- Academic Editor
†These authors contributed equally.
Percutaneous coronary intervention (PCI) has made significant progress as one of the main treatments for coronary artery disease (CAD), but the risk of major adverse cardiovascular events (MACE) after PCI remains high. Therefore, early identification of high-risk CAD patients after PCI and improvement of risk factors are crucial for patient prognosis. Although various prognostic biomarkers related to CAD have been identified, most of them have not been widely applied in clinical practice. Recent studies have found that some simple and easily obtainable metabolic indicators have early predictive value for the prognosis of CAD patients after PCI, mainly including four categories: blood lipids and related metabolites, blood glucose and related metabolites, nutrition-related metabolites, and kidney-related metabolites. This review synthesizes the four aforementioned categories of indicators with the aim of integrating their unique characteristics to enable precise prognostication in patients after PCI, deepen mechanistic insights, and furnish evidence-based guidance for clinical decision-making.
Coronary heart disease (CHD) is one of the leading diseases globally, with a complex pathogenesis involving multiple risk factors. To date, no single biomarker has been identified that can comprehensively assess disease severity and accurately predict prognosis. Dysregulation of metabolic indicators such as blood lipids, blood glucose, serum albumin, and uric acid is closely related to the onset, progression, and long-term prognosis of CHD. Particularly after percutaneous coronary intervention (PCI), the impact of these metabolic indicators on patient prognosis is more pronounced. For instance, elevated levels of low-density lipoprotein cholesterol (LDL-C) promote foam cell formation, increased triglyceride (TG) levels affect lipoprotein metabolism and fuel inflammatory responses, and reduced levels of high-density lipoprotein cholesterol (HDL-C) can further exacerbate atherosclerosis. Additionally, insulin resistance (IR) can accelerate CHD progression by promoting inflammation, oxidative stress, and dyslipidemia. Other metabolic indicators, such as low serum albumin (SA) levels, may reflect chronic inflammation and malnutrition, while elevated serum uric acid (SUA) levels can lead to increased oxidative stress, endothelial dysfunction, and enhanced inflammatory responses, thereby promoting the development of atherosclerosis [1, 2, 3, 4, 5]. Lipid indices are directly linked to long-term plaque stability [6]. Glycemic indices reflect metabolic control [7]. Albumin and uric acid–related indices primarily indicate acute-phase stress and microcirculatory status, conferring greater sensitivity for predicting short-term complications such as length of stay and collateral circulation [8, 9, 10]. Therefore, strict control of blood lipids, blood glucose, and uric acid levels is crucial for improving the prognosis of patients after PCI.
Epidemiological evidence indicates that mortality remains high and prognosis unfavorable despite timely and aggressive PCI [11]. Moreover, unplanned readmissions after PCI impose a substantial burden on healthcare systems and are attracting increasing attention [6]. Consequently, there is a pressing need to develop effective prognostic biomarkers to predict and optimize post-procedural management [12, 13]. This review synthesizes several categories of metabolic indicators that offer early insight into post-PCI outcomes. It aims to integrate their distinctive characteristics and elucidate underlying mechanisms. Additionally, it provides an evidence-based reference for clinical decision-making.
A study has established a causal relationship between LDL-C and coronary artery disease (CAD) [14]. Despite many CAD patients achieving target LDL-C levels with statin therapy, the incidence of major adverse cardiovascular events (MACE) remains high [15]. This indicates that solely reducing LDL-C levels is insufficient to fully prevent MACE, especially after PCI.
Research indicates that the role of LDL-C in cardiovascular disease risk is not
only related to its current level but also to its cumulative exposure over time.
Previous studies, often focusing on single-time-point LDL-C measurements (usually
in middle and old age), underestimate the impact of long-term cumulative effects.
A cohort study found that the cumulative exposure to LDL-C (calculated as age
In addition to the traditional risk factor LDL-C, Lp(a) has been confirmed as an
independent risk factor for all-cause mortality and MACE in post-PCI patients
[18, 19]. Lp(a) is a complex formed by one molecule of apolipoprotein (a) and one
molecule of LDL-C through covalent bonds. As shown in Fig. 1, Lp(a) can
significantly increase cardiovascular risk by inducing vascular inflammation,
promoting atherosclerosis, calcification, and thrombosis [20, 21, 22, 23]. In 2018, the
American Heart Association (AHA) suggested that Lp(a)
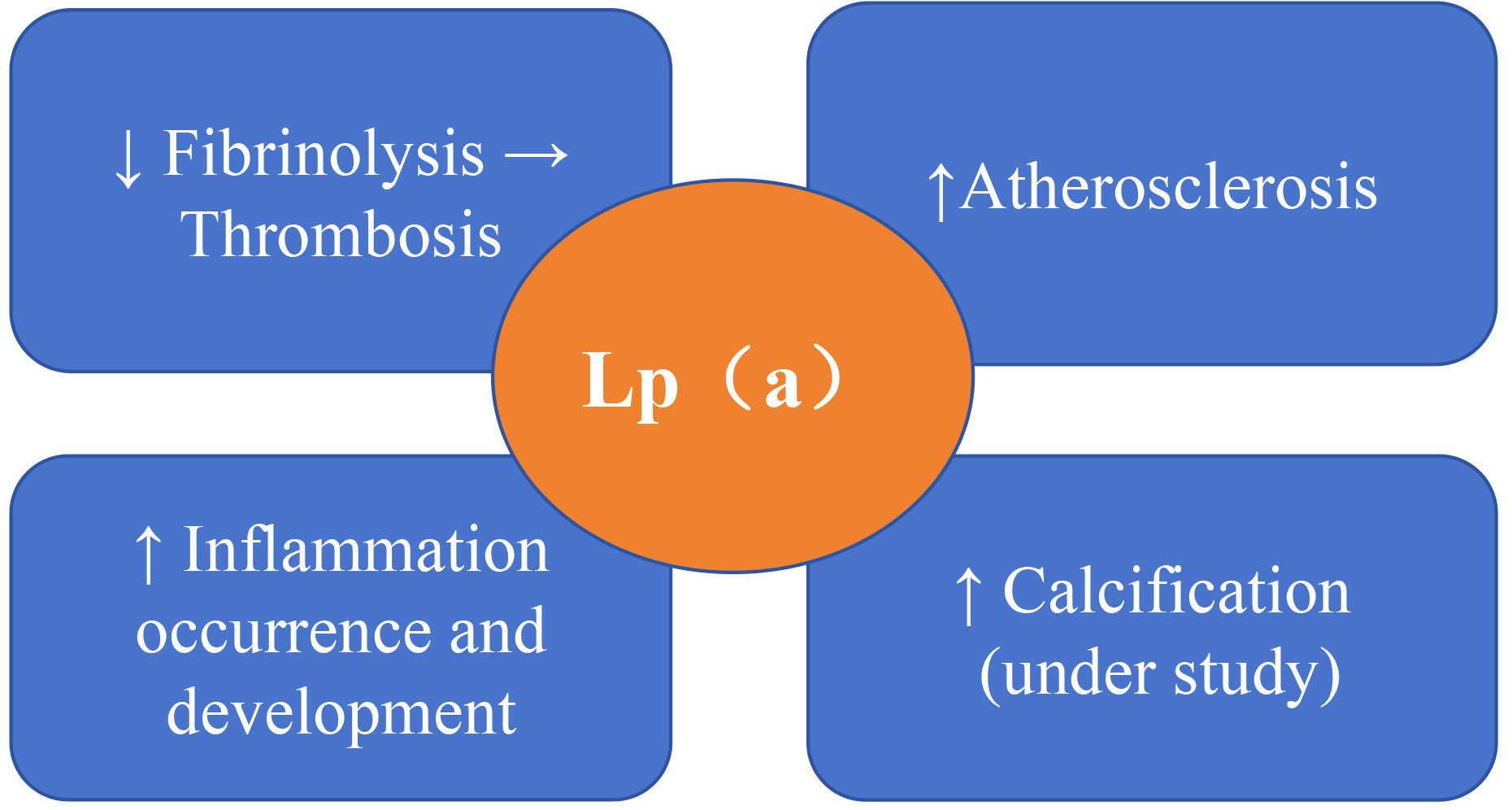 Fig. 1.
Fig. 1.
Mechanisms by which lipoprotein (a) (Lp(a)) affects the prognosis of cardiovascular disease. Lp(a) can increase cardiovascular risk by inducing vascular inflammation, atherosclerosis, calcification, and thrombosis, acting as a novel biomarker for cardiovascular disease (CVD) [20, 21, 22, 23]. The upward arrow indicates an increase, the downward arrow indicates a decrease, and the rightward arrow indicates that the reduction in the fibrinolysis process leads to thrombosis.
The pathological basis of cardiovascular diseases (CVD) is inflammation and lipid metabolism abnormalities. Compared to other indicators, the monocyte-to-high-density lipoprotein ratio (MHR) and the neutrophil-to-high-density lipoprotein ratio (NHR) can more comprehensively reflect the patient’s inflammatory state and lipid metabolism [28, 29, 30]. Studies have shown that the severity of CAD is positively correlated with MHR and NHR [21], which are more accurate prognostic indicators for post-PCI patients. For example, Yu et al. [31] demonstrated that MHR in ACS patients after PCI is positively correlated with the Gensini score. It can serve as an independent predictor of in-hospital MACE events. Meanwhile, the sensitivity and specificity of NHR for predicting adverse events related to ACS after PCI are as high as 77.6% and 74.2% [32, 33]. Previous studies have shown that MHR and NHR can be used for risk stratification of post-PCI CAD patients and for predicting short-term and long-term prognosis. However, how to quantitatively assess their utility remains an important direction for future research. Single-center designs and abbreviated follow-up periods may introduce heterogeneity in the distribution of clinical parameters across diverse geographic and ethnic populations. Therefore, large-scale, multicenter studies with extended follow-up are needed to enhance the generalizability and clinical applicability of lipid-related biomarkers.
As shown in Fig. 2, IR can accelerate the progression of CAD through mechanisms
such as inflammation, oxidative stress, dyslipidemia, and endothelial dysfunction
[34]. Although the “hyperinsulinemic-euglycemic clamp” is the “gold standard”
for measuring IR, its complexity limits its clinical application [35]. In recent
years, studies have found that the triglyceride-glucose index (TyG index = ln
[fasting triglycerides (mg/dL)
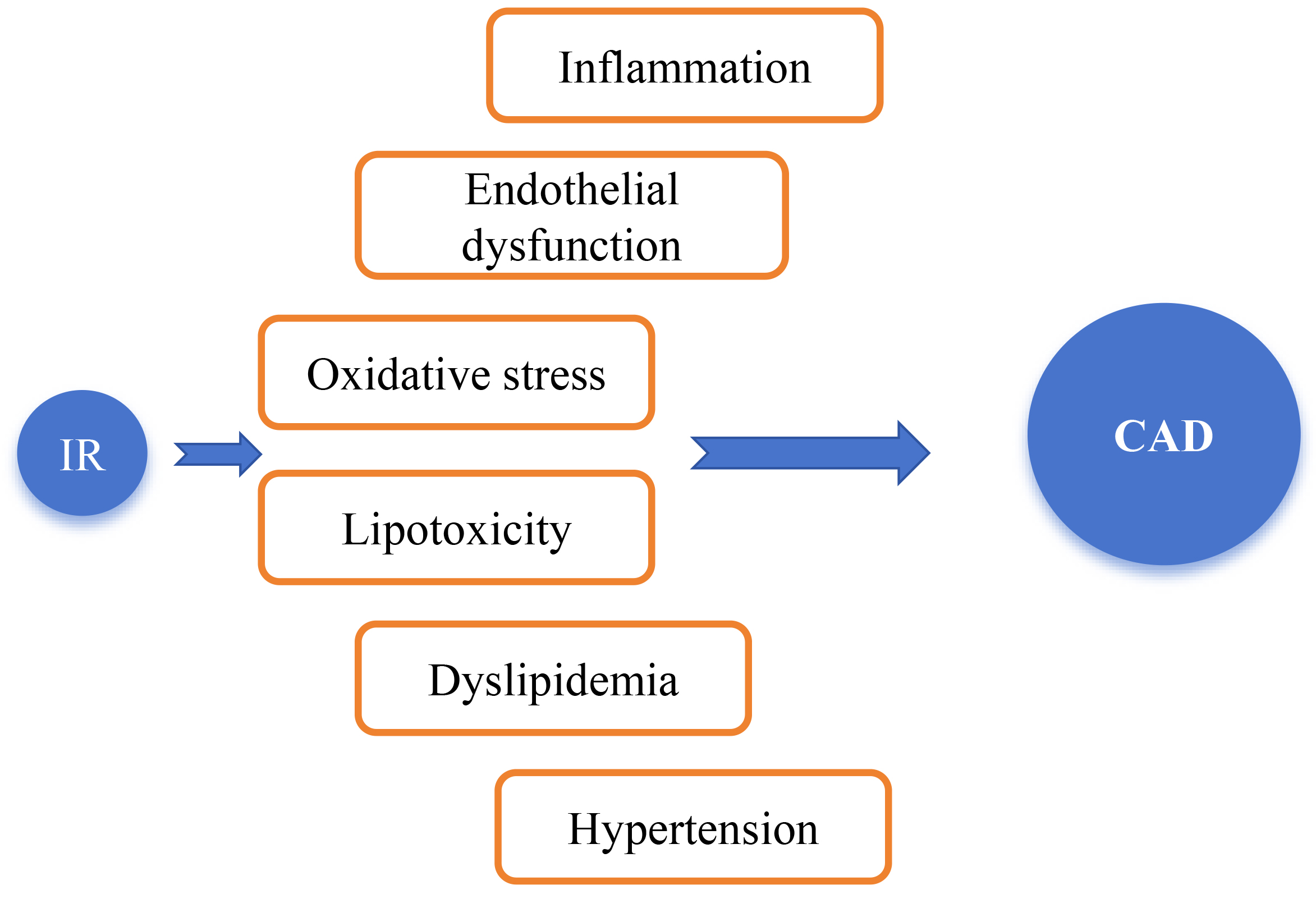 Fig. 2.
Fig. 2.
Mechanisms by which insulin resistance (IR) leads to the occurrence and development of coronary artery disease (CAD). IR was identified many years ago as a key mediator of metabolic disorders, type 2 diabetes mellitus (T2DM), and CVD. IR can accelerate the progression of CAD through mechanisms such as inflammatory responses, oxidative stress, dyslipidemia, hypertension, and endothelial dysfunction [34].
The TyG index is easily obtainable and is a better cardiovascular risk predictor
than fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) [38]. It can
effectively predict the long-term prognosis of CAD patients after PCI [39].
Multiple studies have confirmed that the TyG index performs significantly in
predicting recurrent adverse cardiovascular events in ACS patients [40, 41]. Chen
et al. [39] first demonstrated that patients with a higher TyG index
(
Combinations of the TyG index with obesity indicators (such as
triglyceride-glucose-body mass index ratio (TyG-BMI = ln [Fasting TG (mg/dL)
Cumulative TyG exposure is significantly associated with MACE, all-cause mortality, and ISR following PCI [51, 52]. A prospective cohort study further demonstrated that greater cumulative TyG exposure is linked to an increased risk of post-PCI MACE [53]. Considering the advantages of the improved TyG index-related indicators in assessing IR, future research should explore their application value in post-PCI patients. This will provide more accurate risk assessment tools for clinical use.
The SHR assesses the state of relative hyperglycemia by comparing admission blood glucose levels with the average glucose levels estimated from HbA1c. It has been reported that SHR should be regarded as a high-risk prognostic indicator for patients with ST-segment elevation myocardial infarction (STEMI) following PCI. A study has shown that in patients with STEMI undergoing PCI, SHR is significantly associated with increased in-hospital mortality and all-cause mortality risk, regardless of whether the patient has diabetes [54]. A multicenter observational study further demonstrated that each tertile increase in SHR was associated with a 28% rise in 30-day MACE risk. This metric independently predicted prognosis after PCI in STEMI patients [55]. Additionally, it has been proven that GHR can independently predict the risk of adverse outcomes in post-PCI CAD patients without diabetes [56]. To date, studies examining the prognostic utility of the SHR and the GHR specifically in patients after PCI are scarce. Existing evidence is primarily derived from populations in intensive care units or general medical wards. Data for older adults, women, and individuals with comorbid anxiety or depression remain limited.
Albumin is the main protein in the human body, involved in a variety of important physiological functions. As shown in Fig. 3 (Ref. [9, 10, 57, 58, 59, 60, 61, 62, 63]), these include: (1) maintaining plasma oncotic pressure and capillary permeability; (2) acting as a carrier for many endogenous and exogenous substances, participating in their transport and distribution; (3) affecting the pharmacokinetics of many drugs, regulating the absorption, distribution, metabolism, and excretion of drugs; (4) inhibiting platelet aggregation, protecting endothelial function of blood vessels, and maintaining vascular homeostasis; (5) having anti-inflammatory and antioxidant effects, reducing oxidative stress and inflammatory reactions [9, 10, 57].
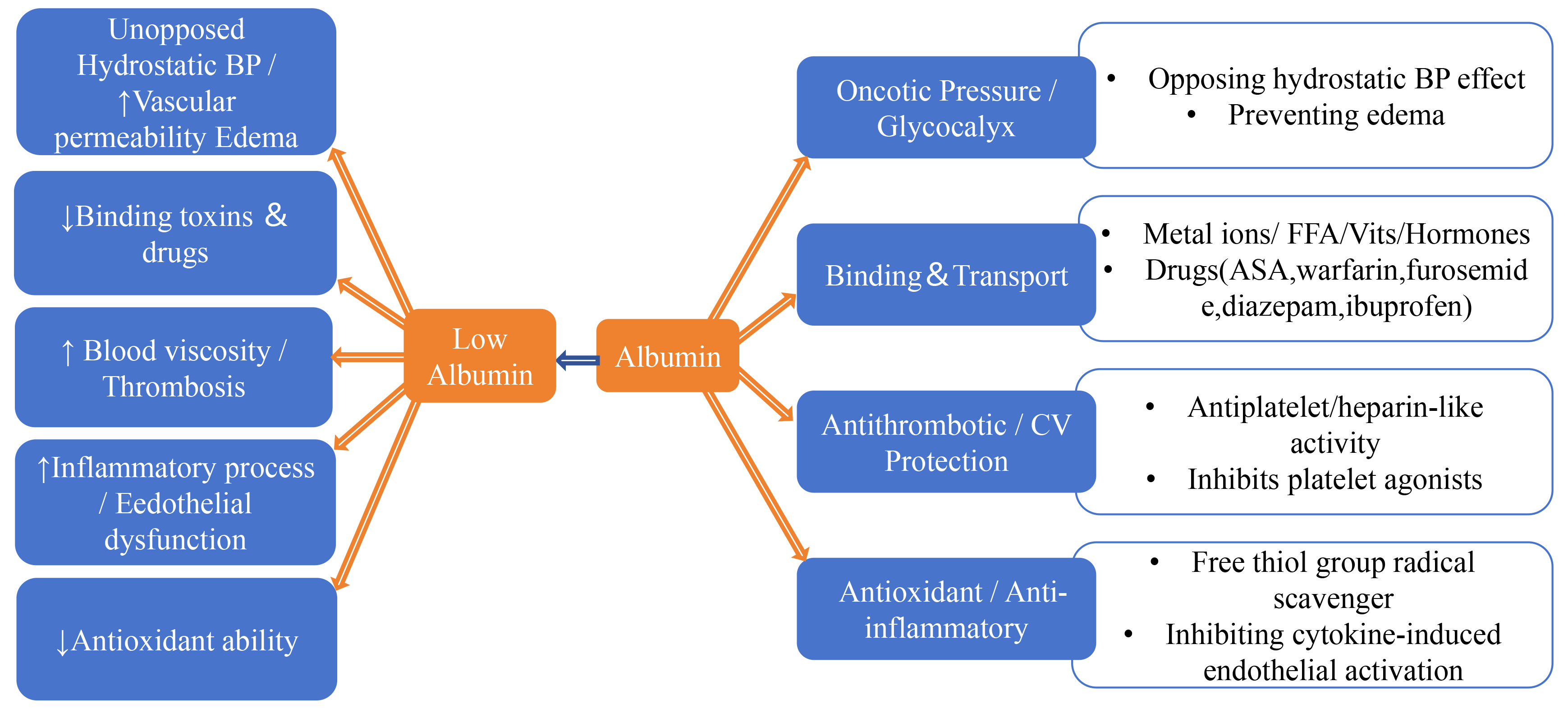 Fig. 3.
Fig. 3.
Mechanisms by which albumin contributes to the occurrence and development of CVD and the impact of decreased albumin levels. Low levels of serum albumin (SA) can accelerate the occurrence and development of CVD through the following mechanisms: (1) Refractory hypertension/vessel permeability increase leading to edema; (2) Reduced binding to toxins and drugs; (3) Increased blood viscosity/thrombosis; (4) Accelerated inflammatory processes/endothelial dysfunction; (5) Decreased antioxidant capacity [9, 10, 57, 58, 59, 60, 61, 62, 63]. The upward arrow indicates an increase, the downward arrow indicates a decrease. CV, cardiovascular; BP, blood pressure; FFA, free fatty acids; ASA, aspirin; CVD, cardiovascular disease.
SA functions as a natural antiplatelet and anticoagulant, as well as the principal antioxidant in plasma. It also acts as a scavenger of inflammatory mediators, thereby exerting critical effects across the pathophysiology of CVD [58]. Experimental data indicate that reduced SA concentrations diminish platelet activation–aggregation inhibition by neutralizing adenosine 5′-diphosphate (ADP), thromboxane A2, and coagulation factors. This subsequently prevents glycoprotein IIb/IIIa complex (GPIIb/IIIa) activation and fibrinogen binding. Additionally, reduced SA levels amplify inflammatory and oxidative stress, decrease nitric oxide bioavailability, and ultimately lead to endothelial dysfunction [59, 60, 61, 62, 63]. Collectively, these alterations promote coronary plaque progression and thrombus formation.
It has been confirmed as an important risk factor for the progression of
cardiovascular diseases and is also an economical, simple, and easily obtainable
prognostic predictor [64]. In a prospective study involving 734 individuals, it
was found that hypoalbuminemia (
Studies have shown that when albumin is combined with other risk factors (such
as C-reactive protein, neutrophils, alkaline phosphatase, etc.), its predictive
value is significantly enhanced [58]. Results from a study involving 2164
patients showed that the ratio of low SA to high-sensitivity C-reactive protein
(hs-CRP) (SA
Elevated plasma levels of Hcy have been confirmed to be closely related to
endothelial dysfunction, vascular inflammation, and the progression of
atherosclerosis. Studies have shown that the risk of vascular disease is
significantly associated with elevated Hcy levels. A study involving 39,242
participants demonstrated that patients with total homocysteine (tHcy)
Numerous studies have shown that SUA is an independent predictor of all-cause and cardiovascular mortality in CAD patients [72]. As shown in Fig. 4 (Ref. [8, 73, 74, 75]), Elevated SUA levels can promote oxidative stress, inflammatory responses, and thrombotic risk. While low SUA levels impair endothelial integrity through three mechanisms: antioxidant depletion, immune-inflammatory dysregulation, and iron-catalyzed oxidative stress. Additionally, UA regulates T-cell activation and cytokine release, so low SUA may disrupt immune-inflammatory homeostasis, attenuate immune surveillance, and thereby impair endothelial repair and plaque stability.
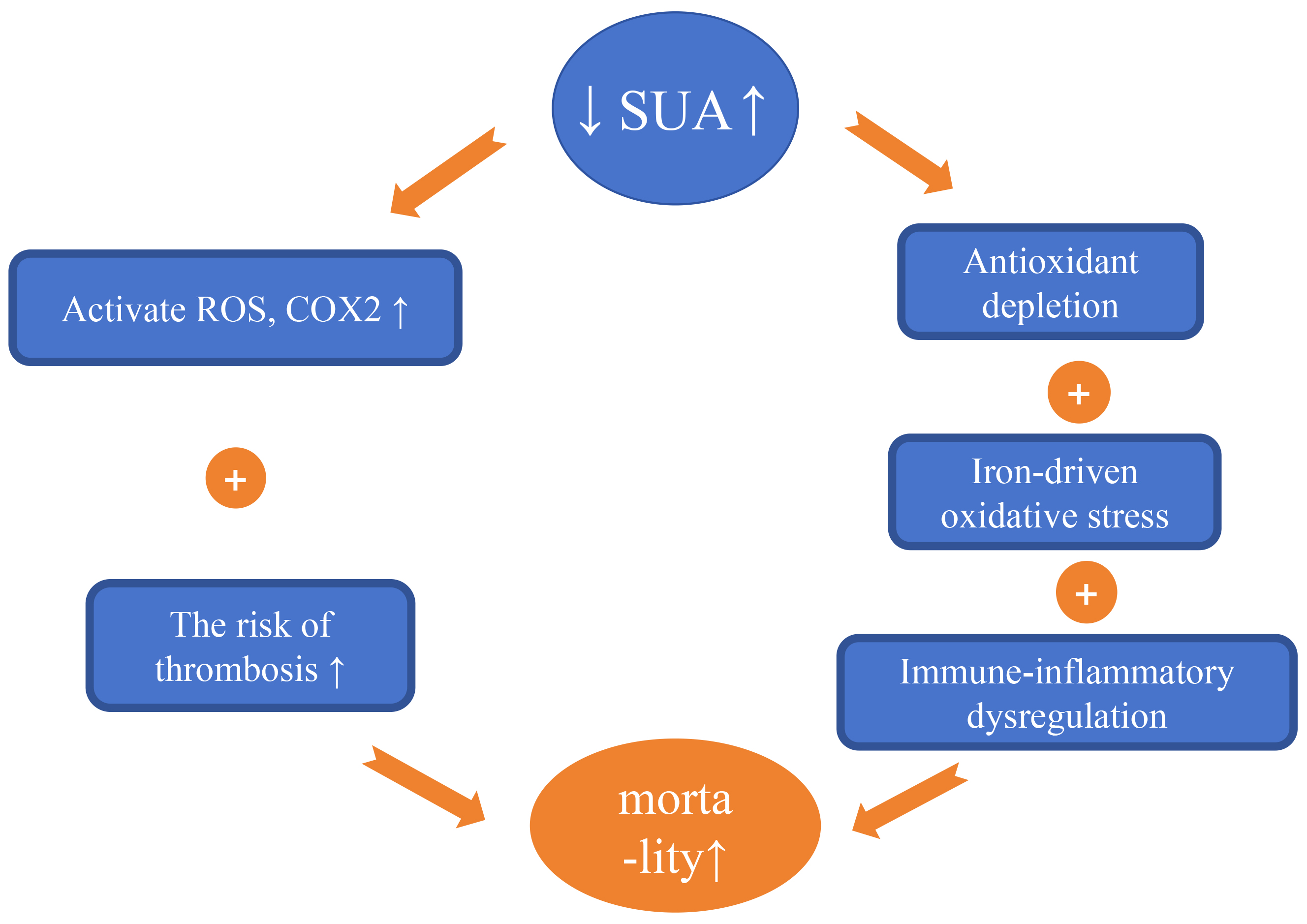 Fig. 4.
Fig. 4.
Mechanisms by which fluctuations in serum uric acid (SUA) levels affect the occurrence and development of cardiovascular disease. UA serves as an independent predictor of all-cause and cardiovascular mortality in patients with CAD. Elevated SUA levels can lead to: (1) Increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), as well as the activation of cyclooxygenase 2, resulting in inflammatory stress. (2) Increased mortality associated with thrombotic diseases. Conversely, low levels of SUA can result in: (1) Antioxidant depletion. (2) Immune-inflammatory dysregulation. (3) Iron-catalyzed oxidative stress—collectively impairing endothelial integrity and elevating MACE risk in CAD [8, 73, 74, 75]. The upward arrow indicates an increase, the downward arrow indicates a decrease. CAD, coronary artery disease; UA, uric acid; MACE, major adverse cardiovascular events.
For example, a study has shown that SUA has a U-shaped relationship with
long-term all-cause mortality risk in CAD patients, with those in the optimal SUA
range (5.59 mg/dL
In recent years, the serum uric acid to creatinine ratio (SUA/SCr) ratio has emerged as a novel biomarker reflecting endogenous uric acid levels, gaining considerable attention for its ability to mitigate the confounding effects of estimated glomerular filtration rate (eGFR) variability on SUA. Existing research has shown its association with the occurrence risk and mortality of metabolic syndrome [79]. A growing body of evidence has established that the SUA/SCr ratio surpasses SUA alone in predicting cardiovascular events. However, no large-scale, dedicated studies have yet investigated the prognostic implications of the SUA/SCr ratio in patients with CAD following PCI.
Evidence indicates that post-procedural outcomes in patients with CAD following PCI are influenced by multiple factors. These include lipid status, glycaemic control, nutritional state, renal function, and chronic low-grade inflammation. Elevated levels of Lp(a), MHR, NHR, TyG index, SUA, and low levels of SA are all independent risk predictors. In particular, combined indicators such as TyG-BMI, TyG-WC, TyG-WHtR, NAR, and AAR have shown stronger prognostic predictive capabilities. Moreover, long-term exposure to adverse metabolic states, such as persistently elevated LDL-C or TyG index, is also associated with higher cardiovascular risks. The present study proposes using routine laboratory parameters to predict long-term risk, thereby advancing the decision window. We recommend integrating these four indices into a risk-assessment table. This tool can be applied at hospital discharge and during outpatient follow-up visits to enable dynamic prognostic evaluation and guide tailored therapeutic adjustments.
Whereas the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score primarily quantifies lesion complexity to guide PCI strategy, the multiparametric metabolic prediction models outlined herein are intended for post-procedural risk management during longitudinal follow-up. Existing conclusions are largely derived from single-center retrospective cohorts within specific populations. Therefore, their generalizability and clinical value must be validated in more diverse populations. Future studies should employ harmonized, standardized assays alongside multi-ethnic, large-scale cohorts and establish ethnicity-specific cut-off values to minimize confounding attributable to potential racial differences in metabolic biomarkers. It is anticipated that future iterations of novel predictive models will exhibit enhanced discriminative accuracy and prognostic utility for patients following PCI.
CVD, cardiovascular disease; ASCVD, atherosclerotic cardiovascular disease; PCI, percutaneous coronary intervention; CAD, coronary artery disease; MACE, major adverse cardiovascular events; CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; TG, increased triglyceride; HDL-C, high-density lipoprotein cholesterol; Lp(a), lipoprotein (a); MHR, monocyte-to-high-density lipoprotein ratio; NHR, neutrophil-to-high-density lipoprotein ratio; IR, insulin resistance; TyG, triglyceride-glucose index; TyG-BMI, triglyceride-glucose-body mass index ratio; TyG-WHtR, triglyceride-glucose-waist-to-height ratio; TyG-WC, triglyceride-glucose-waist circumference ratio; hs-CRP, high-sensitivity C-reactive protein; SHR, stress hyperglycemia rate; GHR, glucose-to-high-density lipoprotein cholesterol ratio; SA, serum albumin; NAR, neutrophil-to-serum albumin ratio; AAR, alkaline phosphatase to serum albumin ratio; Hcy, homocysteine; tHcy, total Homocysteine; SUA, serum uric acid; SUA/SCr, serum uric acid to creatinine ratio; ISR, in-stent restenosis; T2DM, type 2 diabetes mellitus; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; CLIDAS, Clinical Depth Data Accumulation System; STEMI, ST-segment elevation myocardial infarction; SII, systemic inflammatory-immune index; ROS, reactive oxygen species; RNS, reactive nitrogen species; AHA, American Heart Association; OCT, optical coherence tomography; ADP, adenosine 5′-diphosphate; GPIIb/IIIa, glycoprotein IIb/IIIa complex.
ZZ was responsible for the literature screening and drafting the initial manuscript. JL, GQ, and HZ provided suggestions for modifications. HZ revised the important knowledge content. All authors contributed to the editorial revisions and conceptualization of the manuscript. All authors have read and approved the final manuscript. All authors have participated sufficiently in the work and agree to be accountable for all aspects of the work.
Not applicable.
We thank each reviewer of this article, who has undergone a very careful and thorough review that greatly improved the original paper.
Funded by the Hebei Provincial Medical Science Research Project (No. 20250403) and the Hebei Provincial Key Research and Development Program (No. 20377741D).
The authors declare no conflict of interest.
During the preparation of this work, the authors used ChatGPT to check spelling and grammar. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
