies”. Alternatively, ACE was replaced with “angiotensinogen” or “AGT” for studies related to angiotensinogen M235T genotype and DCM. Details of the Embase search strategy are described in Supplementary Material I. Language was not a limiting factor in our search.
Two authors independently reviewed all studies and collected the data using a standard information extraction approach following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [39, 40]. The studies included met with the following criteria: (1) a cohort study highlighting the ACE I/D allele polymorphism or AGT M235T gene polymorphism; (2) the case group involved DCM patients. (3) A healthy control group was included in all studies. The exclusion criteria were as follows: (1) reviews, comments, case reports, meta-analysis and animal experiments; (2) other studies in which neither ACE nor AGT gene polymorphism was explored in DCM or a control group in a DCM or control group.
The following data were collected: the first author of the studies, year of publication, genotypes of patients and controls, p-values to calculate Hardy-Weinberg equilibrium (HWE) in the control group, the source of control subjects, and diagnosis methods or criteria in DCM patients.
The quality of studies was independently assessed by two authors using a revised bias assessment score (Supplementary Material II) [41]. Total scores ranged from 0 (worst) to 13 (best). Any dissension was resolved by discussion.
All data for statistical analysis were obtained from the published paper or meeting abstracts. RevMan Software 5.4.1 (Cochrane Collaboration, https://www.cochrane.org/products-and-services/review-writing-software) was used for pooling the odds ratio (OR) in the meta-analysis. Meta-regression and calculation of genetic models were performed with Stata 12.0 software (StataCorp LP, College Station, TX, USA). The most appropriate genetic models were calculated following protocols described previously [41, 42]. Continuity correction by adding 1 into the 0 genotype was applied. For ACE I/D gene polymorphism, a recessive genetic model was used. For the role of AGT M235T genotype in DCM, a dominant model was used. Meta-regression was used to explore potential sources of heterogeneity. A p-value less than 0.10 and I2 greater than 50% were considered to be significant for statistical heterogeneity. The random-effect model was used in the analysis [43, 44]. Sensitivity analysis was also performed to test the robustness of the results by excluding studies that deviated from HWE. In addition, a subgroup analysis to determine the origin of the patients was also performed. Begg’s test, Egger’s test and funnel plots were used to assess and avoid any publication bias.
A total of 242 studies were retrieved for ACE gene polymorphism and 26 studies for AGT gene polymorphism from the databases. Among them, 29 studies were included in the analysis. Of these, 27 studies [4, 5, 6, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36] were included in the meta-analysis to show an association between ACE I/D gene polymorphism and DCM (Fig. 1a); 7 studies [11, 15, 19, 20, 26, 28, 30] correlated AGT M235T gene polymorphism and DCM (Fig. 1b). Among these, five studies [11, 19, 20, 28, 30] were included in both the ACE I/D and AGT M235T gene polymorphism analysis. Fig. 1 shows the flow diagram of the criteria used in the study selection. Table 1a (Ref. [4, 5, 6, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]) and Table 1b (Ref. [11, 15, 19, 20, 26, 28, 30]) list the selected studies and the main characteristics in the control and DCM Group. The quality of the selected studies is shown in Supplementary Material III.
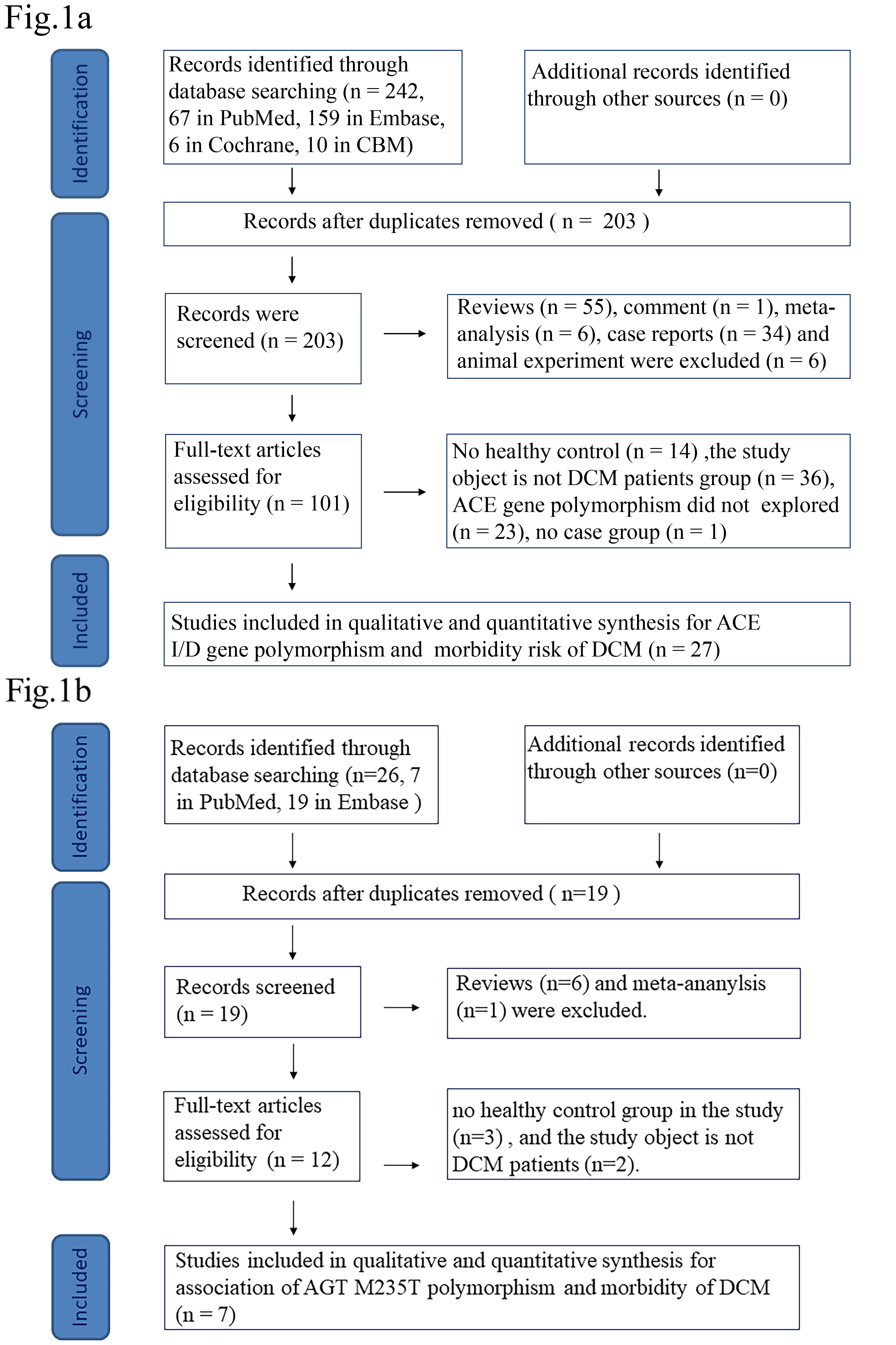 Fig. 1.
Fig. 1.
Flow diagram highlighting the criteria for selection of the studies. (a) ACE I/D flow diagram. (b) AGT flow diagram. DCM, dilated cardiomyopathy; ACE, angiotensin converting enzyme; AGT, angiotensinogen; I/D, insertion/deletion; CBM, the China Biology Medicine disc.
| First author, Year | Ethnic | DCM | Control | HWE in control p value | Control subjects | Diagnosis methods/criteria in DCM patients | ||||||
| Genotypes | Genotypes | |||||||||||
| N | II | ID | DD | N | II | ID | DD | |||||
| Kose et al. 2014 [22] | Turkey | 36 | 8 | 17 | 11 | 104 | 16 | 47 | 41 | 0.6780 | Healthy subjects | Echocardiography |
| Kong et al. 2012 [34] | Han Chinese | 101 | 21 | 48 | 32 | 105 | 30 | 53 | 22 | 0.8744 | Healthy individuals | WHO/ISFC diagnostic criteria of DCM in 1995 |
| Mahjoub et al. 2010 [5] | Tunisia | 76 | 12 | 38 | 26 | 151 | 46 | 83 | 22 | 0.1162 | Age, sex and ethnicity matched controls without any previous history of cardiovascular disorders | Criteria provided by the World Health Organization. |
| Shan et al. 2001 [18] | Chinese | 83 | 27 | 25 | 31 | 155 | 50 | 80 | 25 | 0.4564 | Healthy individuals | WHO/ISFC diagnostic criteria of DCM in 1995, echocardiography |
| Zou et al. 2003 [17] | Chinese | 43 | 12 | 18 | 13 | 53 | 28 | 20 | 5 | 0.6095 | Age and gender matched healthy individual and blood donor | WHO/ISFC diagnostic criteria of DCM in 1995, echocardiography |
| Wu et al. 2002 [35] | Chinese | 43 | 14 | 22 | 7 | 63 | 23 | 28 | 12 | 0.5092 | Healthy individuals | WHO/ISFC diagnostic criteria of DCM in 1995, echocardiography |
| Küçükarabaci et al. 2008 [23] | Turkey | 29 | 5 | 18 | 6 | 20 | 7 | 9 | 4 | 0.7229 | Healthy subjects | Echocardiography |
| Rai et al. 2008 [6] | India | 51 | 8 | 33 | 10 | 164 | 47 | 87 | 30 | 0.3532 | Healthy, age, sex, and ethnicity matched controls without any previous history of cardiovascular disorders | Echocardiography |
| Jurkovicova et al. 2007 [19] | Caucasian population of Slovakia | 110 | 21 | 50 | 39 | 156 | 38 | 78 | 40 | 0.9984 | Healthy control subjects matched to patients by gender and age | Not defined |
| Covolo et al. 2003 [36] | Italy | 122 | 17 | 62 | 43 | 230 | 39 | 105 | 86 | 0.4744 | Born in Italy, had no clinical symptoms or signs suggesting the presence of HF, and no history of CHD or IDC | Echocardiography |
| Tiago et al. 2002 [20] | South Africa | 157 | 26 | 60 | 71 | 225 | 18 | 105 | 102 | 0.2050 | Healthy, unrelated Black South Africans were recruited from the general population of surrounding districts | Echocardiography |
| Tiret et al. 2000 [28] | France | 422 | 94 | 200 | 128 | 387 | 71 | 190 | 126 | 0.9662 | Age matched French population without clinical history of cardiovascular disease or insulindependent diabetes | Radionucleotide angiography or echocardiography |
| Straburzynska-Migaj 2005 [32] | Poland | 52 | 14 | 19 | 19 | 110 | 28 | 48 | 34 | 0.1910 | Healthy pregnant women | Echocardiography |
| Candy et al. 1999 [21] | Black South Africans | 171 | 27 | 72 | 72 | 106 | 13 | 46 | 47 | 0.7376 | Age matched, unrelated black South Africans free of cardiovascular disease | Echocardiography and radionuclide ventriculography |
| Vancura et al. 1999 [29] | Czech | 90 | 27 | 33 | 30 | 287 | 70 | 146 | 71 | 0.7677 | Residents from 1 district in central Bohemia | Not defined |
| Yamada et al. 1997 [30] | Japanese | 88 | 36 | 35 | 17 | 122 | 50 | 55 | 17 | 0.7640 | Healthy individuals | Echocardiography, coronary angiography and left ventriculography |
| Sanderson et al. 1996 [27] | Chinese | 51 | 20 | 25 | 6 | 183 | 71 | 88 | 24 | 0.6882 | Healthy subjects and patients without heart disease | The criteria set by the World Health Organization, Echocardiography, and cardiac catheterization |
| Montgomery et al. 1995 [25] | United Kingdom | 99 | 18 | 50 | 31 | 364 | 84 | 168 | 112 | 0.1729 | Local general practice group | The criteria recommended by the WorldHealth Organization, Echocardiography |
| Raynolds et al. 1993 [14] | USA | 112 | 22 | 50 | 40 | 89 | 20 | 50 | 19 | 0.2431 | Actual or prospective heart donors and healthy volunteers with normal ECG and echocardiographic studies | Echocardiogram |
| Ozhan et al. 2004 [31] | Turkey | 35 | 4 | 17 | 14 | 88 | 11 | 28 | 49 | 0.0411 | Healthy unrelated age-and sex-matched subjects | Transthoracic echocardiogram |
| Kurbanov et al. 2014 [24] | Uzbekistan | 102 | 33 | 45 | 24 | 60 | 34 | 14 | 12 | 0.0004 | Healthy subjects | The diagnostic criteria for DCM (WHO, 1995), echocardiography |
| Harn et al. 1995 [4] | Chinese | 35 | 2 | 13 | 20 | 35 | 2 | 24 | 9 | 0.0112 | Patients with normal donor-screening echocardiograms and normal coronary arteriograms | Echocardiography |
| Rani et al. 2017 [11] | India | 177 | 15 | 120 | 42 | 200 | 72 | 86 | 42 | 0.0891 | Healthy, ethnicity-matched unrelated subjects without any family history of heart disease, hypertension, diabetes or any other chronic ailments | Echocardiography |
| Schmidt et al. 1996 [33] | Austria | 14 | 4 | 7 | 3 | 95 | 21 | 38 | 36 | 0.0801 | Healthy control group | Ultrasonography |
| Chen et al. 2017 [16] | Chinese | 64 | 17 | 29 | 18 | 120 | 51 | 57 | 12 | 0.4957 | Healthy individuals | Diagnostic criteria of Chinese Society of Cardiology |
| Goncalvesova et al. 2005 [12] | Slovak | 70 | 15 | 29 | 26 | 103 | 28 | 51 | 24 | 0.9336 | General Slovak population | Echocardiography |
| Berg et al. 2012 [13] | Bashkortostan | 27 | 10 | 9 | 8 | 82 | 32 | 41 | 9 | 0.4394 | Healthy people, age, gender and ethnicity matched, without chronic diseases as well as without pathology of cardiovascular system in the anamnesis | WHO classification criteria, echocardiogram, and coronarography |
ACE, angiotensin-converting enzyme; I/D, insertion/deletion; DCM, dilated cardiomyopathy; HWE, Hardy-Weinberg equilibrium; WHO/ISFC, World Health Organization/International Society of Forensic Genetics; CHD, coronary Heart Disease; IDC, idiopathic dilated cardiomyopathy; ECG, electrocardiograph.
| First author, Year | Ethnic | DCM | Control | HWE in control p value | Control subjects | Diagnosis methods in DCM patients | ||||||
| Genotypes | Genotypes | |||||||||||
| N | MM | MT | TT | N | MM | MT | TT | |||||
| Jurkovicova et al. 2007 [19] | Caucasian population of Slovakia | 110 | 31 | 51 | 28 | 156 | 62 | 69 | 25 | 0.4339 | Healthy control subjects matched to patients by gender and age | Not defined |
| Tiago et al. 2002 [20] | South Africa | 157 | 0 | 55 | 102 | 225 | 0 | 58 | 167 | 0.0265 | Healthy, unrelated Black South Africans were recruited from the general population of surrounding districts | Echocardiography |
| Tiret et al. 2000 [28] | France | 428 | 157 | 200 | 71 | 398 | 131 | 195 | 72 | 0.9695 | Age matched French population without clinical history of cardiovascular disease or insulin-dependent diabetes | Radionucleotide angiography or echocardiography |
| Pávková Goldbergová et al. 2011 [26] | Czech Republic | 91 | 23 | 55 | 13 | 203 | 65 | 101 | 37 | 0.8377 | Not define | Not defined |
| Yamada et al. 1997 [30] | Japan | 88 | 3 | 29 | 56 | 122 | 2 | 44 | 76 | 0.1190 | Healthy individuals | Echocardiography, left ventriculography, and coronary angiography |
| Rani et al. 2017 [11] | India | 177 | 15 | 120 | 42 | 200 | 72 | 86 | 42 | 0.0891 | Healthy, ethnicity-matched unrelated subjects without any family history of heart disease, hypertension, diabetes or any other chronic ailments | Echocardiography |
| Ullah et al. 2019 [15] | Pakistan | 35 | 20 | 0 | 15 | 42 | 38 | 0 | 4 | 0.0000 | Ethnically matched healthy controls without any history for heart abnormality, hypertension and diabetes | Not defined |
DCM, dilated cardiomyopathy; HWE, Hardy-Weinberg equilibrium; AGT, angiotensinogen.
Calculated HWE values in the control group are shown in Table 1a and Table 1b. Table 2a (Ref. [4, 5, 6, 11, 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]) and Table 2b (Ref. [11, 15, 19, 20, 26, 28, 30]) represent the ACE I/D and AGT M236T gene polymorphism (minor allele). In the studies associating ACE I/D gene polymorphism with DCM, the minor allele in the control group (D allele) had an allele frequency of 48.86% (95% CI: 44.25%, 53.47%). The minor allele in the control group for the AGT genotype was the T allele with a frequency of 49.04% (95% CI: 24.49%, 73.59%).
| First Author, Year | D allele frequency | Total frequency | Allele D allele percent (%) |
| Kong et al. 2012 [34] | 97 | 210 | 46.1905 |
| Shan et al. 2001 [18] | 130 | 310 | 41.9355 |
| Zou et al. 2003 [17] | 30 | 106 | 28.3019 |
| Wu et al. 2002 [35] | 52 | 126 | 41.2698 |
| Kose et al. 2014 [22] | 129 | 208 | 62.0192 |
| Mahjoub et al. 2010 [5] | 127 | 302 | 42.0530 |
| Küçükarabaci et al. 2008 [23] | 17 | 40 | 42.5000 |
| Rai et al. 2008 [6] | 147 | 328 | 44.8171 |
| Jurkovicova et al. 2007 [19] | 158 | 312 | 50.6410 |
| Covolo et al. 2003 [36] | 277 | 460 | 60.2174 |
| Tiago et al. 2002 [20] | 309 | 450 | 68.6667 |
| Tiret et al. 2000 [28] | 442 | 774 | 57.1059 |
| Straburzynska-Migaj et al. 2005 [32] | 116 | 220 | 52.7273 |
| Candy et al. 1999 [21] | 140 | 212 | 66.0377 |
| Vancura et al. 1999 [29] | 288 | 574 | 50.1742 |
| Yamada et al. 1997 [30] | 89 | 244 | 36.4754 |
| Sanderson et al. 1996 [27] | 136 | 366 | 37.1585 |
| Montgomery et al. 1995 [25] | 392 | 728 | 53.8462 |
| Raynolds et al. 1993 [14] | 88 | 178 | 49.4382 |
| Ozhan et al. 2004 [31] | 126 | 176 | 71.5909 |
| Kurbanov et al. 2014 [24] | 38 | 120 | 31.6667 |
| Harn et al. 1995 [4] | 42 | 70 | 60.0000 |
| Rani et al. 2017 [11] | 170 | 400 | 42.5000 |
| Schmidt et al. 1996 [33] | 110 | 190 | 57.8947 |
| Chen et al. 2017 [16] | 81 | 240 | 33.7500 |
| Goncalvesova et al. 2005 [12] | 99 | 206 | 45.0583 |
| Berg et al. 2012 [13] | 59 | 164 | 35.9756 |
Note, Pooled D allele prevalence (%): 48.63 (95% CI: 44.05, 53.20). ACE, angiotensin converting enzyme; I/D, insertion/deletion.
| First Author, Year | T allele frequency | Total frequency | Allele T allele percent (%) |
| Jurkovicova et al. 2007 [19] | 119 | 312 | 38.1410 |
| Tiago et al. 2002 [20] | 392 | 450 | 87.1111 |
| Tiret et al. 2000 [28] | 339 | 796 | 42.5879 |
| Pávková Goldbergová et al. 2011 [26] | 175 | 406 | 43.1034 |
| Yamada et al. 1997 [30] | 196 | 244 | 80.3279 |
| Rani et al. 2017 [11] | 170 | 400 | 42.5000 |
| Ullah et al. 2019 [15] | 8 | 84 | 9.5238 |
Note, Pooled T allele prevalence (%): 49.044 (95% CI: 24.49, 73.59). AGT, angiotensinogen.
The 27 eligible studies, connecting ACE I/D allele polymorphism with
the risk of DCM, included 2460 medical cases and 3857 healthy subjects as the
control for the meta-analysis. The recessive genetic model was selected for the
case-control studies, in which the comparison of DD vs. DI+II
was made. The pooled OR as per the regressive genetic model was 1.37 with the
random-effect model (95% CI: 1.13, 1.66; p
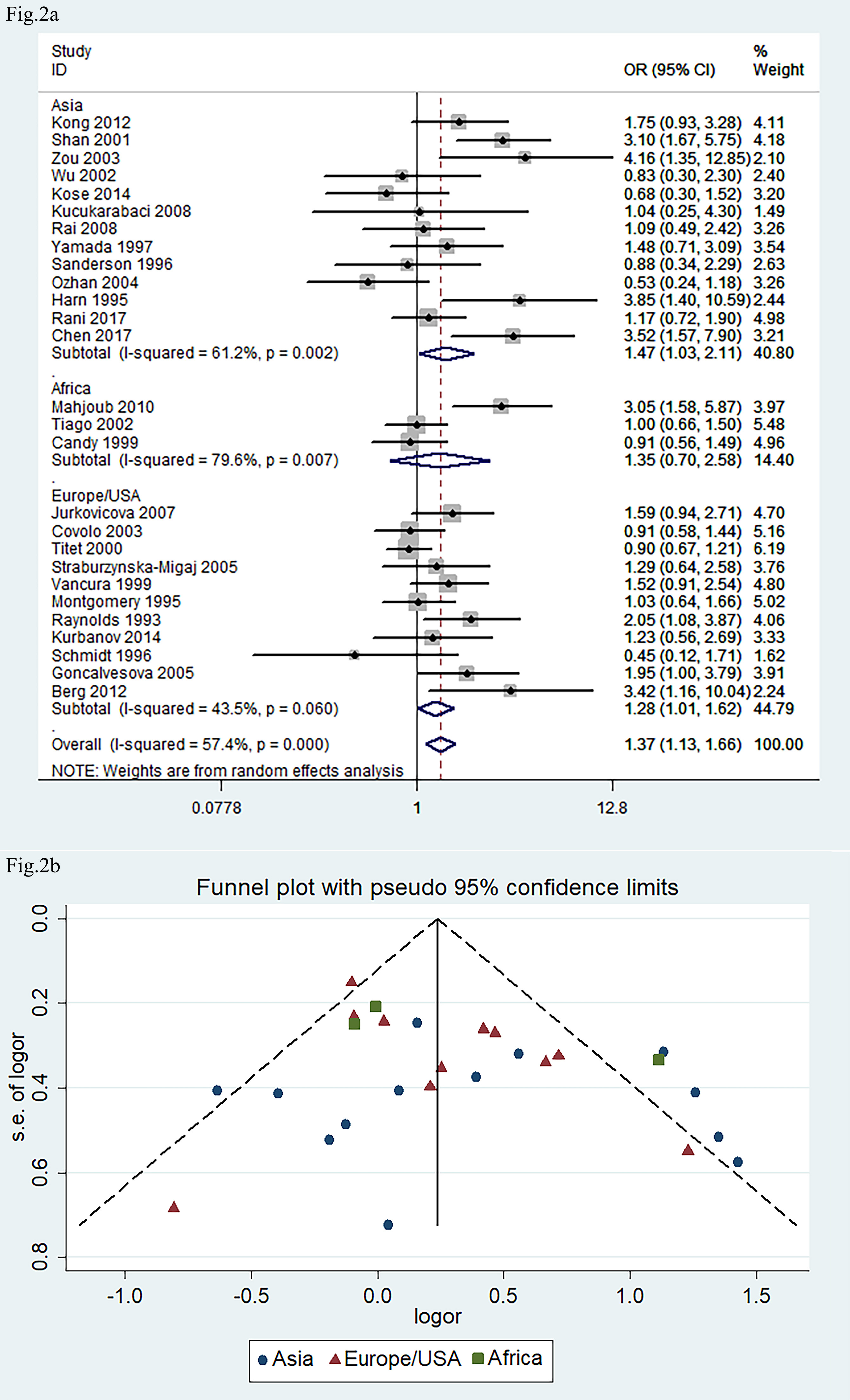 Fig. 2.
Fig. 2.
Forest plot and funnel plot of meta-analysis about ACE
I/D gene polymorphism in association with risk of DCM phenotype. (a) Forest
plot. (b) Funnel plot. The pooled OR in (a) indicated the OR of DD vs.
DI+II genotypes. The pooled OR was 1.37 (95% CI: 1.13, 1.66; p
The seven eligible studies, associating AGT M235T gene polymorphism with DCM, included 1086 DCM patients and 1346 healthy controls. A dominant model (genetic model) was selected, and the comparison of MT+TT vs. MM was made for the meta-analysis.
The pooled OR was 1.83 (Fig. 3a: 95% CI: 0.90, 3.73; p
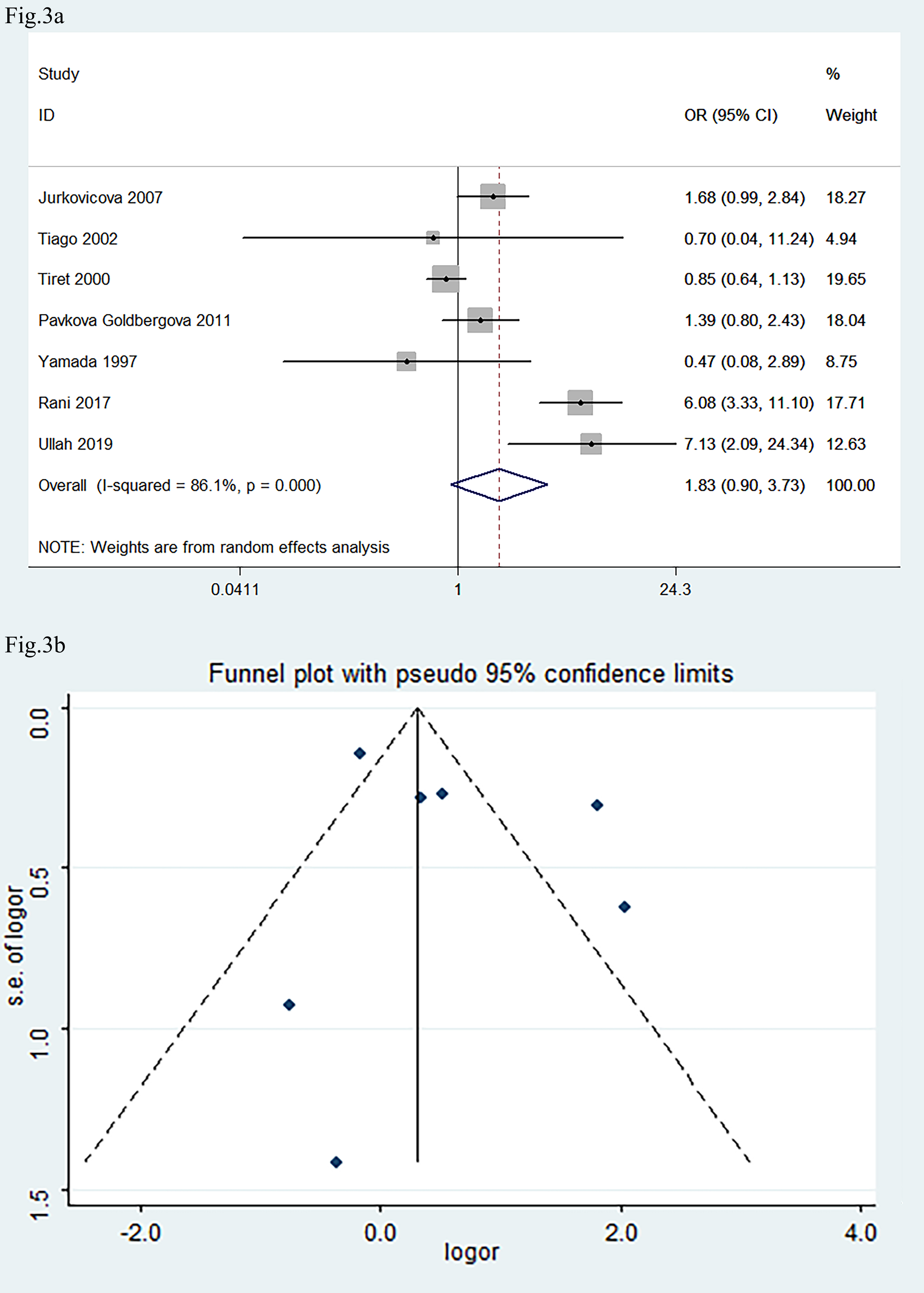 Fig. 3.
Fig. 3.
Forest plot and funnel plot of the meta-analysis correlating
AGT M235T gene polymorphism with the development of DCM. (a) Forest
plot. (b) Funnel plot. OR in (a) indicated the OR of MT+TT vs.
MM genotypes. The pooled OR was 1.83 (95% CI: 0.90, 3.73; p
Our meta-analysis revealed that ACE DD genotype frequency was higher in DCM patients, indicating that ACE I/D gene polymorphism might be associated with the risk of DCM. The subgroup analysis indicated that DD genotype frequency is higher in the Asian and European/USA population. However, it is not significant in Africans. There are just three studies from the African population. Therefore, this lack of association may be due to the small number of studies involving African populations, which limits the statistical power.
DCM is a disease of unknown etiology characterized by ventricular dilation and impaired systolic function [3]. It clinically manifests in heart pump failure or sudden death [45] and is a major indication for heart transplantation [19].
Mutations in genes encoding sarcomeric structural proteins are known contributors to DCM [46]. However, clinical evaluation of families with DCM often reveals the absence of disease in individuals carrying these mutations [2].
The number of rare variants implicated in DCM in the Exome Variant Server (EVS) database was at least double than reported in genetic studies [2]. In addition, the extent of genetic defects varies even among people with the same mutation within the same family. A fixed predictable genotype-phenotype correlation for a specific mutation has not been reported [3]. It has been proposed that clinical heterogeneity in DCM patients is a result of multiple factors, including age, disease-causing gene mutations, environmental effects, and genetic modifiers [47].
Several genes, including those encoding the components of the RAS, are considered potential modifiers in DCM [5]. RAS is a major regulator of cardiovascular and renal functions, including sodium extraction/reabsorption and water balance [26]. Thus, the systemic or local cardiovascular RAS system contributes to the pathophysiology of various cardiovascular diseases and may play an autocrine or paracrine role in cardiac remodeling and fibrosis [48, 49].
In RAS, renin cleaves a terminal decapeptide from angiotensinogen to form angiotensin I [50], which is further catalyzed (enzymatic removal of a dipeptide) into angiotensin II by ACE. ACE is present on the surface of vascular endothelial cells as a membrane-bound enzyme and circulates in plasma. Cloning of the ACE gene revealed a 287 base pair (bp) Alu repeat sequence with an I/D polymorphism in intron 16 resulting in three genotypes: II, ID, and DD [51, 52]. This polymorphism was strongly associated with increased expression of ACE and high levels of angiotensin II. The mean plasma ACE level in individuals with the DD genotype was almost double that of the II genotype, while subjects with ID genotype had intermediate levels [9]. The modulating effect of the DD genotype on DCM is due to increased ACE activity [6].
Tan and coworkers [53] reported that both endogenous and exogenous angiotensin II lead to myocyte necrosis, abnormal sarcolem permeability, myocytolysis, fibroblast proliferation, and subsequent replacement fibrosis in vivo. In addition, angiotensin II stimulation in cardiac fibroblasts of adult rats in vitro results in a higher synthesis of extracellular matrix proteins [54]. This increased extracellular matrix synthesis is a key feature of cardiac fibrosis, a condition where the heart tissue becomes stiff and less elastic [55]. Subsequent myocardial remodeling and increased arterial stiffness may result due to the reduction in left ventricular ejection fraction [6]. Elevated angiotensin II levels are associated with an increased mortality rate in heart failure patients [56].
Cardiac collagen deposition in rats may be regulated by RAS activity [57], and this accumulation can be prevented by non-hypotensive doses of ACE inhibitors. Candy and coworkers [21] asserted that the D allele is associated with worsening of left ventricular (LV) systolic function as well as an increase in left ventricular cavity size that occurs in idiopathic DCM. DD genotype is an independent predictor of higher mortality, LV systolic performance, as well as cavity size in idiopathic DCM [21]. Clinical trials have underscored the therapeutic importance of ACE inhibitors in heart failure [58]. Experimental data in animals and preliminary studies in humans have demonstrated that early administration of captopril, an ACE inhibitor, attenuated progressive LV dilatation [59].
In the present study, we did not confirm an association between the AGT M235T polymorphism and DCM. It is reported that the AGT haplotype, which carries the A (-6) G variation in the promoter, and M235T polymorphism, is associated with higher plasma AGT levels [26, 60]. Bloem and colleagues [61] also found that the T235 allele frequency was higher in black compared to white children, which correlated with the 19% higher mean angiotensin levels in blacks than in whites. Polymorphism of the AGT gene is thus race-specific. It is reported that there was almost complete linkage disequilibrium of G (-6) A with the M235T of AGT gene [26]. The null finding for AGT M235T may reflect low statistical power (small sample size, n = 7 studies, among them, one study did not take part in the pool OR due to the number of MM genotype was zero in both the control and DCM group) or population-specific linkage disequilibrium (e.g., AGT haplotypes with promoter). Future studies should prioritize haplotype analysis and larger sample sizes for AGT-related endpoints.
Our result is distinct from a previous genome-wide association study (GWAS) on DCM as we have identified an association between ACE I/D single nucleotide polymorphism and DCM [62]. To our knowledge, the criterion of assessing statistical significance in GWAS is stricter than in general comparative studies.
There are some limitations in our meta-analysis. First, the studies included are smaller, especially studies associating AGT M235T with DCM (only 7 studies), and ACE I/D gene polymorphism in Africa, which may lack statistical power to detect true associations. Second, several studies in this meta-analysis reported only a few patient cases. Finally, the qualities of some studies were not satisfactory; for example, three studies deviated from HWE for ACE I/D gene polymorphism. In addition, DCM diagnosis across studies used WHO criteria, echocardiography, or a combination, which may introduce heterogeneity. Standardizing diagnostic thresholds (e.g., left ventricular ejection fraction cutoffs) in future could improve consistency. In the end, the review was not registered. All of these limitations may have affected the results of the present study. Further investigations are required to explain the effect of AGT M235T and ACE I/D polymorphisms in the pathogenesis of DCM.
In conclusion, despite the above limitations, the present study has suggested that ACE I/D, but not AGT M235T gene polymorphism, might be a risk factor for DCM. Additional large-scaled and more rigorous case-control studies are needed to further confirm the role of ACE I/D and AGT M235T polymorphisms in DCM.
DCM, dilated cardiomyopathy; ACE, angiotensin converting enzyme; AGT, angiotensinogen; RAS, renin-angiotensin system; HCM, hypertrophic cardiomyopathy; EVS database, Exome Variant Server database.
Data involved in statistics are presented in Table 1a and Table 1b. For further information, please contact the corresponding author.
SD and NJ, Data collecting and formal analysis; YH and ZL, checking the data and analysis; CL, writing-review & editing the manuscript; RL, Conceptualizing and writing-original draft, funding acquisition. All authors contributed to the conception and editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This study was supported by grant from the National Natural Science Foundation of China (No.32171182); the Sichuan Provincial Natural Science Foundation (No. 2024NSFSC0553); Development and Regeneration Key Laboratory of Sichuan Province (No.23LHNBZZD02).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/RCM39763.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
