Academic Editor: Giuseppe Santarpino
Cardiac surgery-associated acute kidney injury (CSA-AKI) is a critical
complication associated with mortality and morbidity. This study aimed to clarify
the impact of CSA-AKI on activities of daily living (ADL) at discharge in elderly
cardiac surgery patients. We included 122 cardiac patients who underwent coronary
artery bypass surgery, valve surgery, or combined surgery by mid-line incision
followed by postoperative cardiac rehabilitation (CR) from March 2015 to May
2020. CSA-AKI was based on KDIGO criteria. The index of ADL was the Functional
Independence Measure (FIM). We compared background factors, clinical parameters,
activity level before hospitalization, CR progress, and FIM in patients with or
without CSA-AKI. Multiple regression analysis was performed with FIM at discharge
as the dependent variable and items with p
Against the backdrop of an aging society, an estimated 61,506 cardiac surgery operations have been performed so far in Japan in 2020, and the age of the patients undergoing surgery is rising [1]. Because patients undergoing cardiac surgery are increasingly older, the reserve capacity of organs in these many patients is deteriorated due to comorbidities such as diabetes, high blood pressure, renal failure, and lung disease, in addition to heart disease [2]. Cardiac surgery-associated acute kidney injury (CSA-AKI) has been reported to be a complication in up to 14–30% of post-cardiac surgery patients and to result in poor short- and long-term prognoses [3, 4]. CSA-AKI prolongs the lengths of stay in the intensive care unit (ICU) and hospital that lead to increased medical costs [5]. We have experienced clinically that CSA-AKI patients take time to reacquire activities of daily living (ADL) by the time of discharge.
One purpose of cardiac rehabilitation (CR) in patients after cardiac surgery is the restoration of ADL [2]. Preoperative low ADL predicts late postoperative acquisition of early walking [6]. In addition, the Functional Independence Measure (FIM) score, which is an index of ADL at discharge, predicts whether a patient can be discharged to home [7]. Therefore, it is meaningful to clarify both the time required to improve ADL until discharge of elderly cardiac surgery patients to home and the predictors of ADL decrease at discharge. However, no reports have clarified the effect of CSA-AKI on ADL at discharge in these patients.
Previous studies of factors that delay the progression of early CR in cardiac surgery patients have reported these factors to be prehospital renal function, start day of postoperative walking, CSA-AKI, postoperative positive fluid balance, and postoperative atrial fibrillation (POAF) [8, 9, 10]. Furthermore, we found that the factors delaying the acquisition of early walking in elderly patients with heart failure (HF) were walking level before hospitalization and renal function at admission, and the factors decreasing ADL at discharge were walking level before hospitalization, renal function at admission, worsening renal function during hospitalization, and the start day of standing [11, 12].
Therefore, we hypothesized that the factors predictive of ADL decline at discharge in elderly cardiac surgery patients would be activity level before hospitalization, CSA-AKI, POAF, and postoperative start day of walking. Thus, the purpose of the present study was to determine the effects of CSA-AKI on ADL at discharge in elderly cardiac surgery patients.
This was a retrospective, single-center, and observational study. We included 231 patients who underwent coronary artery bypass surgery and valve surgery by mid-line incision and postoperative CR from April 2015 to May 2020. Exclusion criteria were patients younger than 65 years old, those who could not walk 50 m before hospitalization, those undergoing reoperation for postoperative cardiac tamponade, those who died after surgery, and those with infective endocarditis, postoperative stroke, or preoperative dialysis. After determining whether the patients met the definition of CSA-AKI, we divided them into two groups according to the presence or absence of CSA-AKI and investigated and compared various patient characteristics and clinical parameters (Fig. 1).
 Fig. 1.
Fig. 1.Diagram of the process to select the elderly cardiac surgery patients for the cardiac surgery-associated acute kidney injury (CSA-AKI) group and Non-CSA-AKI group.
CSA-AKI was defined according to the KDIGO (Kidney Disease: Improving Global
Outcomes) creatinine-based definition criteria [13] of an increase by 0.3 mg/dL
or more within 48 hours postoperatively or an increase by 1.5 times or more from
the baseline level within 7 days postoperatively. eGFR was determined by a
laboratory technician at admission and discharge. The eGFR was calculated using
the following formula: 194
Characteristics of the patients and clinical parameters evaluated included age, sex, body mass index, walking level before hospitalization, comorbidities before surgery and POAF, duration of drain placement, duration of oxygen administration during hospitalization, number of days until return to preoperative weight, CR progress status, FIM at discharge, length of stay (LOS) in the ICU and postoperative LOS, intraoperative and surgery-related factors, and duration of intubated ventilator management. Laboratory data evaluated included hemoglobin, albumin, C-reactive protein, and serum creatinine levels, and eGFR at admission and discharge. Under a physician’s direction, a laboratory technician used echocardiography to measure the left ventricular ejection fraction (LVEF) at admission in the physiology laboratory. Each test was performed using a standard protocol, and the LVEF was calculated using a modified Simpson method. All survey items were evaluated retrospectively from the electronic medical records by one physical therapist (PT) [12].
Patients in the present study participated in phase I CR in accordance with the Japanese Circulation Society guidelines for rehabilitation in patients with cardiovascular disease [2, 12]. After confirming the attending physician’s orders for each patient’s rest and assessing the patient’s subjective symptoms and vital signs, we initiated breathing practice for intubated patients, and early mobilization exercises consisting of getting out of bed (sitting on the edge of the bed), standing at bedside in the ICU, and then walking around the bed after drain removal [12]. If patients were able to walk in the ward corridor, resistance training such as sit-to-stand exercise and calf raises was conducted by a PT using the patient’s own weight, and then the patients participated in an aerobic exercise program assisted by PTs, nurses, and trainers. During the aerobic exercise, the patient was monitored with an ECG monitor [12].
ADL at discharge was assessed by the FIM, which consists of a motor area (motor FIM) that includes 13 items such as self-care, excretion control, movement, and exercise, and a cognitive domain (cognitive FIM) comprising five items that cover communication and social cognition [12]. The 7-point ordinal scale of the FIM indicates the burden of care, with 1 point indicating full support; 2 points, maximum support; 3 points, medium support; 4 points, minimal contact support; 5 points, supervision; 6 points, modified independence; and 7 points, complete independence. FIM scores ranged from a minimum of 18 points to a maximum of 126 points, whereas motor and cognitive FIM minimum scores were 13 and 5 points and maximum scores were 91 and 35 points, respectively [12, 15]. The FIM in this study was measured by any one of eight PTs in charge of the patients [12].
We classified ADL before hospitalization into the following three categories. (1) independent outdoor activity level, in which the subject could walk outdoors independently without any support; (2) assisted outdoor activity level, in which the subject could walk outdoors using a cane or buggy, etc.; and (3) indoor activity level, in which the subject could walk indoors with or without a cane. Information on activity levels was collected by the PT in charge directly from the patient, family, nurse, or care manager. Alternatively, this information was obtained by the primary doctor or nurse, by a medical social worker, who obtained it from the patient’s family, or by the care manager, who obtained it from the electronic medical record [11, 12].
The duration of postoperative intubation was measured from the time of
postoperative admission to the ICU until extubation. The duration of drain
placement was measured from the time of drain insertion at the end of the
surgical procedure until removal of the drain. After removal of the patient from
the ventilator and extubation, oxygen therapy was administered via nasal cannula
or oxygen mask to maintain an oxygen saturation above 95% [12, 16]. The duration
of oxygen administration during hospitalization included the time O
The period required for the patient’s weight after cardiac surgery to return to its preoperative level was calculated as the index of postoperative weight change.
Scheduled elective surgery was defined as scheduled surgery, scheduled surgery after hospitalization for acute HF or acute exacerbation of chronic HF was defined as semi-scheduled surgery, and surgery performed immediately after or on the day after emergency admission was defined as emergency surgery.
We performed statistical analysis after using the Shapiro-Wilk test to determine
whether the data were normally distributed. To evaluate patient characteristics
and clinical parameters in the CSA-AKI group and non-CSA-AKI group, we performed
the Mann-Whitney U test, t-test, Chi-square test, and Fisher’s exact
test, as appropriate, on the data from the two groups [12]. In addition, stepwise
multiple regression analysis was performed to evaluate the effects of CSA-AKI in
the early postoperative period on ADL at discharge. In the multiple regression
analysis, discharge motor FIM was used as the dependent variable, with the
independent variables selected on the basis of previous studies [6, 7, 8, 9, 10, 11, 12], or the
items with bivariate correlation with discharge motor FIM of p
A flowchart of the patients included in this study is shown in Fig. 1. Of the 562 consecutive cardiovascular surgery patients admitted to Yodogawa Christian Hospital from April 2015 to May 2020, the criteria for participation were patients who underwent CR after coronary artery bypass surgery and/or valve surgery by midline incision. Among the 231 patients who were prescribed phase I CR after cardiac surgery, we excluded 109 patients who met the exclusion criteria. Therefore, 122 patients were ultimately included in the final analysis and were divided into the CSA-AKI group (n = 84) and non-CSA-AKI group (n = 38).
Table 1 shows clinical characteristics of the patients in the non-CSA-AKI group (male 58.3%; median age 75.0 [70.5–80.0] years) and CSA-AKI group (male 60.5%; median age 76.5 [68.0–81.7] years) before surgery. Clinical characteristics and comorbidities in the CSA-AKI group, including complications of CKD and serum creatinine, were high, but hemoglobin, albumin, and eGFR values were low.
| Non-CSA-AKI | CSA-AKI | t or Z or |
p value | ||
| N = 84 | N = 38 | ||||
| Age, years (range) | 75.0 (70.5–80.0) | 76.5 (68.0–81.7) | 0.92 | ||
| Male, n (%) | 49 (58.3) | 23 (60.5) | 0.05 | 0.81 | |
| Body mass index, kg/m |
22.7 (20.3–25.2) | 23.8 (21.6–25.6) | 0.13** | 0.13 | |
| Preoperative LVEF | 63 (51.5–70.5) | 56 (41.0–66.0) | 0.14** | 0.11 | |
| Preoperative complications/Medical History | |||||
| Diabetes mellitus, n (%) | 32 (38.0) | 18 (47.4) | 0.93 | 0.33 | |
| Chronic kidney disease, n (%) | 29 (34.5) | 26 (68.4) | 12.14 | ||
| Dyslipidemia, n (%) | 43 (51.2) | 17 (44.7) | 0.43 | 0.51 | |
| Preoperative atrial fibrillation, n (%) | 17 (20.2) | 9 (23.7) | 0.18 | 0.66 | |
| Hypertension, n (%) | 53 (63.1) | 29 (76.3) | 2.08 | 0.15 | |
| Respiratory disease, n (%) | 4 (4.8) | 1 (2.6) | 0.50 | ||
| Orthopedic disease, n (%) | 13 (15.5) | 8 (21.1) | 0.57 | 0.45 | |
| Laboratory data before surgery | |||||
| CRP (mg/dL) | 0.14 (0.06–0.52) | 0.16 (0.08–0.30) | 0.01** | 0.91 | |
| Hemoglobin (g/dL) | 12.2 |
11.5 |
2.01* | 0.04 | |
| Albumin (mg/dL) | 3.8 |
3.5 |
2.81* | 0.005 | |
| Creatinine (mg/dL) | 0.9 (0.7–1.1) | 1.1 (0.9–1.6) | 0.28 | 0.001 | |
| eGFR (mL/min/1.73 m |
56.7 |
43.7 |
3.54* | 0.004 | |
| CSA-AKI, cardiac surgery-associated acute kidney injury; LVEF, left
ventricular ejection fraction; CRP, C-reactive protein; eGFR, estimated
glomerular filtration rate. All values are presented as number (%), mean | |||||
Table 2 shows POAF within one week after surgery, laboratory data at one week
after surgery, and intraoperative and surgery-related factors. Compared to the
non-CSA-AKI group, the CSA-AKI group had significantly higher serum creatinine
values (p
| Non-CSA-AKI | CSA-AKI | t or Z or |
p value | ||
| N = 84 | N = 38 | ||||
| POAF within one week, n (%) | 23 (27.4) | 20 (52.6) | 10.0 | 0.001 | |
| Laboratory data at one week after surgery | |||||
| CRP (mg/dL) | 3.9 (3.1–6.1) | 6.0 (4.1–7.8) | 0.23** | 0.009 | |
| Hemoglobin (g/dL) | 10.3 (9.6–11.1) | 9.8 (8.9–10.7) | 0.19** | 0.02 | |
| Albumin (mg/dL) | 3.0 (2.8–3.2) | 2.9 (2.7–3.2) | 0.01** | 0.84 | |
| Highest creatinine level (mg/dL) | 0.9 (0.7–1.1) | 1.6 (1.3–2.8) | 0.63** | ||
| Lowest eGFR level (mL/min/1.73 m |
55.2 |
27.5 |
9.95* | ||
| Scheduled surgery/Semi emergency/Emergency (n, %) | 73/9/2 (86.9/10.7/2.4) | 27/11/0 (71.0/28.9/0) | 7.01 | 0.03 | |
| On pump, n (%) | 80 (95) | 38 (100) | 0.21 | ||
| Duration of surgery (min) | 319 (266.5–379.0) | 349 (292.7–417.0) | 0.21** | 0.01 | |
| Duration of extracorporeal circulation (min) | 157 (123.5–192.0) | 167 (144.7–241.7) | 0.16** | 0.06 | |
| Bleeding volume (ml) | 144 (106–217.5) | 219 (124–306.5) | 0.18** | 0.04 | |
| Types of surgery, n (%) | |||||
| Single CABG/Single Valve/Composite valve/CABG + Valve/CABG + Valve + Vascular replacement, n (%) | 27/27/19/4/7 (32.1/32.1/22.6/4.7/8.3) | 11/14/4/7/2 (28.9/36.8/10.5/18.4/5.3) | 0.10 | ||
| Intravenous medications | |||||
| Furosemide, n (%) | 23 (27.4) | 18 (47.4) | 4.68 | 0.03 | |
| hANP, n (%) | 16 (19.4) | 19 (50.0) | 12.25 | ||
| Catecholamine, n (%) | 14 (16.7) | 17 (44.7) | 10.87 | ||
| CSA-AKI, cardiac surgery-associated acute kidney injury; eGFR, estimated glomerular filtration rate; POAF, postoperative atrial
fibrillation; hANP, human atrial natriuretic peptide. All measurements are
presented as number (%), mean | |||||
Table 3 shows activity levels before hospitalization, rehabilitation progress,
postoperative length of stay, FIM scores, and home reversion ratio.
Activity level before hospitalization was significantly lower in the CSA-AKI
group (p = 0.01). Regarding rehabilitation progress, start day of
sitting on the edge of the bed and start day of walking around the bed were
significantly delayed in the CSA-AKI group (sitting: p = 0.01, walking:
p
| Non-CSA-AKI | CSA-AKI | t or Z or |
p value | ||
| N = 84 | N = 38 | ||||
| FIM at discharge (points) | 125 (120–125) | 116 (110.5–124.7) | 0.37** | ||
| Motor FIM score | 90 (85.0–90.0) | 83 (76.2–89.7) | 0.37** | ||
| Cognitive FIM score | 35 (35–35) | 35 (35–35) | 0.20** | 0.02 | |
| Activity levels before hospitalization (n, %) (Outdoor independence/Outdoor assistance/Indoor) | 72/10/2 (85.7/11.9/2.4) | 25/8/5 (65.8/21.1/13.2) | 0.01 | ||
| Postoperative rehabilitation progress | |||||
| Start day of sitting on the edge of the bed (days) | 1 (1–2) | 1 (1–3) | 0.22** | 0.01 | |
| Start day of standing at the bedside (days) | 2 (1–3) | 2 (1–3.7) | 0.12** | 0.15 | |
| Start day of walking around the bed (days) | 4 (2.0–5.0) | 4 (3.25–6.0) | 0.27** | ||
| Day of reacquisition of 50 m walking (days) | 4 (4.0–6.0) | 7.5 (4.2–11.0) | 0.32** | ||
| Postoperative ventilator intubation period (h) | 5 (4.0–9.0) | 9 (5.2–13.7) | 0.24** | 0.007 | |
| Drain insertion period (days) | 2 (2.0–3.0) | 3 (2.0–5.0) | 0.15** | 0.09 | |
| Oxygen administration period (days) | 6 (3.5–8.5) | 8 (5.0–13.7) | 0.24** | 0.007 | |
| Number of days to return to preoperative weight | 5 (3.0–9.0) | 7.5 (5–14.7) | 0.23** | 0.008 | |
| Length of ICU stay (days) | 2 (1.0–2.0) | 2 (2.0–3.0) | 0.29** | 0.001 | |
| Length of stay after surgery (days) | 17 (11.0–25.0) | 26 (19.2–31.7) | 0.35** | ||
| Home reversion ratio (%) | 83 (98.8) | 37 (97.3) | 0.52 | ||
| ADL, activities of daily living; FIM, Functional Independence Measure; ICU,
intensive care unit. All values are presented as number (%), mean | |||||
Table 4 shows the results of the univariate and multiple regression analysis for
predicting discharge motor FIM. Among the clinical and laboratory-related
factors, rehabilitation progression-related factors, and surgery-related factors
in Tables 1,2,3 of this study, based on previous studies, the variables that had
a bivariate correlation (p
| Bivariate correlation | Stepwise multiple regression | |||
| Pearson r (Spearman r) | p value | p value | ||
| Age | –0.32 | 0.001 | ||
| CSA-AKI | –0.42 | –0.24 | 0.009 | |
| eGFR before surgery | 0.22 | 0.01 | ||
| Lowest eGFR within one week | 0.26 | 0.003 | ||
| POAF within one week | –0.29 | –0.17 | 0.01 | |
| Postoperative ventilator intubation period | –0.29 | |||
| Start day of walking around the bed | –0.47 | –0.30 | ||
| Activity levels before hospitalization | –0.45 | –0.35 | ||
| R |
0.56 | |||
| Adjusted R |
0.52 | |||
| FIM, Functional Independence Measure; CSA-AKI, cardiac surgery-associated acute kidney injury; eGFR, estimated glomerular filtration rate; POAF, postoperative atrial fibrillation. | ||||
This is the first study, to our knowledge, to report a relationship between CSA-AKI and ADL at discharge in elderly cardiac surgery patients. Even after adjusting for confounding factors such as age, preoperative eGFR, lowest eGFR within one week after surgery, and postoperative period of intubation and ventilator use, the factors of CSA-AKI, start day of walking, POAF, and activity levels before hospitalization were found to be potentially predictive of ADL decline at discharge.
In our previous study, we clarified the factors that delay early ambulation in elderly HF patients to be their walking level before hospitalization and renal function at hospital admission [11]. Furthermore, we also clarified that the factors of ADL decline at discharge of elderly HF patients are walking level before hospitalization, start day of standing, eGFR at admission, and worsening renal function during hospitalization [12]. In the present study, however, CSA-AKI was an independent factor for ADL at discharge in elderly cardiac surgery patients, whereas preoperative and postoperative renal function was not. This supports the study of Saitoh et al. [8] who reported that in their non-CSA-AKI group, postoperative CR progression was delayed in accordance with the severity of preoperative renal dysfunction, but in their CSA-AKI group, regardless of preoperative renal dysfunction, postoperative CR progression was delayed compared to that in the non-CSA-AKI group. In elderly patients with HF, the effect of renal function at admission was one of the factors influencing ADL at discharge, but the effects of surgery and CSA-AKI are greater in elderly cardiac surgery patients, which may offset the effects of preoperative renal function [8, 9]. Furthermore, the present study revealed that CSA-AKI was a stronger influential factor than renal function within 1 week after surgery, whereas activity levels before hospitalization and delay in CR progress were predictive factors of ADL at discharge. This indicates that a decrease in preoperative activity levels reflects the degree of patient frailty and that patients with advanced frailty preoperatively may experience delayed postoperative progression of CR and may also have lower ADL at discharge (Fig. 2).
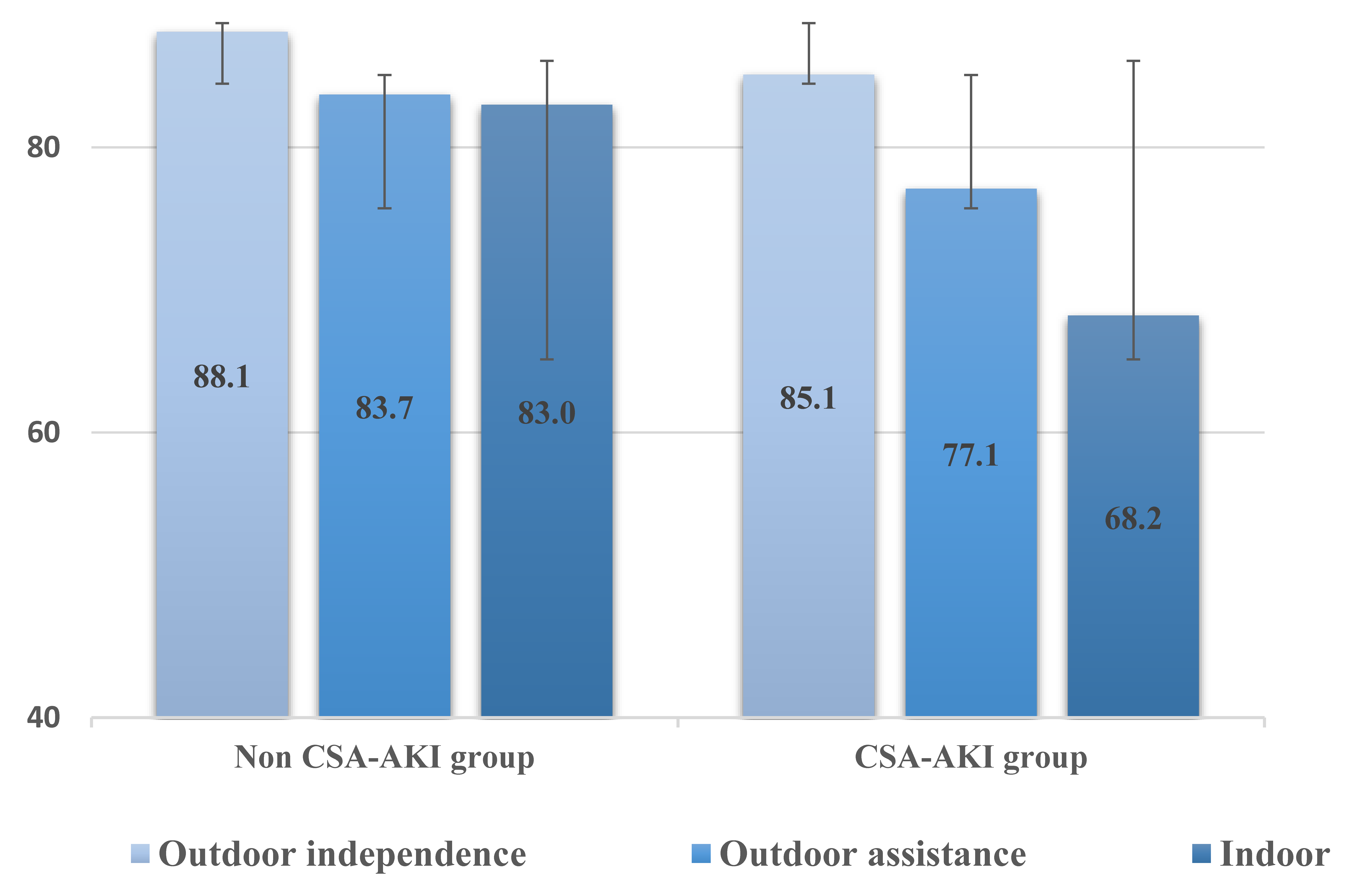 Fig. 2.
Fig. 2.Comparison of discharge FIM by pre-hospital activity level in the Non-CSA-AKI and CSA-AKI groups.
Previous studies have reported that early postoperative POAF is a factor in delaying postoperative progression of CR [10]. In a study by Echahidi et al. [17], POAF tended to occur within 2–4 days after the surgery, and 94% of patients developed this dysrhythmia before the end of postoperative day 6. The onset of POAF in the present study occurred within 1 week after surgery, and it is possible that the appearance of POAF delayed the start of walking. In addition, the predictive factor for ADL decline at discharge was the start day of walking but not the day when the patient could walk 50 m. Previous studies of muscle weakness and exercise intolerance due to bed rest for one day reported that muscle strength was reduced by 1–4% [18] and exercise tolerance by 0.9% [19]. Thus, an early postoperative start day of walking may prevent reductions in muscle strength and exercise capacity needed to re-acquire the preoperative level of ADL.
As a result of this study, 31% of patients developed CSA-AKI, supporting the findings of previous studies [3, 4]. The development of CSA-AKI has been reported to have a variety of causes, including the use of cardiopulmonary bypass, low cardiac output, hypoperfusion/reperfusion injury associated with cardiac surgery, oxidative stress, and increased systemic inflammatory response [3, 4]. Furthermore, postoperative inflammatory reaction, increased oxidative stress, and mitochondrial dysfunction are reported conditions that can easily cause POAF [20, 21, 25]. The present results showed that the CSA-AKI group had a significantly higher value of CRP at 1 week after surgery and higher incidence of POAF within 1 week after surgery. Moreover, in the CSA-AKI group, the postoperative oxygenation period and the number of days to return to preoperative weight were significantly extended. The reason for this is that as postoperative inflammation increases vascular permeability, fluid moves to the third space and intravascular dehydration can easily occur [22]. Therefore, fluid management such as intravenous infusion is performed to maintain cardiac output and tissue perfusion, resulting in an increase in body fluid content and an increase in postoperative body weight of 2.5 to 5% [8, 23]. In particular, in the CSA-AKI group, a decrease in postoperative diuretic reaction was also present, which resulted in a positive fluid balance [8, 9]. To correct these symptoms, the CSA-AKI group received increased administration of intravenous furosemide and human atrial natriuretic peptide and catecholamine preparations 1 week after surgery. Excessive retention of body fluid for several days after surgery can cause HF symptoms such as fatigue, dyspnea, orthopnea, and nighttime paroxysmal dyspnea, in addition to a gain in body weight [24]. Therefore, in the CSA-AKI group, it can be inferred that the start day of walking after surgery was delayed due to the appearance of both POAF and symptoms of HF.
Interestingly, compared to the non-CSA-AKI group, the CSA-AKI group may have experienced rapid muscle weakening and atrophy, even though the period of bed rest was the same in both groups. A recent report found that systemic inflammation from cardiac surgery increases oxidative stress and causes cardiac mitochondrial dysfunction [25]. Oxidative stress and renal mitochondrial dysfunction were shown to play a major role in all stages of disease development during uremia followed by AKI, chronic kidney disease, and their associated comorbidities [26]. Systemic inflammation and excessive oxidative stress cause various types of cell damage and dysfunction of skeletal muscle mitochondria [27, 28]. Furthermore, dysfunctional skeletal muscle mitochondria cause rapid muscle weakening and muscle atrophy [29].
From the above, it is possible that in the CSA-AKI group, oxidative stress and mitochondrial dysfunction, in addition to deconditioning due to the delayed start day of walking, contributed to muscle weakness and muscle atrophy in the early postoperative period. As a result, ADL at the time of discharge was significantly reduced in the CSA-AKI group.
As a result of this study, the Total FIM score at patient discharge was 124
(118–125). Meanwhile, Sasanuma et al. [7] reported that the total FIM
score at discharge of cardiac surgery patients (n = 346; male: 63.6%; mean age:
71.1
To prevent the decline of ADL on discharge in elderly cardiac surgery patients, it is important that patients not develop CSA-AKI. However, CSA-AKI carries various risk factors including intraoperative risk [3, 4], and as a result, CSA-AKI can often develop. Sasanuma et al. [7] reported that the cut-off value of the total FIM score at discharge, which predicts the discharge of patients after cardiovascular surgery to their homes, is 115/116 points for motor and cognition together. In the present study, the total FIM score at discharge in the CSA-AKI group was 116 (110.5–124.7) points, which was the same as the cutoff value reported by Sasanuma et al. [7]. The LOS after surgery was 17 (11.0–25.0) days in the non-CSA-AKI group but was 26 (19.2–31.7) days in the CSA-AKI group. The rates of discharge to home between the two groups were 98.9% and 97.3%, respectively, which were not significantly different. This means that most patients can be discharged to home if there are no complications such as postoperative cerebral infarction. Also, compared to the non-CSA-AKI group, the CSA-AKI group took an average of 8 days more to reacquire the minimum ADL needed for home discharge. From these results, it is important for postoperative CR in patients with CSA-AKI to progress individually according to the patient’s condition taking into account the appearance of POAF and the increased risk for HF. In addition, to aim for discharge without a lowering of the ADL at discharge, it is important to perform preoperative CR at home and increase the patient’s activity level before hospitalization while paying attention to symptoms of HF.
There were several limitations in the present study. This was a single-center, retrospective cohort study, and a small sample size. We did not investigate that leg muscle strength, muscle mass, and physical function before surgery or at discharge. The details of fluid management during and after surgery were not evaluated, nor did we investigate urine output according to the KDIGO AKI definition. Differences in postoperative CR start dates between the two groups were also not evaluated. The duration of POAF was not evaluated accurately. The amount of change in FIM from before surgery to discharge was not investigated. It is difficult to compare preoperative FIM because some patients have undergone preoperative rehabilitation and some have not. We did not investigate the daily dose of diuretics and catecholamine. As a result, the number of patients with CKD in the CSA-AKI group was higher than that in the non-CSA-AKI group, and the effects of preoperative CKD and renal dysfunction were not adjusted for. All study patients underwent postoperative CR, so they cannot be compared with patients who did not undergo postoperative CR. This study included a mixture of patients with coronary artery bypass and valve surgery and patients undergoing on-pump and off-pump surgery, and thus it could not be narrowed down to studying patients undergoing one type of surgery. We consider it a future task to perform propensity score matching or to investigate the effect of CSA-AKI on ADL at discharge by separating patients according to preoperative renal function. In addition, we believe that continuous follow-up of CSA-AKI over the medium to long term, and not just during hospitalization, will be necessary in the future.
CSA-AKI, including the effects of preoperative renal dysfunction in elderly cardiac surgery patients, was a factor affecting ADL at discharge as were activity levels before hospitalization, start day of walking around the bed, and POAF. It will be a future task to investigate the effect of CSA-AKI on ADL and physical function at discharge after excluding the effect of pre-hospital renal dysfunction.
Conceptualization—IK and KPI; formal analysis—IK; investigation, IK, NK; data curation—IK; writing—original draft preparation—IK, AO, MO and MK; writing—review and editing—KPI, HM, DM and PHB; supervision—KPI, HM and PHB; project administration—KPI and SS; funding acquisition—KPI. All authors approved the manuscript for submission.
The protocol of the present study was approved by the institutional review board for ethics at Yodogawa Christian Hospital (approval no. 2020-040). Written informed consent was obtained from each patient.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Kazuhiro P. Izawa is serving as one of the Guest editors of this journal. We declare that Kazuhiro P. Izawa had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Federico Ronco.
