Academic Editor: Efstratios Georgakarakos
Late open conversion in our center has been reviewed in the past 8 years,
comparing 1997–2011 (first period group A) with 2012–2020 (second period group
B). A retrospective analysis of patients treated at our centre by standard EVAR
for infrarenal aortic aneurysm requiring late open conversion between January
1997 and February 2020 was performed. All stent grafts were implanted according
to their current IFU all patients. The data concerning intra and postoperative
complications were collected. Post-operative evaluated data include: ICU
(Intensive Care Unit) stay, major peri-operative (
Endovascular aneurysm aortic repair (EVAR) represents the treatment constituting more than 80% of elective approach [1]. This treatment method, even though enormously successful, is not without its risks, particularly when it is used in morphologic conditions different from the standard of use (IFU) [2, 3].
Common adverse conditions are migration and consequently endoleaks, which can cause elevation of intraluminal sac pressure. The improvement in the materials field and, at the same time, skill of operators, made endoluminal methods suitable to treat the most of the complication [4]. Sometimes if left untreated, these complications increase the risk for aneurysm rupture making it necessary a late open conversion (LOC) [5]; this is the last resort and it may occasionally remain as the ultimate choice to treat EVAR demanding open surgery [4, 6].
Despite the evolution of materials, with the growing and wider application of EVAR, the amount of patients requiring new operation after EVAR and conversion rate appears to be higher than before [3, 7], with an overall incidence of 5.3% in recent experiences by Goudegetting and Davidovic et coll [8, 9].
The objective of our paper is to report the experience of late open conversion in the past 8 years, compared to previous years. In our paper, we focus on the late open conversions that took place in the past 8 years in comparison to the previous experiences.
This was a retrospective monocentric observational study extrapolating information from our recorded data. Informed consent for the study was obtained from all patients.
Data of patients treated at our Centre by standard EVAR for infrarenal aortic aneurysm requiring late open conversion between January 1997 and February 2020 were collected. All stent grafts were implanted according to their current IFU. The study was conducted according to the Ethical board rules and after their approval, all patients expressed their consent to participate in follow up surveillance. The period of observation was divided into two: the first study period (Group A) comprising patients submitted to open surgical conversion between January 1997 and December 2011; the second period (Group B) including patients treated with open conversion between January 2012 and February 2020. Incidence of EVAR conversion and outcome of open conversion were compared between the two groups.
All data concerning epidemiological data and perioperative information were analyzed. The maneuvers necessitating open access for aortic cross-clamping, with partial, complete, or sac manipulation with no removal of the stent graft were recorded. Intra-operative details included the aortic clamp site, the size of stent graft removal, and the type of replacement. In all our cases aneurysms were infrarenal treated with Standard-EVAR.
Graft removal was executed in scheduled or as in a urgent context. Urgent procedures took place because of ruptured or painful aneurysms. The preliminary CT (Computed Tomography) scan at the admission was recorded in all cases. Frequency of operative factors including respecting of IFU, aneurysm morphology, stent graft details and time between stent graft implantation and open surgical conversion were recorded. Indications for open surgery, type of surgical approach, and methods were analyzed.
Data concerning perioperative adverse events were documented. Post-operative
analyzed data included ICU stay, major peri-operative (
The incidence of conversions and the 30-day mortality rate were compared with that of previous years, from January 1997 to December 2011 (first study period, Group A).
IBM SPSS Statistics (IBM Corp., Armonk, New York, NY, USA, Version 20) was used
for actuarial analysis. We used percentage as a mean to define reported
parameters. Groups were compared with non-parametric statistical tests;
categorical variables were compared with the Fisher exact test (considering a
p
First study period – Group A - Among January 1997 and December 2011, a total of
268 EVAR were performed. During this period surgical conversion had been
performed in 14 patients (5.2%). Among these, three patients (3/14, 21.4%)
underwent graft excision after Vanguard Endovascular Stent-Graft (Boston
Scientific Ltd, St Albans, Herts). Three patients underwent graft excision (3/14,
21.4%) and one partial excision (1/14, 7.1%) after Zenith
| ID | IFU followed | Device (Fixation) | Indication for conversion | Time to conversion (months) | Previous endovascular treatment tentative | Modality | Operation | Operative details |
| 1 | Yes | Vanguard | Graft occlusion | 36 | Yes | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 2 | Yes | Talent | Type 1 endoleak with sac expansion | 6 | No | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 3 | Yes | Vanguard | Migration with Type 1 endoleak | 26 | Yes | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 4 | Yes | Vanguard | Type 1 endoleak and graft kinking | 82 | Yes | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 5 | Yes | Gore Excluder | Type 2 Endoleak with sac expansion | 53 | Yes | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 6 | Yes | Talent | Type 3 Endoleak with sac expansion | 75 | Yes | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 7 | Yes | Gore Excluder | Type 1 endoleak with sac expansion | 18 | No | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 8 | Yes | Gore Excluder | Type 2 endoleak with sac expansion | 19 | Yes | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 9 | Yes | Gore Excluder | Type 2 endoleak with sac expansion | 40 | Yes | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 10 | Yes | Zenith | Type 1 endoleak | 17 | No | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 11 | Yes | Zenith | Type 1 endoleak with sac expansion | 1 | Yes | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 12 | Yes | Gore Excluder | Type 1 Endoleak with sac expansion | 15 | No | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 13 | Yes | Zenith | Type 1 Endoleak with migration | 26 | Yes | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 14 | Yes | Zenith | Type 1 Endoleak with migration | 7 | No | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
The mean time between the stent implant and late open conversion was 30 months (range 1–82 months). The average original diameter before EVAR was 54.9 mm (45–67), and the mean aneurysm sac enlargement between device implantation and graft excision was 8.4 mm (3–25).
Nine out of fourteen patients (64.2%) underwent open conversion for type I endoleak with the migration of the endografts or abdominal pain.
In seven cases, the patients underwent treatment for intact aneurysms electively and seven was performed urgently for abdominal pain or rupture. In all our cases, aneurysms were infrarenal and all devices had been implanted according to the IFU. The mean age was 73.9 years (56–91 years IQR [InterQuartileRange]), 13 patients were male. Associated medical comorbidities included coronary artery disease (6/14, 43%), hypertension (10/14, 71.4%), hypercholesterolemia (10/14, 71.4%), diabetes mellitus (3/14, 21.4%), chronic kidney disease (4/14, 28.5%), COPD (Chronic obstructive Pulmonary Disease) (9/14, 64.3%) and previous nicotine abuse (12/14, 85.7%). The American Society of Anesthesiologists’ physical classification status was class II in 5 patients, class III in 7 patients and class IV in 2 patients. Eight out of fourteen patients (57%) having already undergone a previous failed secondary endovascular re-interventions (coil embolization, relining with new endograft, proximal placement of Palmaz stent).
The surgical approach was in all cases the median laparotomy, with incision of the retroperitioneum. In one case a supraceliac clamping and in two cases suprarenal clamping was necessary to remove the graft. One partial excision and all prosthetic aorto-bi-iliac graft reconstruction using standard Dacron was performed. Mortality at 30 days after conversion was 21.4%: a patient died for acute mesenteric ischemia, and 2 patients for multi-organ failure. At 30 days no patients required reoperation two patients presented pneumonia, one patient presented kidney disease and one atrial fibrillation.
At a mean follow-up of 20 months, there was no degeneration of the residual infrarenal aortic neck, no signs of anastomotic pseudoaneurysm, no graft occlusion and no deaths recorded. One of the patients suffered from an incisional hernia during follow-up.
Second study period – Group B - Between January 2012 and February 2020, 481
EVAR were performed in our institute, 8 cases with endoleak were treated with
late open conversion (1.7%). Between these, four patients (4/8, 50%)
experienced graft excision after Nellix (Endologix Inc, Irvine, Calif)
Endovascular Aneurysm Sealing (EVAS). Two patients underwent partial graft
excision after Zenith
| ID | IFU followed | Device (Fixation) | Indication for conversion | Time to conversion (months) | Previous endovascular treatment tentative | Modality | Operation | Operative details |
| 1 | Yes | Gore Excluder (Infrarenal) | Type 2 and then Type 1 Endoleak with sac expansion | 72 | Yes | Urgent | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| Abdominal pain | ||||||||
| 2 | Yes | Nellix | Migration with Type 1 endoleak | 18 | No | Emergency | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| Abdominal pain | ||||||||
| 3 | Yes | Nellix | Migration with Type 1 endoleak | 57 | No | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 4 | Yes | Endurant II (Suprarenal) | Expanding sac without endoleak | 36 | Yes | Elective | Endograft preservation | Aneurysm sac opened and explored, closure by plication of aneurysm sac over endograft |
| 5 | Yes | Nellix | Type 1 Endoleak with sac expansion | 36 | No | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 6 | Yes | Zenith (Suprarenal) | Type 3 Endoleak with sac expansion | 88 | No | Urgent | Partial explant | Bifurcated surgical graft to bilateral common iliac arteries |
| Rupture of the graft | ||||||||
| 7 | Yes | Nellix | Migration with Type 1 endoleak | 72 | No | Elective | Explant | Bifurcated surgical graft to bilateral common iliac arteries |
| 8 | Yes | Zenith (Suprarenal) | Type 1 endoleak | 12 | No | Emergency | Partial explant | Bifurcated surgical graft to bilateral common iliac arteries |
| AAA rupture |
Four patients were treated for intact aneurysms electively and four of these were performed urgently for abdominal pain or rupture.
In all our cases aneurysms were infrarenal and all devices had been implanted according to the IFU.
The median age was 78.8 years (IQR, 70–83 years) and all patients were male. Associated medical comorbidities included coronary artery disease (3/8, 37.5%), hypertension (7/8, 87.5%), hypercholesterolemia (6/8, 75%), diabetes mellitus (2/8, 25%), chronic kidney disease (2/8, 25%), COPD (5/8, 62.5%) and previous nicotine abuse (7/8, 87.5%). The classification status was class II in 1 patient, class III in 6 patients and class IV in 1 patient, according to the American Society of Anesthesiologists’ physical status category.
The median time from index EVAR to open conversion was 48 months (range 12–88 months). The mean aneurysm original diameter before EVAR/EVAS was 57.2 mm (48–74) and the mean aneurysm sac enlargement between device implantation and graft excision was 17.4 mm (14–24).
Six patients (75%) underwent open conversion for type I endoleak with the migration of the endografts or abdominal pain.
Two patients (25%) having already undergone a previous failed secondary endovascular re-interventions (attempt of relining with cuff and coil embolization).
In all cases, a transperitoneal approach via a midline laparotomy was used.
In 7 cases the proximal aortic cross-clamping was infrarenal. In one case suprarenal clamp just to remove the proximal part of the endograft was performed. The stent graft was completely detached in 5 cases. One patient underwent sacotomy without explant (the clamp was not positioned, but the aorta was prudentially prepared in case of need, such case of dislocation during sac evacuation) and 2 cases underwent partial graft explant with preservation of the first proximal covered stent of the endograft, used as a “neo-neck” for proximal anastomosis.
Prosthetic aortic reconstruction was aorto-bi-iliac graft in every instance employing standard Dacron graft (Fig. 1).
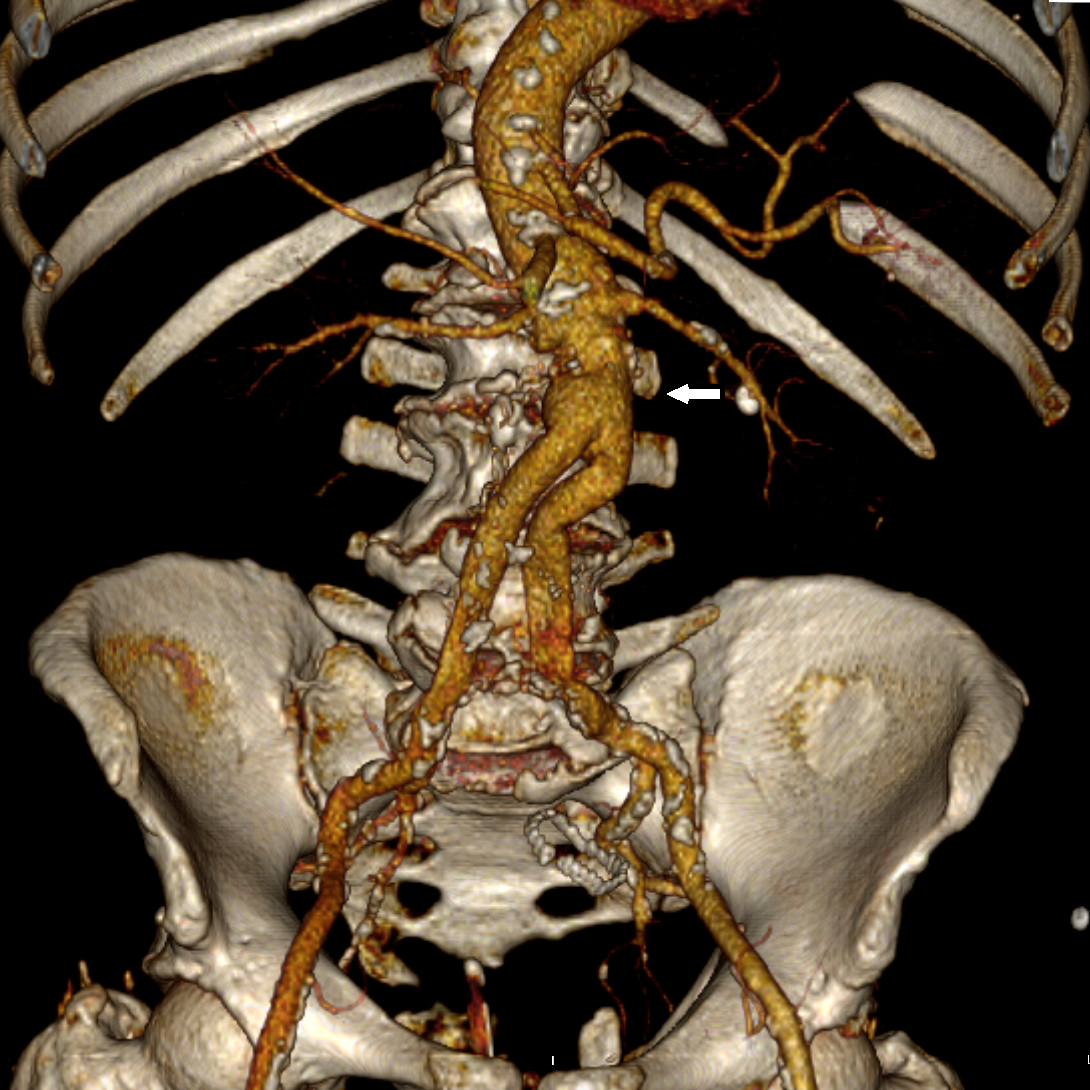 Fig. 1.
Fig. 1.Explantation of the endoprosthesis and prosthetic aortic reconstruction through aorto-bi-iliac Dacron graft. White arrow: proximal anastomose in the neck of the Dacron graft.
The entire 30 days mortality in the hospital was 12.5% and the global incidence of moderate to severe complications was 37.5%. One renal failure and no major cardiac event were reported. Mean intensive care unit stay and hospital stay were 6.5 days and 18.4 days, respectively (Table 3).
| ID | ICU (days) | LOS (days) | Complications | Re-interventions | Follow-up (months) | Outcome |
| 1 | 1 | 10 | Uncomplicated | /// | 79 | Good condition |
| 2* | 16 | 16 | Aspiration pneumonia, death | /// | /// | Exitus |
| 3 | 1 | 9 | Uncomplicated | /// | 17 | Good condition |
| 4 | 1 | 8 | Uncomplicated | /// | 16 | Good condition |
| 5 | 10 | 48 | Anastomosis bleeding | Covered stenting | 13 | Good condition |
| 6 | 3 | 16 | Delirium | /// | 12 | Good condition |
| 7 | 1 | 7 | Uncomplicated | /// | 4 | Good condition |
| 8 | 25 | 31 | Acute Kidney Disease, Pneumonia | /// | 2 | Alive at 2 months in a rehabilitation clinic |
| Mean value | 6 | 18.4 | 20 | |||
| ICU, intensive care unit stay; LOS, length of hospital stay. * Not considered for mean values. | ||||||
No death was registered in elective cases, but in patient who underwent surgery urgently, there was listed a post-operative death. An 80-years-old male developed a large proximal type I endoleak with the migration of a Nellix in the context of abdominal pain (Fig. 2). Hemodynamically unstable at presentation, he died in the intensive care unit from aspiration pneumonia in the 16th post-operative day. In one case, covered stenting was needed for postoperative bleeding in the site of distal anastomosis of an aorto-bi-iliac bypass.
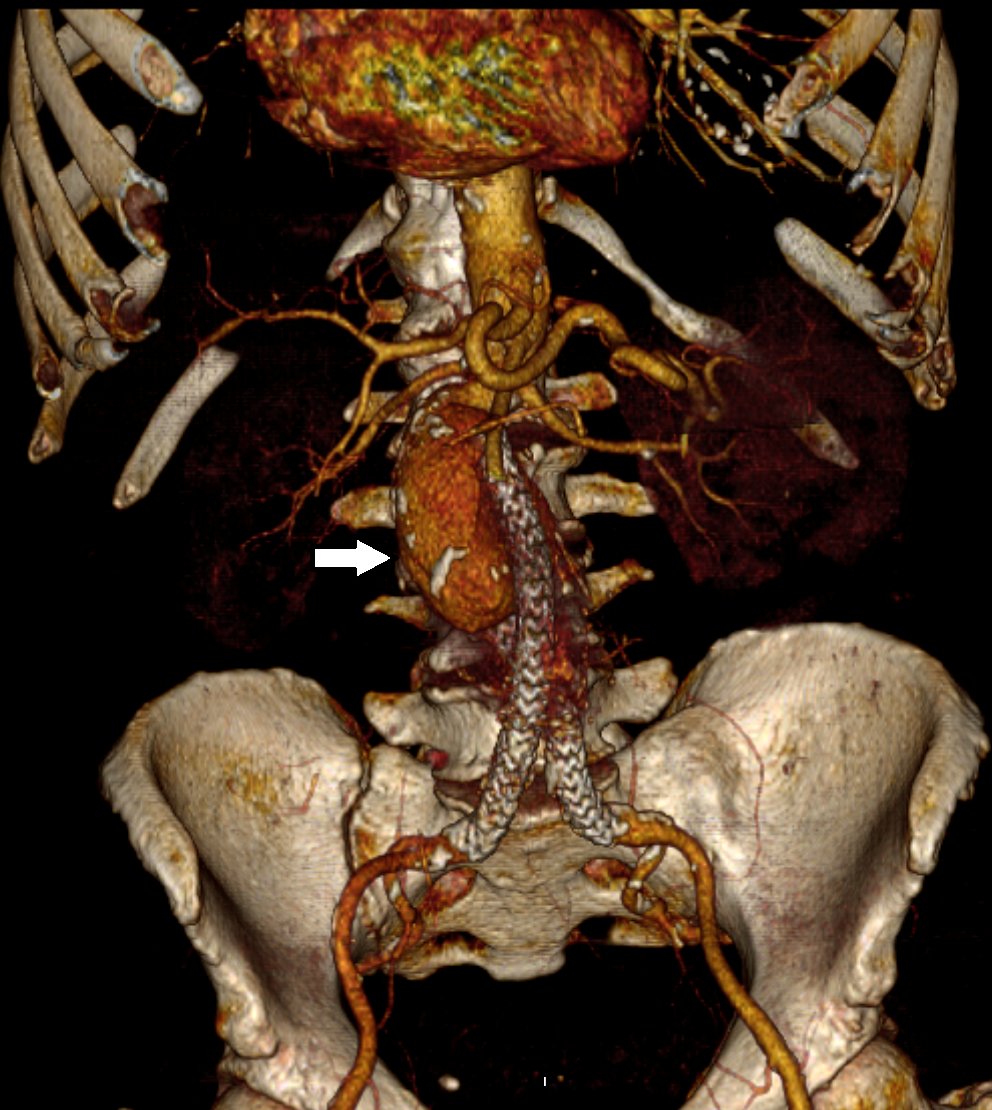 Fig. 2.
Fig. 2.CTA Volume rendering reconstruction documented blood collection (white arrow) due to a proximal Endoleak type I after EVAS with Nellix device.
At a mean follow-up of 20 months, there were no late complications or deaths verified. Doppler ultrasonography (DUS) during the follow-up, showed the intact proximal anastomosis with no degeneration of the residual infrarenal aortic neck and no signs of anastomotic pseudoaneurysm and good results in short-mid term with well-preserved primary patency and freedom of re-intervention in all seven cases. None of the patients suffered from incisional hernia during follow-up.
The incidence of conversions between the two groups was statistically significant (p = 0.0118) (Table 4).
| EVAR | Conversion | % | |
| 1997–2011 | 268 | 14 | 5.2 |
| 2012–2020 | 481 | 8 | 1.7 |
The most demanding of the non-immediate complications of the EVAR treatment, despite the advances obtained in the materials and the experience acquired by the operators [10].
In the papers published in the past, the mortality of long-term conversions is higher than in the OPEN surgery [10, 11]. Overall the 30-day mortality rate in this cohort was 18.1% (4/22), this is consistent with current literature [7]; and mortality rates were different between elective and urgent operation [12, 13].
The suprarenal clamping was used more in Group A (3/14, 21.4%) than in the most recent period (1/8, 12.5%). In Group B, open conversion for type Ia endoleak with the migration of the endografts or abdominal pain was performed in six out of eight patients, and nine out of fourteen in Group A. This category of type I endoleak, associated with worrying symptoms and non-fit for endovascular treatment, was judged as an indication for open conversion in our center. An endovascular approach to repair a proximal endoleak represents the first choice treatment in the literature [7, 14]; however, in selected cases such as patients fit for open surgery with unfavorable anatomy for advanced EVAR, primary LOC can be considered.
The removal of the endograft and aortic clamp site are important problems during LOC. No clear recommendations exist regarding the management of an endograft by complete or partial removal, and this issue is controversial. Complete removal is an absolute need in infection cases. Complete removal, especially an endograft with suprarenal fixation, may increase the risk of an aortic wall injury [12, 15]. Preservation of the proximal covered stent of an endograft with suprarenal fixation used as an infrarenal “neo-neck” with the incorporation of the aorta to the suture line during elective surgical explantation simplifies the procedure [15, 16]. In our series, we performed infrarenal aortic cross-clamping including the main body and proximal anastomosis using the neo-neck technique and iliac endoclamping through Fogarty catheter (Figs. 3,4).
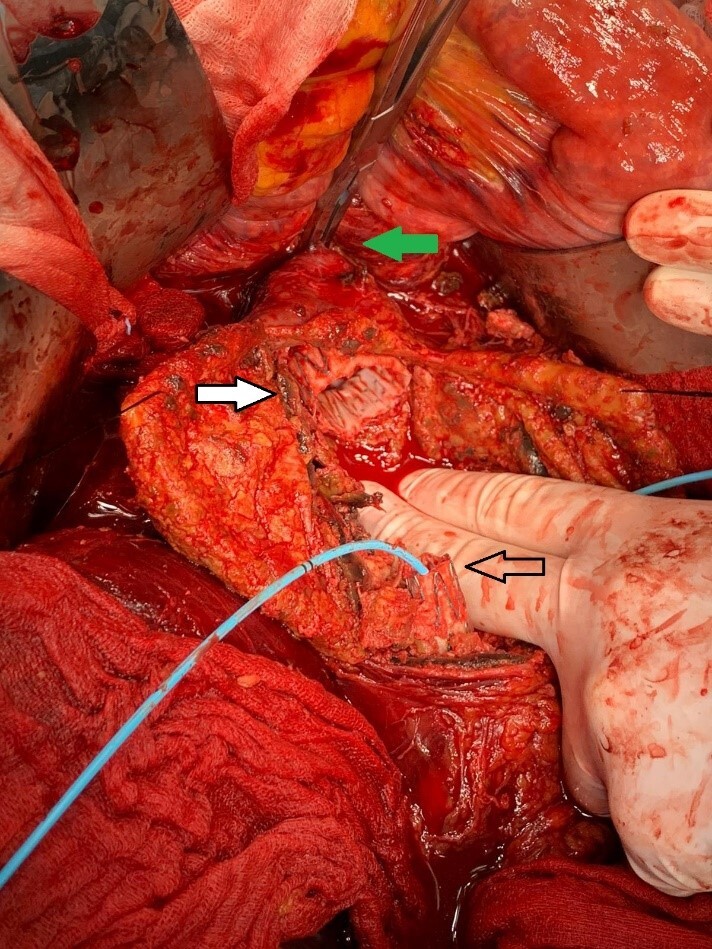 Fig. 3.
Fig. 3.Infrarenal aortic cross-clamping (green arrow), partial removal of the endografts (white arrow indicate the “neo neck” technique) and iliac endoclamping through Fogarty catheter (black arrow).
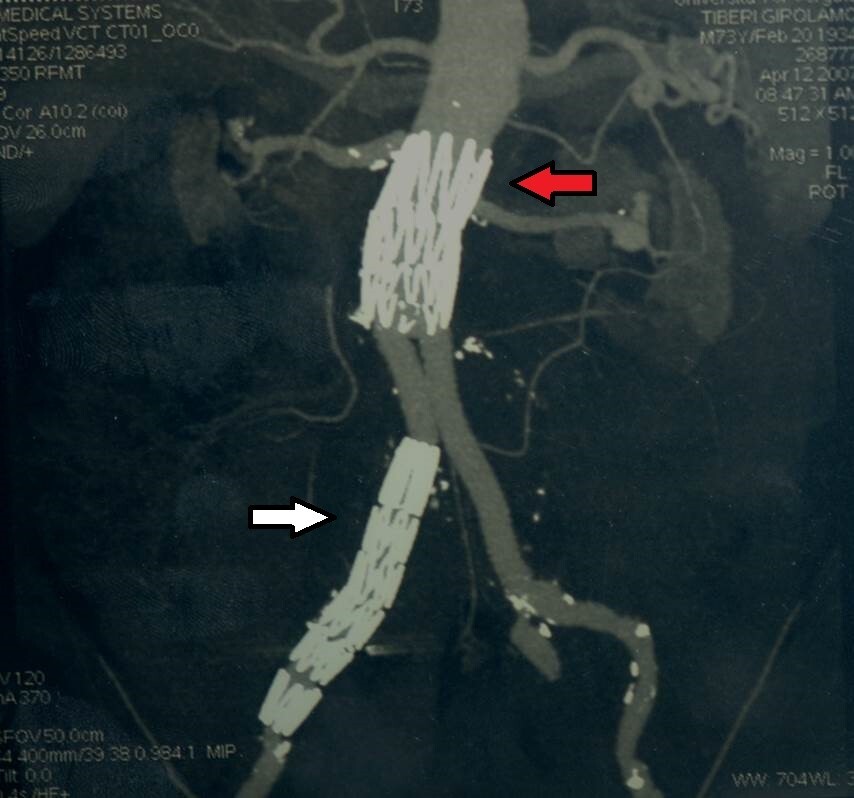 Fig. 4.
Fig. 4.Partial reconstruction with preservation of the proximal covered stent and right distal iliac stent graft (white arrow) of an endograft with suprarenal fixation (red arrow).
In this occurrence, four patients with EVAS implantations underwent a complete
removal for relentless proximal type I endoleak, because no endovascular options
were available. The specific structure and conformation of this device is are
predisposed to proximal sealing defects with a high rate of conversion to open
surgery treatments or re-intervention. Although, initial data on this device have
been promising [17], Nellix has also proved to undergo adverse events, mostly the
non-negligible migration due to sac rupture and proximal graft failure rate,
particularly during mid-term follow up (
EVAR is accomplished in higher volumes and at more centers; this can lead to an increase in patients undergoing surgery outside the IFU with a consequent increase in additional procedures and consequently the need for OPEN conversions [25, 26]. Contrarily, we noted a smaller volume of open conversion at our center during the past few years. In the literature, The percentage of explants in recent years has increased with the need for conversion for EVAR failures with an average incidence of 3.7% [2, 22, 26]. In the past eight years, our institution’s EVAR conversion rate of 1.7% compares favorably with that in the literature, instead, between 1997 and 2011, our conversion rate was 5.2%. In addition, the 30-day mortality rate after conversion also decreased: 12.5% vs 21.4%.
These figures are probably due to greater respect for current IFU in recent years and the evolution of material employed.
Multivariate analysis is not applicable due to the small number of patients considered and this constitutes a limitation of the study.
Contrary to the literature, we noted a smaller volume of open conversion at our centre during the past few years.
The greatest recurrent open surgery indication in this series is the Endoleak type 1 and migration in both considered periods. Adherence with current IFU and the technical progress in Endoprosthesis design, maintain lower rate incidence in the recent period. Open conversion after EVAR for AAA repair may be achieved without suprarenal clamping in most patients. Partial endograft removal in selected patients facilitates the open conversion and appears durable. The results are influenced by multiple comorbidities; in both periods emergency graft excision appears to increase mortality and morbidity, compared to elective surgical settings.
AAM and FMO contributed equally to the conception of this work and the acquisition of data, and drafted the article. FMO, LT, CC, AR, MB, researched literature and contributed to the acquisition of data. AAM, SF and AI critically revised the article for intellectual content. All authors approved the final version of the article.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee (approval number cod. 19121).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
