Academic Editors: John Lynn Jefferies and Karim Bendjelid
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is used as
mechanical circulatory support in cardiogenic shock (CS). It restores peripheral
perfusion, at the expense of increased left ventricle (LV) afterload. In this
setting, Impella can be used as direct unloading strategy. Aim of this
meta-analysis was to investigate efficacy and safety of LV unloading with Impella
during ECMO in CS. A systematic search on Medline, Scopus and Cochrane Library
was performed using as combination of keywords: extracorporeal membrane
oxygenation, Impella, percutaneous micro axial pump, ECPELLA, cardiogenic shock.
We aimed to include studies, which compared the use of ECMO with and without
Impella (ECPELLA vs. ECMO). Primary endpoint was short-term all-cause mortality;
secondary endpoints included major bleeding, haemolysis, need for renal
replacement therapy (RRT) and cerebrovascular accident (CVA). Five studies met
the inclusion criteria, with a total population of 972 patients. The ECPELLA
cohort showed improved survival compared to the control group (RR (Risk Ratio):
0.86; 95% CI (Confidence Interval): 0.76, 0.96; p = 0.009). When
including in the analysis only studies with homogeneous comparator groups, LV
unloading with Impella remained associated with significant reduction in
mortality (RR: 0.85; 95% CI: 0.75, 0.97; p = 0.01). Haemolysis (RR:
1.70; 95% CI: 1.35, 2.15; p
Cardiogenic shock (CS) remains a major cause of in-hospital mortality when caused by acute myocardial infarction [1, 2]. In addition, epidemiological reviews of contemporary coronary care units revealed that up to 70% of cases of CS are secondary to conditions other than acute coronary syndrome (ACS), such as non-ischemic and chronic ischemic cardiomyopathies, valvular dysfunction and shock sustained by arrhythmias [3]. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is widely used as mechanical circulatory support (MCS) in refractory CS and peri-arrest scenarios leading to maintained peripheral perfusion and restored end-organ function [4]. Despite its beneficial effect on organ perfusion, VA-ECMO increases left ventricular (LV) afterload, therefore potentially damaging further an already failing myocardium. Several strategies have been used to decrease LV afterload or directly unload the left heart: surgical procedures (atrial septostomy, direct LV, left atrial or pulmonary artery venting), use of the intra-aortic balloon pump (IABP) and use of percutaneous left ventricular assist devices (pLVAD). One of the commonly used pLVADs is the micro axial pump, Impella (Abiomed, Danvers, MA, USA).
In the last few years, the use of Impella has markedly increased in the setting of ACS complicated by cardiogenic shock, with promising results [5]. However, there is still limited data regarding the use of Impella in patients with CS during ECMO support. Few recent meta-analyses tried to explore this setting, however they included heterogeneous populations and support strategies [6, 7].
The aim of this meta-analysis was to investigate the impact on clinical outcomes of LV unloading with Impella during VA-ECMO vs. isolated ECMO, selecting homogeneous populations in order to provide more reliable results.
This systematic review was conducted in accordance to the PRISMA guidance (Preferred Reported Items for Systematic Reviews and Meta-Analysis) [8, 9]. We performed a systematic search of the MEDLINE, Cochrane Central Register of Controlled Trials and Scopus databases from January 2000 to September 2021 for all trials comparing ECMO unloading strategies. Our search strings included ‘extracorporeal membrane oxygenation’, ‘Impella’, ‘percutaneous micro axial pump’, ‘ECPELLA’ (concomitant use of ECMO and Impella), ‘cardiogenic shock’ (Supplementary Table 1). The resulting citations were imported into Endnote X7 and duplicates removed by manual inspection.
We aimed to include randomised controlled trials, propensity matched studies and observational case-control studies with no differences in baseline characteristics, which compared the use of ECMO with and without Impella (ECPELLA vs. ECMO). Case reports or series, systematic reviews and studies with unmatched populations or no uniform patient characteristics in the compared groups were excluded from the analysis.
Primary endpoint was short-term all-cause mortality (defined as mortality during hospital stay or within 30 days); secondary endpoints included major bleeding, haemolysis (defined as increase of lactate dehydrogenase serum level above 1000 U/L or greater than two and one-half times the upper limits of normal range), need for renal replacement therapy (RRT) and cerebrovascular accident.
Included studies were assessed using the “The Risk Of Bias In Non-randomized Studies of Interventions” (ROBINS-I) tool (Supplementary Material 2) [10].
Outcomes were analysed on an intention to treat basis. The risk ratios were
computed with the Mantel-Haenszel model using the random and fixed effect models,
as appropriate. Heterogeneity among the selected studies was assessed using the
I
Of a total of 1897 studies identified in the original search only 5 met the inclusion criteria [11, 12, 13, 14, 15] (Fig. 1). Funnel plot for asymmetry was used to look for publication bias the studies included (Fig. 2). There were no available randomised clinical trials. The characteristics of the included studies are described in Table 1 (Ref. [11, 12, 13, 14, 15]). As per our study design, we selected only propensity matched retrospective studies (with a 2:1 and a 1:1 ratio) [11, 13] or retrospective case-control trials with similar baseline characteristics [12, 14, 15]. Where matched and unmatched data were described, we analysed only figures from propensity matched cohorts.
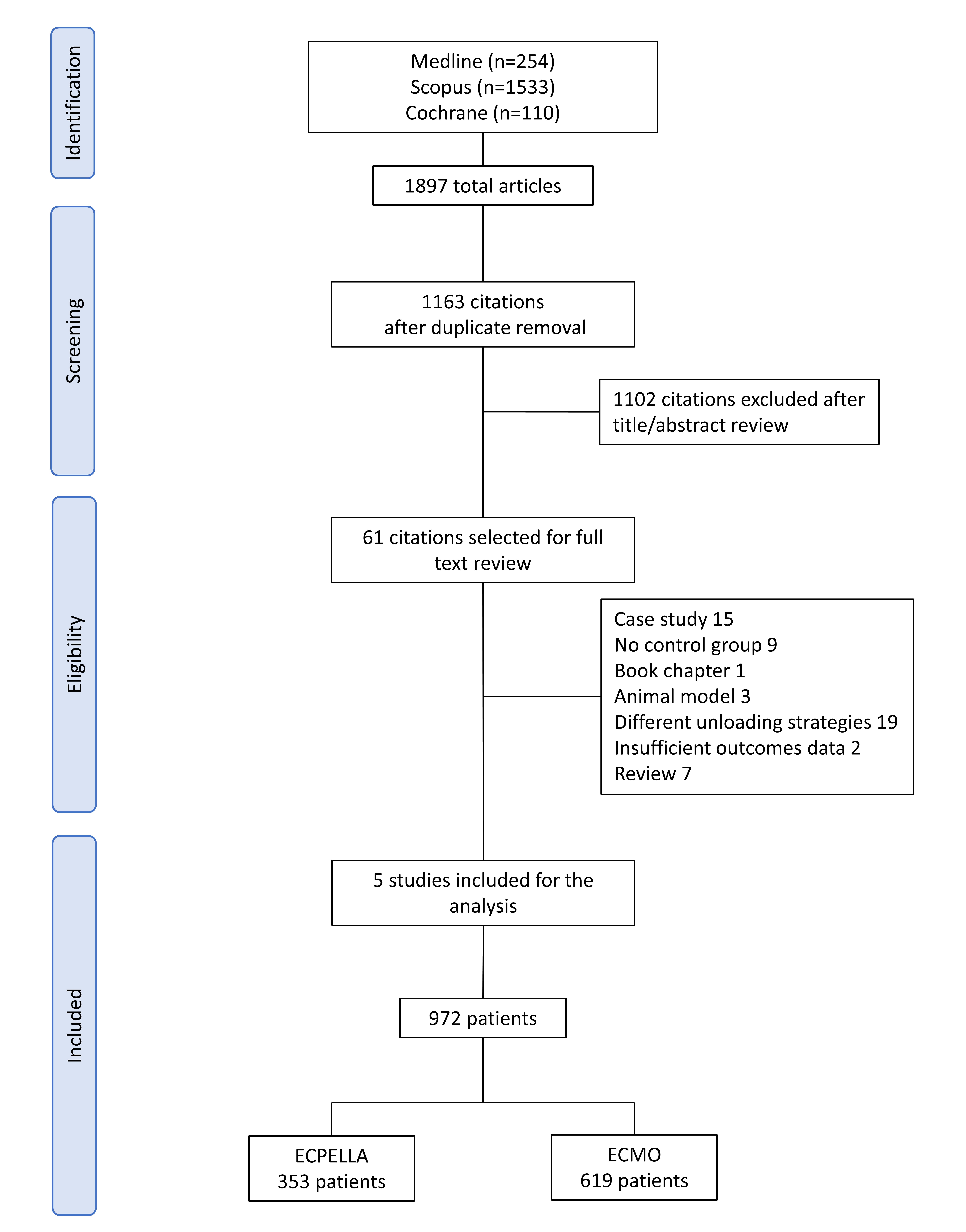 Fig. 1.
Fig. 1.Search flow diagram. Systematic search of Medline, Scopus and Cochrane library was performed with identification of 1897 articles. After duplicate removal and title/abstract review, 61 citations were selected for full text review. Five studies were included in the analysis with a total population of 972 patients, 353 in the ECPELLA and 619 in the ECMO cohort.
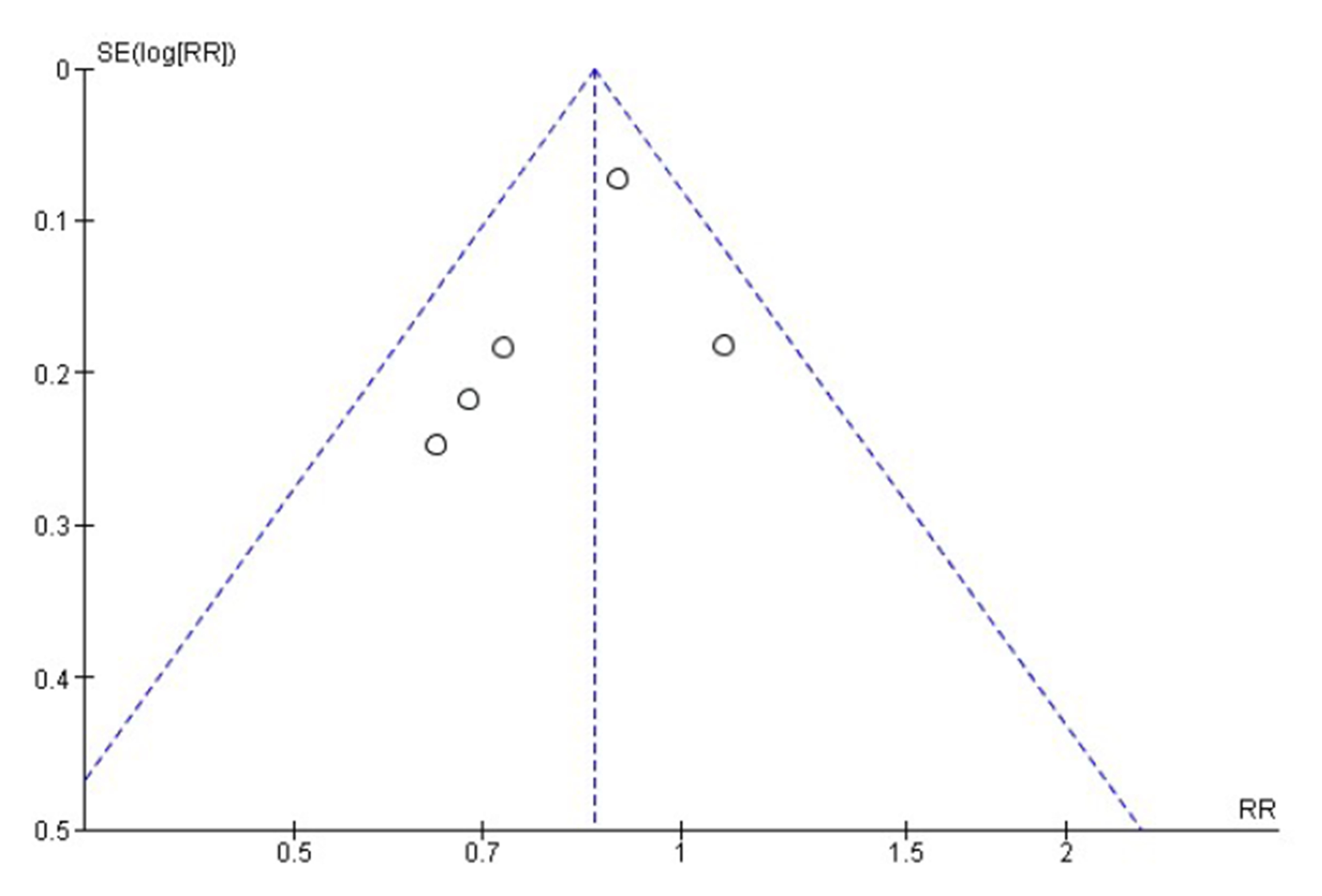 Fig. 2.
Fig. 2.Funnel plot analysis for publication bias. RR, Risk ratio; SE, Standard error.
| Reference | Country (n. centres) | Study durantion | Study design | Cardiogenic shock definition | Mortality |
| Pappalardo 2017 [11] | Italy, Germany (2) | January 2013–April 2015 | Retrospective | - | Hospital mortality |
| Propensity match | |||||
| 2:1 ratio | |||||
| Akanni 2019 [14] | United States (1) | February 2011–October 2014 | Retrospective | (1) SBP |
30-day |
| Case-control | (2) inability to wean off cardiopulmonary bypass for post-cardiotomy shock despite maximal support | ||||
| Patel 2019 [12] | United States (1) | 2014–2016 | Retrospective | SBP |
30-day |
| Case-control | |||||
| Homogeneous populations | |||||
| Schrage 2020 [13] | Germany, United States, France, Italy (16) | 2005–2019 | Retrospective | - | 30-day |
| Propensity match | |||||
| 1:1 ratio | |||||
| Gaisendrees 2021 [15] | Germany (1) | January 2016–December 2020 | Retrospective | - | Hospital mortality |
| Case-control |
There was not significant heterogeneity between the selected studies for the named outcomes, except for bleeding complications and need for RRT (Figs. 3,4, Ref. [11, 12, 13, 14, 15]).
 Fig. 3.
Fig. 3.Primary outcome. (A) All cause-mortality assessed with fixed effect meta-analysis including the five selected studies [11, 12, 13, 14, 15]. (B) All cause-mortality assessed with random effect meta-analysis including the five selected studies [11, 12, 13, 14, 15]. (C) All cause-mortality with fixed effect meta-analysis including propensity matched or homogeneous population studies [11, 12, 13]. (D) All cause-mortality with random effect meta-analysis including propensity matched or homogeneous population studies [11, 12, 13]. M-H, Mantel-Haenszel; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ECPELLA, ECMO + Impella.
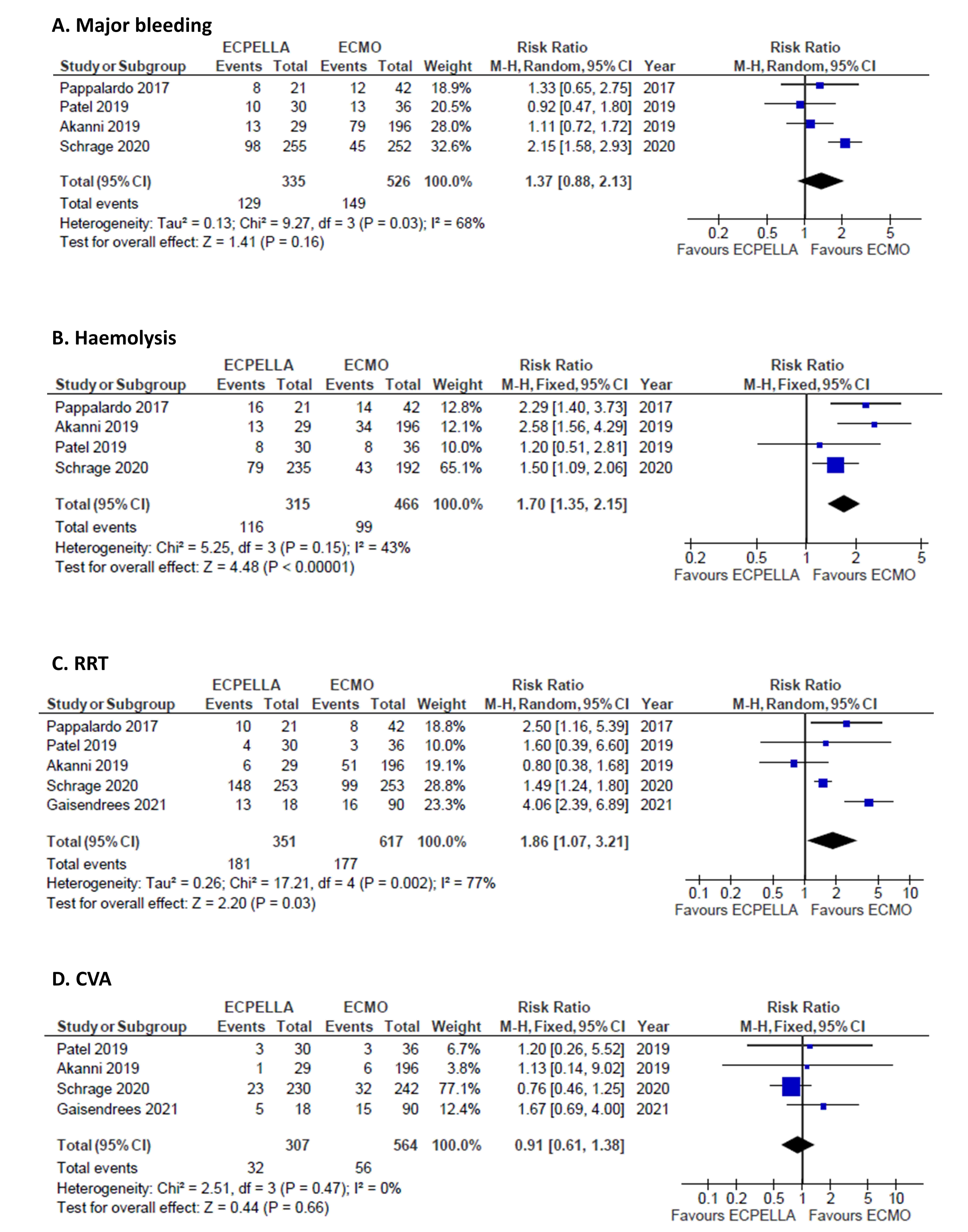 Fig. 4.
Fig. 4.Secondary outcome. (A) Major bleeding. (B) Haemolysis. (C) Renal replacement therapy (RRT). (D) Cerebrovascular accident (CVA). M-H, Mantel-Haenszel; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; ECPELLA, ECMO + Impella.
On a different note, we excluded recent or previously considered studies in other meta-analyses [6, 7, 16] due to heterogeneity in populations and support strategies. In details, in Char’s study the compared cohorts were not uniform [16]; Mourad mainly analysed ECMO vs. Impella [17] and Tepper studied two different venting techniques during ECMO, Impella vs surgical approach [18].
A total of 972 patients were included in the selected studies, 353 in the ECPELLA and 619 in the ECMO cohort. Demographic and clinical characteristics for each study are shown in Table 2 (Ref. [11, 12, 13, 14, 15]). There was no significant difference between baseline clinical data in patients supported with ECMO and Impella (ECPELLA) or with ECMO only.
| Study | Pappalardo 2017 [11] | Akanni 2019 [14] | Patel 2019 [12] | Schrage 2020 [13] | Gaisendrees 2021 [15] | ||||||||||
| Charact. | ECPELLA (n = 21) | ECMO (n = 42) | p value | ECPELLA (n = 29) | ECMO (n = 196) | p value | ECPELLA (n = 30) | ECMO (n = 36) | p value | ECPELLA (n = 255) | ECMO (n = 255) | p value | ECPELLA (n = 18) | ECMO (n = 90) | p value |
| Age, years | 51 (47–61) | 54.5 (46–65) | 0.6 | 63 (53–67) | 57 (48–67) | - | 55 (50–66) | 63 (50–71) | 0.23 | 56.39 ± 12.72 | 56.55 ± 13.21 | 0.07 | 57 (47.5–65) | 56.5 (45–66) | 0.98 |
| Male gender, n (%) | 18 (86) | 37 (88) | 0.5 | 23 (79) | 133 (67.86) | - | 20 (67) | 25 (70) | 1.00 | 195/255 (76.5) | 195/255 (76.5) | 0.99 | 15 (84) | 73 (82) | 0.82 |
| CPR | 12 (57) | 28 (67) | 0.5 | - | - | - | 12 (40) | 15 (42) | 1.00 | 84/255 (32.9) | 88/255 (34.5) | 0.03 | - | - | - |
| STEMI | 10 (48) | 20 (48) | 1 | 16 (55) | 42 (21.43) | - | 15 (50) | 6 (17) | 0.007 | 119/158 (75.3) | 124/162 (76.5) | 0.48 | 12 (67) | 49 (55) | 0.16 |
| PCI | 9 (43) | 18 (43) | 1 | - | - | - | 9 (30) | 1 (2.8) | 0.004 | 129/146 (88.4) | 145/152 (95.4) | 0.26 | 12 (67) | 49 (55) | 0.16 |
| SAVE score | −9 (−12– −2) | −8 (−11– −6) | 0.8 | - | - | - | −6 (−11– −3) | −9 (−14– −3) | 0.47 | −8.68 ± 6.91 | −7.67 ± 6.13 | 0.15 | - | - | - |
| PH | 7.31 (7.08–7.39) | 7.27 (6.98–7.43) | 0.7 | - | - | - | 7.3 (7.2–7.4) | 7.2 (7.1–7.4) | 0.18 | - | - | - | 6.96 (6.81–7.0) | 6.90 (6.8–7.1) | 0.7 |
| Lactate, mmol/L | 9.02 (4.60–11.00) | 9.03 (4.05–14.17) | 1 | - | - | - | 3.6 (2–7) | 7.6 (2.4–12.6) | 0.094 | 8.58 ± 5.67 | 8.55 ± 5.69 | 0.48 | 12.68 (10.74–17) | 13.5 (10.45–16) | 0.84 |
| Values are n (%), mean | |||||||||||||||
In the five selected studies, the overall short-term mortality was 60.9%; crude mortality in the ECPELLA and ECMO groups was 56.1% and 63.7%, respectively. The population supported by ECMO with LV unloading was associated with significantly improved survival compared to the control group (RR: 0.86; 95% CI: 0.76, 0.96; p = 0.009, Fixed effect; RR: 0.84; 95% CI: 0.72, 0.98; p = 0.03, Random effect) (Fig. 3A,B).
Including in the analysis only propensity matched studies or those with no significant differences in baseline characteristics in the comparator groups [11, 12, 13], LV unloading with Impella during ECMO remained associated with significant reduction in mortality (RR: 0.85; 95% CI: 0.75, 0.97; p = 0.01, Fixed effect; RR: 0.83; 95% CI: 0.70, 0.98; p = 0.03, Random effect) (Fig. 3C,D).
Secondary outcomes are reported in Fig. 4. Haemolysis and renal replacement
therapy occurred at a higher rate in the ECPELLA group, 36.8% and 51.6%,
respectively, compared to the ECMO group, 21.2% and 28.7%, respectively
(Haemolysis: RR: 1.70; 95% CI: 1.35, 2.15; p
In the current meta-analysis simultaneous LV unloading of CS patients treated with ECMO leads to improved short-term survival at a cost of higher haemolysis and RRT rates. No differences were observed with regards to major bleeding complications or cerebrovascular accidents.
Cardiogenic shock can complicate several acute pathologies beyond ACS, and its in-hospital mortality remains high despite the progress in interventional and medical therapies [2, 3, 19]. In the context of CS, MCS devices support organ perfusion and provide hemodynamic stability as bridge to recovery or long-term treatment, such as transplant or durable LV assist device [20, 21]. ECMO is widely used in refractory CS and cardiac arrest scenarios [4]. It provides biventricular support and restores peripheral perfusion; however, it does not provide a direct LV unloading effect, increasing, on the contrary, the afterload. LV pressure overload leads to LV dilatation, increase in left atrial (LA) pressure, and refractory pulmonary oedema. Furthermore, LV overload increases wall stress and myocardial oxygen consumption, resulting in myocardial ischemia. If the overload is extreme and LV contractile impairment significant, the aortic valve can remain closed during systole, causing LV blood stasis and thrombotic events [22]. On the contrary, effective LV unloading allows myocardial recovery, and it has been suggested that the degree of reverse remodelling depends on the degree of unloading provided [22, 23, 24]. Furthermore, recent retrospective studies suggest that the use of LV venting during VA ECMO has a potential impact on mortality [13, 25, 26].
Many surgical and percutaneous unloading strategies are available: venting cannula inserted through the right upper pulmonary vein via pre-existing sternotomy, in patients with post-cardiotomy CS; minimally invasive surgical approaches (sub xiphoidal or anterolateral); transeptal venting with creation of an atrial septal defect to decompress the LA; indirect unloading with IABP inserted via femoral arterial access and positioned in the descending aorta; percutaneous transvalvular micro axial pumps (Impella) via peripheral arterial access.
The hemodynamic benefit of Impella during ECMO has been described, with decrease in capillary wedge pressure, pulmonary pressures and vascular resistances [27]; however, there is still limited data regarding the clinical impact of this strategy. Recent metanalyses have tried to review the available studies, however they included heterogeneous populations and no uniform support strategies [6, 7].
Our analysis focussed on the use of Impella as direct unloading device and selected only propensity matched or case-controlled studies with similar baseline characteristics, in order to reduce heterogeneity and provide more reliable results. Furthermore, this is the first metanalysis that includes the recent relevant figures of Schrage et al. [13] on a matched population of 500 patients.
In our meta-analysis LV unloading with Impella was associated with improved survival compared to support with isolated ECMO. However, the use of a second device increased the rate of haemolysis and need for RRT, with no additional risk of major bleeding or cerebrovascular events.
It has been previously reported that the mechanical characteristics of the micro axial pump increases the shear stress on blood components, leading to various degree of haemolysis [28, 29]. In addition, this phenomenon might contribute to a certain extent to the higher rate of RRT. The association between haemolysis and acute kidney injury is well described in literature [30], aggravating in this scenario the critical condition of shock characterised by renal hypoperfusion and venous congestion.
Interestingly, the increased complexity added by a second device did not increase the rate of major bleeding, contrary to previous data on the use of Impella alone in CS [31, 32]. ECMO per se requires large-bore vascular access and therapeutic anticoagulation, therefore, a second device might not entail a significant additional risk of bleeding or other severe complications.
On a different note, timing for LV unloading and its impact on mortality remains controversial; to date there is no expert consensus and decisions on LV unloading escalation relies on physician expertise, and it is often performed as bailout strategy once the consequences of increased LV afterload become apparent. Preliminary data from observational studies and meta-analysis suggest benefit in early LV unloading compared to delayed escalation strategies [13, 33], however no clear thresholds have been identified.
In our institution we advocate early unloading guided by biochemical, echocardiographic, radiological, or hemodynamic evidence of suboptimal LV unloading despite optimal medical therapy (inotropes, vasopressor support, diuretics). We propose a few parameters that should indicate the need for early LV unloading: hemodynamic signs of high filling pressures with wedge pressure above 17 mmHg [34] and central venous pressure above 14 mmHg [35]; biochemical evidence of LV overload and impaired myocardial perfusion (increase in Brain Natriuretic Peptide (BNP) values at 24 and 72 h [36, 37, 38], troponin increase in the first 48 h with no underlying ischemic cause); echocardiographic evidence of overload (LV end-diastolic diameter increase of more than 1 cm from baseline, worsening mitral regurgitation and lack of aortic valve opening); radiological signs of worsening pulmonary oedema.
These parameters could help in the discussion regarding escalation criteria, however, data from the ongoing REVERSE randomised trial (NCT03431467) and future clinical studies will add relevant information to the current practice regarding LV unloading during ECMO.
First important limitation in our analysis is the small number of studies
included, with relatively limited sample sizes. Secondly, the observational and
retrospective design of the original papers could have caused under-reporting of
adverse events and indeed selection bias. Third, the timing for mortality was not
uniform in the five studies, three reported 30-days mortality and two studies
referred to in-hospital mortality. In addition, the definitions used by the
authors for bleeding and its grading scale was different in each paper.
Pappalardo et al. [11] considered major bleeding all intracranial,
retroperitoneal, intraocular and retropharyngeal bleeding, bleeding of the
cannulation site requiring either radiological or surgical intervention,
cannulation site haematoma
In conclusion, LV unloading with Impella during ECMO in patients with CS was associated with improved survival, despite increase in haemolysis rate and need for RRT. Moreover, no additional risk for major bleeding and cerebrovascular events was observed.
FF conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, final approval; VP conception and design of the study, acquisition of data, analysis and interpretation of data, revising the article, final approval. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to all those who assisted in the drafting of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. Vasileios Panoulas is serving as one of the Editorial Board members of this journal. We declare that Vasileios Panoulas had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Karim Bendjelid and John Lynn Jefferies.
