Academic Editor: Efstratios Georgakarakos
Aortic aneurysm is an enlargement of the aorta with a loss of the arterial wall parallelism. There are several possible causes concerning etiology, one of which is the postsurgical presence of a patent distal false lumen. An aortic aneurysm is mainly seen after a surgery for type A aortic dissection and it represents an important late complication. Even more, in some cases the abdominal aortic aneurysm could be complicated by a patent distal false lumen, which makes it challenging for further treatment. We present a case of a 44-year-old male patient with Marfan syndrome and the history of a surgical repair of type A aortic dissection. He was diagnosed with a large abdominal aortic aneurysm and patent distal false lumen. In this report, we describe our technique and the outcome for endovascular fenestration of the persistent intimal flap and the stent-graft implantation for abdominal aortic aneurysm isolation after the surgery for aortic dissection type A. Our technique presents a novelty approach to patients with abdominal aortic aneurysm, complicated with a patent distal false lumen.
Aortic aneurysm is an enlargement of the aorta with a loss of the arterial wall parallelism, and this pathology is the second most frequent disease of the aorta after atherosclerosis [1]. Depending on the anatomical location of the aneurysm, we divide them into thoracic aortic aneurysms (TAA) and abdominal aortic aneurysms (AAA). Many etiology factors can contribute to aortic aneurysm formation, like atherosclerosis, high blood pressure, trauma, or past surgical intervention of the aorta. Surgery for aortic dissections, mainly dissections comprising the ascending aorta, could be a risk factor for the dilatation of the aorta and a late formation of an aortic aneurysm. Aortic dissection is a life-threatening medical emergency, and it is the most common acute aortic condition requiring urgent surgical management. That condition is characterized by separation in the intima-media space, where blood is entering between the aorta layers and forming one true and one false lumen. Classification is based on the anatomical localization of the dissection. The modern, University of Stanford Classification, is dividing aortic dissections in type A dissection (TAAD) involving the ascending aorta and the aortic arch, and Stanford type B dissection (TBAD) involving the descending aorta, distal to the origin of the left subclavian artery [1, 2]. The original, DeBakey Classification, defines three types of aortic dissection. Type I where the intimal tear is in the ascending aorta and extends distally compromising the whole aorta. Aortic valve insufficiency is frequently presented with the dissection. In type II the dissection is limited to the ascending aorta and in type III the dissection begins distally to the left subclavian artery, compromising only the descending aorta [3, 4]. Surgery is a treatment of choice for TAAD aiming to secure the primary entry tear, to prevent from a rupture of the ascending aorta and subsequent death [1]. The success rate of the operations for type A aortic dissection is high. Still, because of the extent of the disease into the entire aorta, most of the patients end up having a patent distal false lumen [1, 5]. The patent distal false lumen could potentially lead to an aneurysmal formation and complications, like ruptures, which are the main cause of late death after surgical intervention [5]. Patients with an abdominal aortic aneurysm are suitable for an endovascular aortic aneurysm repair. If the aneurysm is complicated by a patent distal false lumen, a surgical repair is the main option. On the other hand, surgery of the descending aorta carries a high operative risk and thus these patients will vastly benefit from a possible endovascular approach [1]. In this case report, we present a novelty endovascular technique for treating patients with AAA and patent distal false lumen.
A 44-year-old male patient was presented to our hospital with complaints of
chronic abdominal and flank pain, which had worsened significantly during the
days before the admission. His past medical history was remarkable with a
surgical replacement of ascending aorta, with a prosthesis (Unigraft 28, Braun),
and aortic valve replacement with valve prosthesis (Sorin Bicarbon 27) because of
TAAD. The above was performed 17 years before the admission and there is no
evidence of AAA back then. The patient risk factors had genetically proven Marfan
syndrome, hypertension, dyslipidemia, hyperuricemia and diabetes type I. Upon
presentation, the patient was hemodynamically stable, with a regular heart rate
of 68 bpm, blood pressure of 120/70 and no difference between the two arms. A
Duplex ultrasonography (USG) examination was performed, during which a suspicion
of abdominal aortic aneurysm was raised. According to the recommendations we did
a Computed tomography aortography (CTA), which is the “gold standard” for
diagnosing aortic aneurysms [1]. The examination revealed a persisting aortic
dissection flap, starting from the aortic arch and extending to the descending
and abdominal aorta. The infrarenal part of the abdominal aorta had an aortic
aneurysm with dimensions of 61
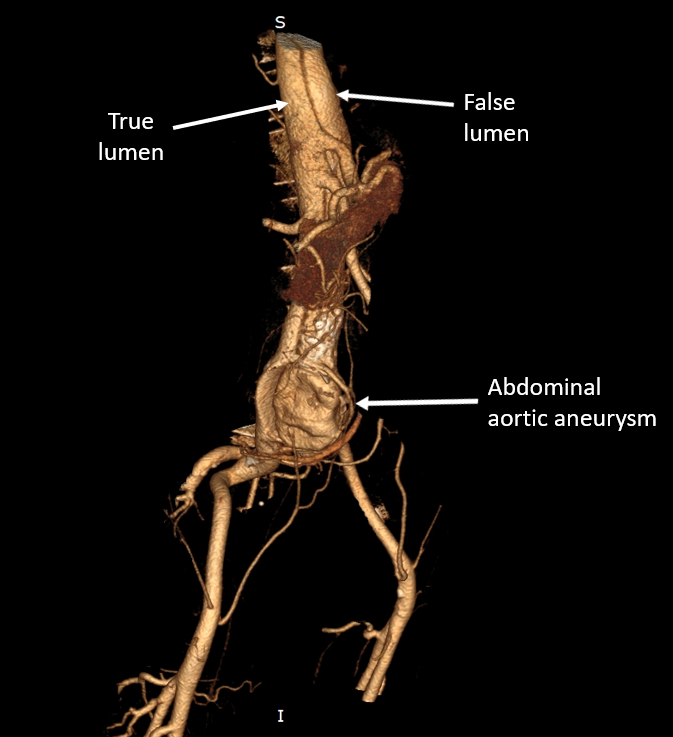 Fig. 1.
Fig. 1.Initial Computed tomography (CT). The true and the false lumen
are clearly distinguished and the large abdominal aortic aneurysm (AAA), sizing
60
The procedure was performed totally with the help percutaneous intervention under a deep awake sedation, local anesthesia with three vascular access sites: 12-Fr right femoral access, 14-Fr left femoral access, for the stent-graft implantations and 5-Fr right radial access for angiographic Pigtail catheter insertion. After placing the JR catheters in the “true” and “false” lumen on the level of the thoracic aorta, we crossed from the false to the true lumen with a hydrophilic Whisper Extra Support guidewire 0.014” (Abbot) with successful introduction of the wire through an intimal flap entry above the renal vessels. The wire was caught with a lasso (Amplatz Goose Neck, EV3) and externalized from the right femoral approach connected to the true lumen. With the help of two parallel supportive microcatheters (Trailblazer, Medtronic, USA) in the two lumens, and with saw-like movements of the Whisper wire, with tension from proximal to distal, a long fenestration (cutting) of the intima on the level of the infrarenal aorta was performed (Fig. 2). After successfully creating a single lumen of the aorta in this segment, a stent-graft Endurant II 36/16/145 mm (Medtronic, MN, USA) was implanted. The oversizing degree of the Endurant stent-graft, implanted in the infrarenal neck wall of the AAA was 25% in order to better sealing. Furthermore, two iliac grafts were implanted—Endurant II 16/20/124 mm (Medtronic, MN, USA)—into the right iliac artery and Endurant II 16/16/82 mm (Medtronic, MN, USA) into the left iliac artery. The sealing effect on the level of the new-formatted single sub-renal lumen was excellent and the aneurysm of the abdominal aorta was successfully isolated (Fig. 3). The left percutaneous femoral approach was closed by Manta closure device 14 Fr (Teleflex, PA, USA) and the right femoral access was closed by Angio-seal 8 Fr (St Jude Medical, St Paul, MN, USA). The CTA after the procedure confirmed a well-isolated aneurysm of the abdominal aorta, without changes in size (Fig. 4). The subsequent control CTAs one month, six months and one-year post-procedure demonstrated full restoration of the aortic flow, isolation of the aneurysm and all the stents implanted were patent and with optimal apposition (Fig. 5).
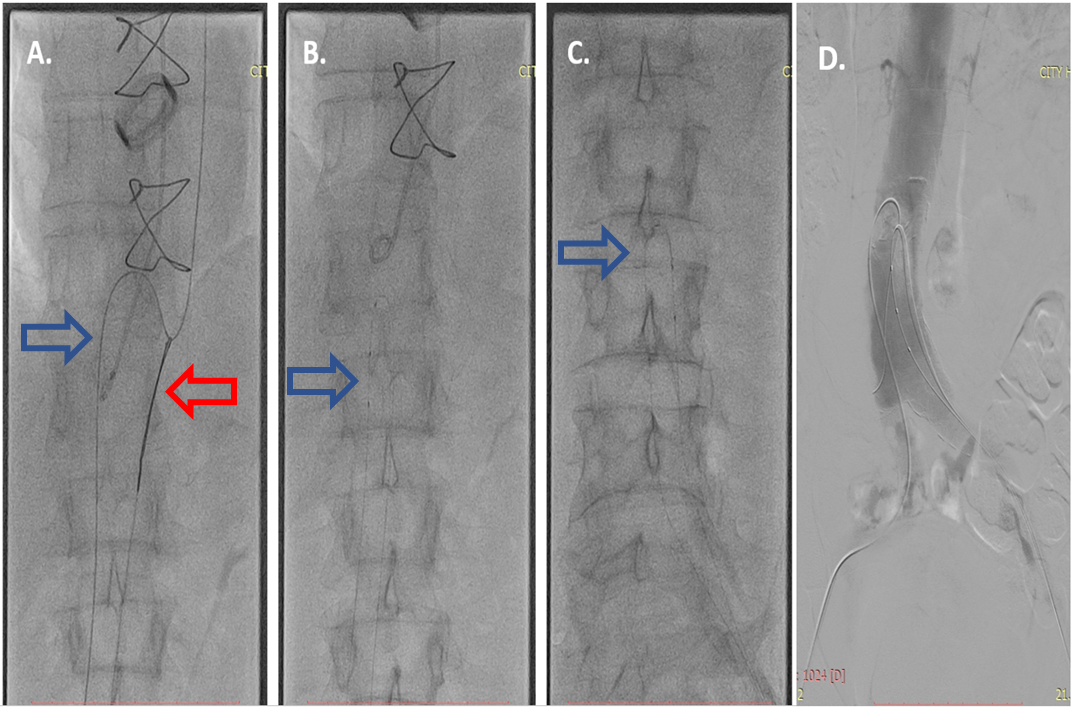 Fig. 2.
Fig. 2.Fenestration of the intima. (A) The hydrophilic Whisper Extra Support guidewire 0.014” (Abbot) in the true lumen (blue arrow) was caught with a lasso (Amplatz Goose Neck, EV3) (red arrow) and was passed from the true to the false lumen. (B) Fenestration of the proximal part of the intima with the help of microcatheters in the true and false lumen (blue arrow). (C) Fenestration of the distal part of the intima (blue arrow) indicating the microcatheters. (D) Showing the successfully created single lumen of the abdominal aorta.
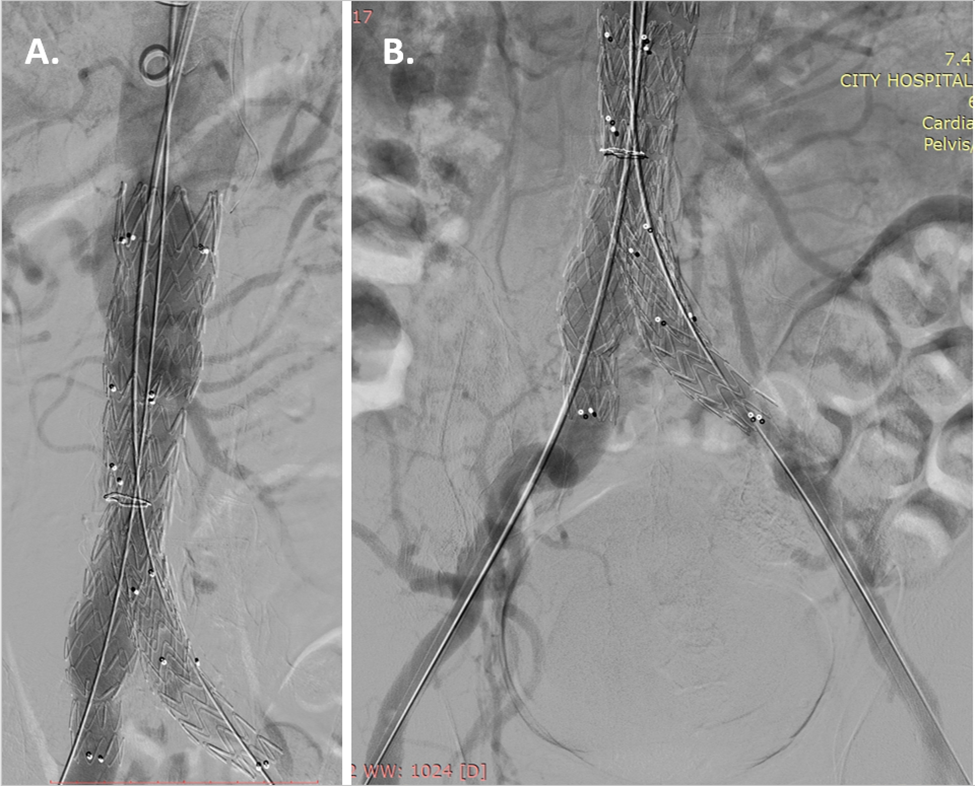 Fig. 3.
Fig. 3.Stent-graft implantation. (A) Stent- graft Endurant II 36/16/145 mm (Medtronic, MN, USA) implantation in the abdominal aorta and (B) extension into the two iliac arteries Endurant II 16/20/124 mm (Medtronic, MN, USA) into the right iliac artery and Endurant II 16/16/82 mm (Medtronic, MN, USA) into the left iliac artery, were implanted.
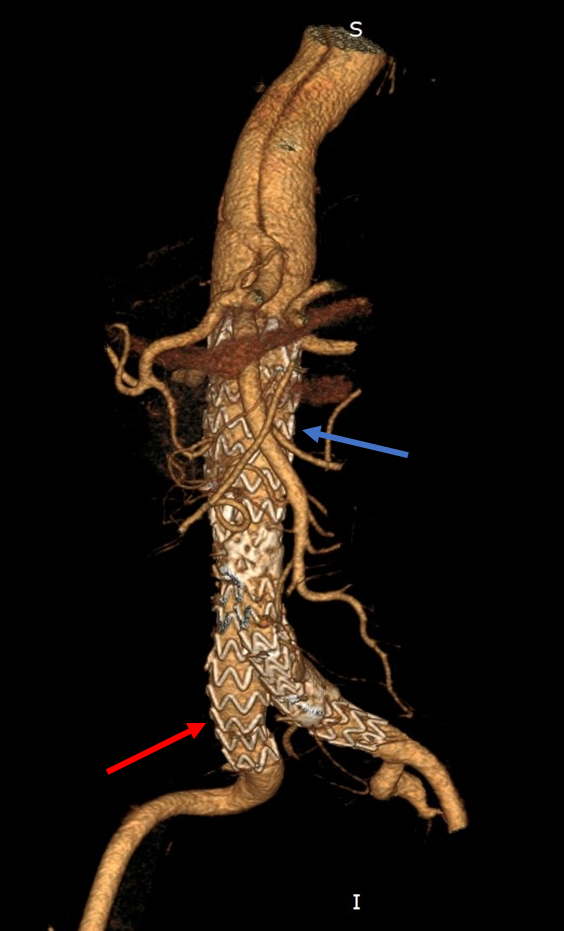 Fig. 4.
Fig. 4.Final result after stent-graft implantation. Stent-graft implanted in the abdominal aorta (blue arrow) and extensions into the iliac arteries (red arrow) with optimal position. Restoration of normal aortic flow and immediate isolation of the abdominal aortic aneurysm.
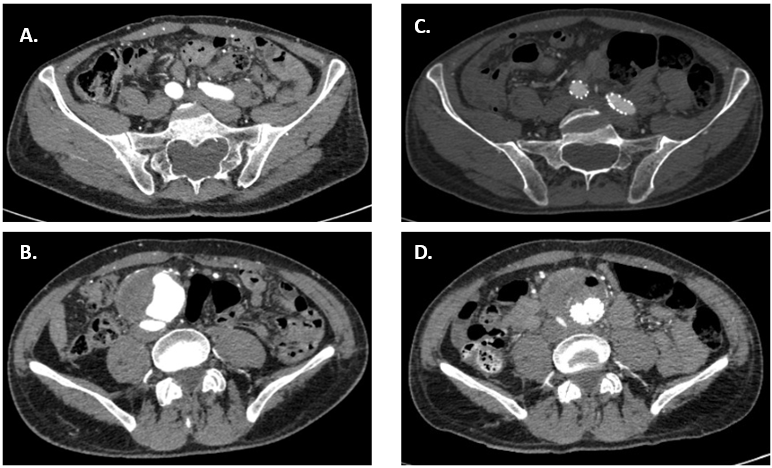 Fig. 5.
Fig. 5.Pre and Postprocedural CT of the aorta and iliac arteries. On the left (A,B), axial views of CTA at aneurysm site (B) and iliac arteries (A), preoperative. On the right (C,D), axial views of the CTA at aneurysm site (D) and iliac arteries (C), postoperative.
Despite the improvements in the surgery techniques, preoperative and
postoperative management of the patients for aortic dissection type A, the
complications rate and mortality are still high. Late complications and
reoperations after surgical repairs of acute type A aortic dissection are
relatively common, ranging from 65% to 85% at 10 years [8]. The complications
are mainly because of the high incidences of patent distal false lumen, ranging
between 75% and 100% [5, 6]. The growth rate of the descending abdominal aorta
after repairs of acute type A aortic dissection is 0.8 mm/year, and an aortic
size greater than 4 cm or less than 4 cm with the presence of a patent distal
false lumen are factors responsible for a greater increase in the diameter of the
aorta [7]. According to Gariboldi et al. [5], Marfan syndrome is a
significant risk factor for the persistence of a false lumen and distal
reoperations. Other predictive factors for residual patent false lumen after
surgery for type A aortic dissection include male sex, younger age, and increased
initial aortic diameter [8]. An aortic diameter greater than 40 mm is an
independent predictor for reoperation. Since our patient had an infrarenal
aneurysm sizing 61
In our case, the patient presented with a large abdominal aortic aneurysm and patent distal false lumen, which was properly treated by fenestration of the false lumen, such creating a single lumen on the level of the proximal landing zone, followed by stent-graft implantation. Intraluminal fenestration was firstly described as a technique used for managing patients with malperfusion syndrome [9]. Even though this technique is not that widely used and until now it was mainly applicable for the treatment of aneurysms developing after chronic type B aortic dissection, in our case, it proved to be a feasible and effective method for dealing with patent distal false lumen and abdominal aortic aneurysm after surgery [10]. Moreover, since patients with Marfan syndrome need multiple intervention in their life-span, we believe that this endovascular approach is more suitable, as it significantly reduces perioperative morbidity and mortality, compared with open surgical repair [1, 6]. In this report, we add to the existing literature, presenting endovascular fenestration of the intima and stent-graft implantation in the settings of a patent distal false lumen with abdominal aortic aneurysm, formed late after surgery for aortic dissection type A. The main advantage of this technique in our case is that it converts the two lumens, separated by the persistent intimal flap into a single one avoiding the possibilities of type 1 endoleak through the persistent false lumen. The formation of a single lumen allows us to implant a stent-graft without compromising the visceral vessel flow [9, 10]. The minimalistic strategy including awake anesthesia, fully endovascular approach, and the closure devices used, all lead to fewer complications in the post-procedural period and more rapid recovery of the patient.
Patent false lumen after surgery for type A aortic dissections is commonly observed, especially in male patients with Marfan syndrome and initially dilated aorta. These patients should be carefully followed and closely examined because they are at risk of an aneurysm formation. Each patient with patent distal false lumen and evident AAA should be managed individually and with great care. In our case, the patient was presented to us for the first time with an already developed abdominal aortic aneurysm and a patent distal false lumen. Approach, combining cutting fenestration of the intimal flap and stent-graft implantation in the newly formatted single lumen was adopted and proved to be the effective and reliable method of choice in this case.
SV, IP, ZS, LY designed the case report study and contributed to the manuscript conceptualization, preparation and draft of the manuscript and writing of the case report. SV contributed to library searches and assembling relevant literature, data acquisition, data interpretation, writing of the case report and were the main contributors to the design of the figures. All authors contributed to the critical review and final approval of the manuscript.
The study fulfills the ethical requirements of the Declaration of Helsinki, with regards to human subjects’ research. Informed consent was obtained from all individual participants included in the study. Patient consent for publication — Obtained.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
