† These authors contributed equally.
Academic Editor: Julio Núñez Villota
Hospitalization for congestive heart failure represents a growing burden for health care systems. Heart failure is characterized by extracellular fluid overload and loop diuretics have been for decades the cornerstone of therapy in these patients. However, extensive use of intra-venous diuretics is characterised by several limitations: risk of worsening renal function and electrolyte imbalance, symptomatic hypotension and development of diuretic resistance. Extracorporealveno-venous ultrafiltration (UF) represents an interesting adjunctive therapy to target congestion in patients with heart failure and fluid overload. UF consists of the mechanical removal of iso-tonic plasma water from the blood through a semipermeable membrane using a pressure gradient generated by a pump. Fluid removal through UF presents several advantages such as removal of higher amount of sodium, predictable effect, limited neuro-hormonal activation, and enhanced spontaneous diuresis and diuretic response. After twenty years of “early” studies, since 2000 some pilot studies and randomized clinical trials with modern devices have been carried out with somehow conflicting results, as discussed in this review. In addition, some practical aspects of UF are addressed.
Despite recent advances in pharmacological treatment of heart failure, hospitalization for acutely decompensated heart failure remains a challenge for healthcare systems representing a significant resource and financial burden [1]. Registry data show that the majority of patients admitted for fluid overload already receive diuretic therapy. The rate of early re-admission remains high [2] and is associated with an adverse prognosis in this patient group [3]. Therefore, the need for adjunctive treatment strategies to standard diuretic therapy in patients presenting with fluid overload in the context of decompensated heart failure is critical. The present review discusses the role of extracorporeal veno-venous ultrafiltration (UF) in the management of fluid overload in patients presenting with decompensated heart failure.
Fluid retention with subsequent congestion may be the principal findings in patients with heart failure [4], mediated by a plethora of mechanisms but principally an impaired natriuretic and renal endocrine response to acute volume expansion, even in asymptomatic patients [5] with potentially detrimental effects. More specifically, an increase in central venous pressure and, consequently, renal venous pressure has a profound negative impact on both renal function and prognosis [6, 7, 8, 9] while is also associated with hepatic dysfunction, intestinal ischaemia and endothelial damage [10, 11]. It has been demonstrated that worsening of renal function or Type 1 cardiorenal syndrome occurs in 25–45% of patients hospitalized for decompensated heart failure. In this context, venous congestion of the kidneys rather than arterial underfilling is associated with decreased renal blood flow and an increase in creatinine [12]. Hence, reducing congestion should be a major therapeutic target in patients with heart failure and fluid overload. Nevertheless, aggressive fluid removal can itself cause an increase in creatinine levels, the origin (i.e., decrease in glomerular filtration rate [GFR] or acute tubular damage) and the prognostic impact of which are still to be clarified [13].
Loop diuretics have been for decades the cornerstone of therapy in patients with congestive heart failure [14, 15]. However, extensive use of intra-venous (i.v.) diuretics in the context of acute heart failure can be limited by several issues such as an increased risk of renal injury, electrolyte imbalance and symptomatic hypotension [16, 17, 18, 19].
The use of high diuretic doses in the context of treatment resistance, can
affect a significant number of patients in the advanced stages of the disease and
is associated with a worse prognosis [20, 21, 22, 23]; several mechanisms may be involved
such as reduced drug absorption and decreased glomerular blood flow [24].
Different definitions have been proposed to describe diuretic resistance with the
most commonly used being the presence of persistent congestion despite an
adequate daily dose of furosemide (
Ultrafiltration refers to the mechanical, adjustable removal of iso-tonic plasma
water from the blood through a semipermeable membrane (haemofilter) mediated by
the application of hydrostatic pressure gradient generated by a pump [28]. The
fluid removed from the intra-vascular compartment is constantly replaced by fluid
from the third space configurating the so called “intra-vascular refill”
phenomenon, thus allowing gradual fluid overload resolution. Several devices have
been developed over the years for this aim, the newer of which are characterized
by small size, low blood flow rates and low extracorporeal blood volume
requirement (
 Fig. 1.
Fig. 1.Functioning of modern ultrafiltration device. The blood pump generates a negative pressure, allowing the suction of blood from a vein through the Intake line of the catheter (red). Blood then passes through the intake line pressure sensor and reaches the blood pump. Afterwards blood is pushed through the air detector and enters the haemofilter. A pre-defined amount of ultrafiltrate is produced and passes through the haemofilter’s pressure sensor, the pump, and finally reaches the collecting bag attached to the weight scale. The remaining blood exits from the haemofilter, passes through the return line pressure sensor, the return line air detector and finally returns to the patient through the return line of the catheter (blue).
Ultrafiltration is a demanding process that requires the use of a dedicated machine connected to a dedicated vascular access with all associated challenges and complications, the presence of trained personnel and anti-coagulation. Nonetheless, fluid removal by UF shows some clear advantages compared with fluid removal achieved by diuretics (Table 1). First, UF produces ultrafiltrate through convection allowing the removal of a significantly larger amount of sodium (ultrafiltrate is almost iso-tonic with plasma) compared with hypotonic urine produced by diuretics [29, 30]. Hence, for any amount of fluid removed a greater amount of sodium is excreted with UF than with diuretics [31]. Furthermore, UF allows a controlled rate of fluid removal set by the clinician, whereas fluid removal is unpredictable and relies on many variables when diuretics are used. This aspect is of utmost importance in patients with labile hemodynamic stability, in whom an exaggerated fluid removal could be deleterious. The use of loop diuretics is associated with neuro-hormonal activation mainly due to renin secretion in response to sodium reabsorption inhibition, causing both the stimulation of sympathetic nervous system and a decrease in renal blood flow and glomerular filtration rate (thus promoting a vicious circle leading to diuretic resistance) [6]. Conversely, UF does not cause neuro-hormonal activation, unless fluid removal is too fast and exceeds plasma refilling rate [32, 33]. Moreover, it has been demonstrated that the mechanical elimination of fluid overload allows reduction of ventricular filling pressures and promotes cardiac performance [34]. Interestingly, those favorable effects were not reproduced removing the same amount of fluid by i.v. diuretic infusion, possibly due to the fact that patients treated with UF had lower hormone levels (renin activity, norepinephrine, and aldosterone) with a therefore, smaller impact on circulation and ventilation [35, 36]. In addition, it has been suggested that lung decongestion may restore the lung’s ability to clear norepinephrine, thus limiting renin release [36]. Lastly, fluid removal through UF in patients with fluid overload despite therapy with diuretics allows enhanced spontaneous diuresis and restores diuretic response [37]. This “reversal braking phenomenon” has not been completely understood yet and may be due to a period of “diuretic holiday” and reduced neuro-hormonal activation occurring during UF therapy [37] combined with the positive effect of progressive renal venous decongestion.
| Fluid removal with diuretics | Fluid removal with ultrafiltration | |
| Advantages | ||
| No need for anticoagulation | No neurohormonal activation | |
| No need for specific training | Production of isotonic ultrafiltrate | |
| No need for a dedicated access | Elimination of predictable and adjustable amount of water | |
| Not expensive | Restoration of diuretic responsiveness | |
| No effect on potassium and magnesium concentration | ||
| Disadvantages | ||
| Neurohormonal activation | Need for anticoagulation with increased risk of bleeding | |
| Induction of hypotonic urine | Need for trained personnel | |
| Elimination unpredictable amount of water | Need for a dedicated access | |
| Risk of diuretic resistance | Expensive | |
| Risk of electrolytes imbalance | ||
Beneficial effects of UF in heart failure with fluid overload are showed in Fig. 2.
 Fig. 2.
Fig. 2.Beneficial effects of ultrafiltration in heart failure with fluid overload. UF removes isotonic fluid, allowing to interrupt the vitious circle between heart failure and fluid overload. Consequently, venous congestion and renal venous pressure are reduced decreasing renal damage with further advantages on fluid overload resolution. Furthermore, UF allows to temporarily stop diuretic administration (“diuretic holiday”), decreasing neurohormonal activation and contributing to restore diuretic responsiveness in diuretic resistant patients.
Earlier studies conducted between 1980–2000 demonstrated the favorable effects of UF in patients with diuretic resistance and/or end-stage congestive heart failure [38, 39, 40, 41]. More recently, over the last two decades, some pilot studies and randomized clinical trials (RCTs) utilizing more modern devices have yielded conflicting results.
Some studies demonstrated a clinical benefit associated with the use of different strategies and protocols of UF in patients with acute heart failure, with favorable effects extending beyond the index hospitalization [37, 42]. However, concerns were raised regarding the use of aggressive fluid removal protocols and it was highlighted that there is little benefit in the use of UF in patients with end-stage heart failure [31, 36, 42]. Marenzi et al. [32] demonstrated that during the initial phases of UF the intravascular volume (estimated using a formula based on hematocrit) does not decrease, suggesting that all the removed fluid is replaced by fluid from the interstitial space according to the “intra-vascular refill” phenomenon. Thereafter, the intravascular volume gradually decreases, suggesting that that the process of intra-vascular refill progressively weans off and, thus, UF rate should be managed accordingly to avoid excessive fluid removal.
Several RCTs, based on the evidence provided by the aforementioned studies, have been conducted (Table 2, Ref. [36, 43, 44, 45, 46, 47, 48]). The RAPID-CHF (Relief for Acutely Fluid-Overloaded Patients with Decompensated Congestive Heart Failure) [43] trial randomized 40 patients admitted with congestive heart failure to receive standard care or early UF via a less invasive UF device (System 100, CHF Solutions Inc., Brooklyn Park, Minnesota) used outside ICU and via peripheral venous access, on top of standard care. A single 8-hour session of UF was administered to the UF arm. UF resulted in significantly larger fluid loss within the first 24 h compared to standard care (4650 mL vs. 2938 mL, p = 0.001) and faster resolution of symptoms. Weight loss at 24 h was larger in the UF group at 2.5 kg as opposed to 1.86 kg in patients who did not receive UF (p = 0.24). Overall, the trial demonstrated that early application of UF in patients with acute heart failure was tolerated well, and might have added benefits to standard of care, but the assessment of the effect was limited due to the small population size.
The UNLOAD (Ultrafiltration versus Intravenous Diuretics for Patients
Hospitalized for Acute Decompensated Congestive Heart Failure) [44] trial
assigned patients admitted with fluid overload due to heart failure to early UF
versus standard diuretic therapy. Ultrafiltration was performed via a Simple
Access Fluid Extraction (SAFE) UFC 100 Console, a dual rotary occlusive pump
device attached to peripheral venous access. The length and rate of UF was left
to the discretion of the treating physician (fluid was removed at an average rate
of 241 mL/h for 12.3
The ULTRADISCO (ULTRAfiltration vs. DIureticS on clinical, biohumoral and
haemodynamic variables in patients with deCOmpensated heart failure) [36]
randomised 30 patients with overload due to decompensated heart failure to UF or
i.v. diuretics to compare the clinical, bio-humoral, and hemodynamic effects of
the two treatments. Ultrafiltration was performed through PRISMA
The CARESS-HF (Cardiorenal Rescue Study in Acute Decompensated Heart Failure)
[45, 46] aimed to assess the role of UF in management of acute heart failure with
cardiorenal syndrome. Patients with acute decompensated heart failure, impaired
renal function and persistent congestion were randomized to receive UF with the
use of the Aquadex System 100 (CHF Solutions, Eden Prairie, MN, USA) at a fixed rate of 200 mL/h or
pharmacological therapy. The primary endpoint was the change in serum creatinine
(intended as a marker of acute tubular injury) and body weight from baseline at
96 hours after randomization. The trial did not show significant difference in
weight loss between the intervention and the control group (loss of 5.7
| Rapid-chf [43] | Unload [44] | Ultradisco [36] | Caress-hf [45, 46] | Cuore [47] | Avoid-hf [48] | |
| Year of publication | 2005 | 2007 | 2011 | 2012 | 2014 | 2016 |
| Study design | Multi-center | Multi-center | Single center | Multi-center | Multi-center | Multi-center |
| Prospective | Prospective | Prospective | Prospective | Prospective | Prospective | |
| Number of Centers | 6 | 28 | 1 | 22 | 2 | 30 |
| Sample size (UF arm vs. diuretic arm) | 40 | 200 | 30 | 188 | 56 | 224 |
| (20 vs. 20) | (100 vs. 100) | (15 vs. 15) | (94 vs. 94) | (27 vs. 29) | (110 vs. 114) | |
| Population characteristics | Hospitalized with HF; | Hospitalized with HF; | Hospitalized for HF; | Hospitalized with HF; | NYHA III or IV; | Hospitalized with HF; |
| 2+ edema; | LVEF |
|||||
| UF device | System 100 | Aquadex System | PRISMA | Aquadex System | Dedyca | Aquadex System 100 |
| Primary EP | Weight loss (Kg) at 24 h | Weight loss (Kg) at 48 h | Hemodynamics changes determined by PRAM (baseline to 36 h) | Change in sCr (mg/d) | Rehospitalization due to HF at 1 year | Time to first HF event at 90 days |
| Dyspnea assessment at 48 h | Change in weight (Kg) at 96 h | |||||
| Adverse events | UF arm: 1 catheter site infection | UF arm: | Not reported | 60-day serious adverse event: | UF arm: 6 premature clotting of filter | At least 1 serious adverse event: |
| 1 catheter infection | UF arm: 72% | UF arm: 66% | ||||
| 5 filter clotting events | Control arm: 57% ( p = 0.03) | Control arm: 60% (p = 0.4) | ||||
| 1 patient hemodialyzed | Serious adverse event of special interest: | |||||
| UF arm: 23% | ||||||
| Control arm: 14% (p = 0.122) | ||||||
| Results (primary EP; UF arm vs. diuretic arm) | –2.5 vs. –1.86 (p = 0.24) | Weight loss: 5 |
Significant hemodynamics changes in favor of UF group (cardiac index, CPO, dP/dtmax, SVR) | 11% vs. 48% (p = 0.002) | 25% vs. 35% (p = 0.11) | |
| Dyspnea score: 5.4 |
||||||
| Legend. EP, endpoint; HF, heart failure; sCr, serum creatinine; UF, ultrafiltration; PRAM, pressure recording analytical method; CPO, cardiac power output; SVR, systemic vascular resistance. | ||||||
The CUORE (Continuous Ultrafiltration for Congestive Heart Failure) [47] trial suggested that there might be some longer-term benefits associated with UF treatment in patients with overload due to congestive heart failure (n = 56) compared to standard diuresis. Ultrafiltration in this trial was performed via Dedyca device (Bellco, Mirandola, Italy)—which is specific for patients with heart failure - via central venous access. The trial showed a lower rate of re-hospitalization at 1 year in patients treated with UF compared to those treated with standard diuretic therapy (hazard ratio 0.14, 95% confidence interval 0.04–0.48; p = 0.002), although there was no significant difference in body weight loss at discharge between the two treatment strategies. Furthermore, at 1 year, death rate in the two groups did not differ significantly (7 deaths (30%) in the UF arm vs. 11 (44%) in the control group; p = 0.33). Interestingly and in contrast with previous studies, diuretic therapy during UF was not discouraged and left to the discretion of the treating physician.
The AVOID-HF (Aquapheresis versus Intravenous Diuretics and Hospitalization for
Heart Failure) study [48] compared adjustable UF with adjustable loop diuretics
treatment in patients with cutely decompensated heart failure with UF
administered via AquadexFlexFlow System (Baxter International, Deerfield,
Illinois). The study was prematurely interrupted by the sponsor in disagreement
with the authors due to slow recruitment when only 224 of the planned 810
patients were enrolled. According to study design, UF and diuretic therapy were
titrated based on vital parameters, urinary output and renal function and the
duration of UF therapy was left at discretion of the clinician. The intervention
group received UF therapy at an average rate of 138
Overall, evidence so far does not allow safe drawing of conclusions with regards to the role UF in the management of fluid overload in patients with decompensated heart failure. Direct comparison of study findings is not possible due to the variable UF protocols utilized as far as devices, access, duration, rate and total dose of UF treatment are concerned. Renal specific findings and, more specifically, concerns raised by CARRESS-HF regarding an UF-induced rise in creatinine levels, need to be interpreted with caution to avoid an unwarranted discouragement of further pursuing a potential clinically valuable use of UF in this setting. The clinical significance of an isolated creatinine rise without accompanying data on other measures of renal function such as urine output and longer term stabilization of creatinine levels cannot be established. Moreover, it would be most interesting to ascertain the value of UF in specific and uniform endpoints across studies; the impact on length of hospital stay, standardized symptom assessment tools and longer term outcomes need to be assessed.
Several meta-analyses have summarized and pooled the result of the mentioned
trials. Among the most recent, Wobbe et al. [52] evaluated the data of 8
studies with a global population of 801 patients, and reported greater mean fluid
removal [1372.5 mL, 95% CI 849.6–1895.4 mL; p
Recently, a RCT has been conducted [53], on the impact of UF (performed in 40 of
the 100 enrolled patients) compared with diuretic therapy with torasemide plus
tolvaptan in AHF patients. Patients who received UF for 3 days evaluated at 4 and
8 days achieved a greater weight loss (respectively –2.94
Finally, the PURE-HF (Peripheral Ultrafiltration for the RElief From Congestion in Heart Failure, ClinicalTrials.gov Identifier: NCT03161158) was designed to provide further insight regarding the impact of peripheral UF (via the CHIARA device) complementary to low-dose diuretics on cardiovascular mortality 90 days after randomization and heart failure events 90 days after discharge compared to usual care including stepped intravenous diuretics in 900 acutely decompensated chronic heart failure patients with fluid overload (not fully responsive to diuretic therapy). Unfortunately, the study was prematurely terminated in 2019 after enrollment of 62 patients.
Ultrafiltration is an interesting therapeutical opportunity for patients with heart failure and over fluid overload. Due to its complexity, cost and potential complications, it should be aimed at patients presenting with diuretic resistance in the setting of decompensated heart failure. Unfortunately, it remains still unclear how to pre-emptively identify patients in whom response to diuretics is expected to be poor and, thus, who could benefit most from UF [26]. According to current guidelines [15], UF should only be considered when titration of the initial i.v. diuretic agent and combination with other diuretics are ineffective to treat fluid overload. Whether a more proactive use of UF may be beneficial in certain patient groups needs to be addressed.
Ultrafiltration is usually provided through dual lumen central venous access (i.e., internal jugular vein or femoral vein). Newer simplified UF devices were designed to allow the use of peripheral accesses, namely brachial antecubital venous access, without the need for and the potential complications of central venous catheters. However, these newer devices are not readily available to all hospital settings and routine use of UF in the management of heart failure, would certainly at least initially involve central venous access to a significant extent. Furthermore, peripheral venous access can also be associated with certain complications such as blood-stream infections, thrombosis etc. In addition, in some patients peripheral veins are inadequate and thus the placement of a central catheter remains the only mean to deliver UF [55].
As UF is an extra-corporeal technique in which blood is pumped through an haemofilter, anticoagulation is required to prevent filter clotting and line thrombosis. Different regimens have been used in the studies according to local protocols and device used, using continuous infusion of unfractionated heparin (monitoring aPTT or ACT) or low molecular weight heparin according to weight and renal function. University of Minnesota suggests a protocol for anticoagulation during UF with AquadexFlexFlow (CHF Solutions, Inc., Brooklyn Park, MN) device based on the infusion of heparin proximal to the filter (in the withdrawal line) following a high nomogram heparin protocol, seeking to achieve a goal aPTT of 72 to105 seconds [55].
The initial UF rate should be set at the discretion of the attending physician.
In general, considering that the entity of the “intra-vascular refill”
phenomenon (i.e., the fluid removed by UF is replaced by fluid from the third
space) gradually decreases over time, it is reasonable not to augment UF rate
once the therapy is started in order to avoid excessive fluid removal
[32, 48]. Thus, once a start rate is chosen according to the patient’s clinical and
hemodynamic profile (initial rate usually advised is
Ultrafiltration can be performed through several devices. Conventional UF
machines require large bore central accesses, a significant blood flow (upwards
of 200–400 mL/min) and around 100 mL of extracorporeal blood volume [60].
Conversely, newer portable dedicated devices require smaller catheters, lower
blood flow (
New insights regarding the key role of lymphatic system in the regulation of myocardial extracellular volume and on the association among lymphatic dysfunction, interstitial edema and elevated central venous pressure have been highlighted [62, 63]. In fact, lymphatic flow is a major compensatory mechanism in heart failure against the development of interstitial oedema. Thus, the lymphatic system should be intended as a potential target in the management of HF [64] and, among the potential approaches to promote its draining function, UF may find a role. Therefore, it is likely that the patients who would benefit the most from UF therapy are patient affected by lymphatic insufficiency in which the use of diuretics would further inhibit lymphatic function thus enhancing central venous pressure and kidney injury. In such patients, on the contrary, implementation of lymphatic function by UF would promote the intra-vascular refill phenomenon and consequently reabsorption of fluid overload.
A crucial aspect in UF is the target fluid removal over a certain period of
time. Thus, assessing fluid status with precision could aid to achieve
homeostasis avoiding the risk of excessive fluid removal [65]. Analysis of
bioelectrical impedance is a technique developed to estimate body composition via
measurement of electrical parameters [66]. In the last years, it has been widely
implemented in clinical practice (in the context of cancer, cardiac surgery,
hospitalized children, cirrhosis, chronic renal failure, anorexia [67, 68, 69, 70, 71, 72, 73]) since
it is noninvasive, safe, portable, inexpensive, appliable both at bedside and in
the outpatient setting [74]. Bioelectrical impedance vector analysis (BIVA) is a
novel approach which allows to assess hydration status in real time without the
need to use regression equations and mathematical assumptions as in the
traditional approach [75]. BIVA has been shown to be adequate for monitoring the
fluid status in patients undergoing peritoneal dialysis, in critically ill
patients and in the setting of congested heart failure [76]. With regards to the
mechanism of BIVA, it is based on the analysis of bioimpedance (Z). Z is defined
as the opposition exerted by a conductor against the flow of an applied
alternating electrical current and is based on the principle that the human body
performs as a circuit in which the tissues have different electrical properties
(fluids and lean tissues are good conductors as opposed to fat tissue) [75]. Z is
calculated from two parameters directly measured: resistance (R), which increases
from intra-cellular to extra-cellular fluids, and reactance (Xc), which is
related to the capacitance of the cell membranes (Fig. 3a). R and X
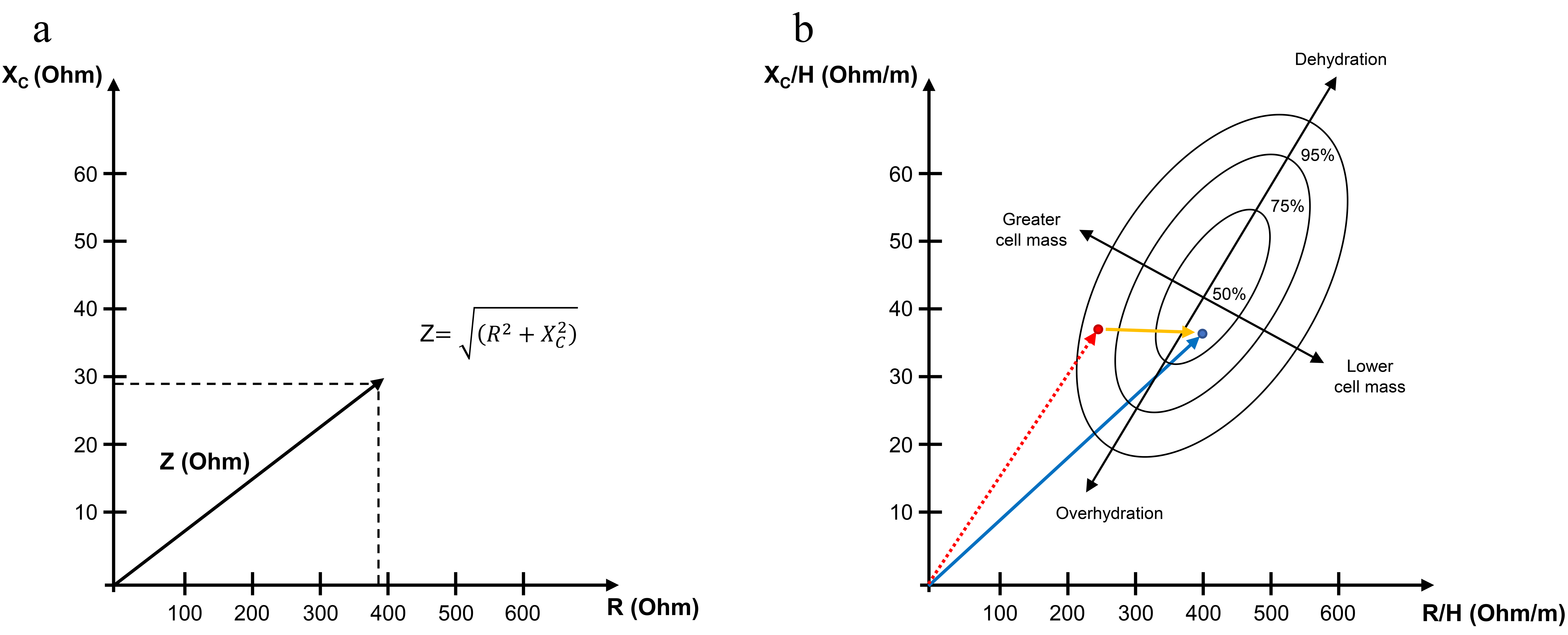 Fig. 3.
Fig. 3.Bioelectrical impedance vector analysis (BIVA) principles of
interpretation. (a) Parameters defining bioimpedance (Z, expressed in
Ohm): X
Natriuretic peptides have also been proposed as a potential tool to guide decongestion. Unfortunately, the fact that their values are influenced by many other variables makes them unsuitable for this role [84]. Creatinine remains the cornerstone for monitoring pharmacological and mechanical decongestion. Nevertheless, its fluctuation during decongestive therapies does not have an unequivocal interpretation as previously pointed out [78]. The neutrophil gelatinase-associated lipocalin (NGAL) is a protein of the lipocalin superfamily secreted both in urine and plasma following kidney damage. It has been shown to be secreted a few hours after kidney injury and its levels are correlated with the entity of the injury [85]. Recent studies on NGAL qualified it as a potential useful tool to assess when an increase in serum creatinine is due to tubular damage and not just a marker of effective decongestion [86].
The role of UF as an adjunctive therapy in patients with fluid overload due to decompensated heart failure is not fully established. Alongside traditional haemofilters, newer heart failure specific devices are safe, easy to use and require only minimal training. Based on the available evidence and guidelines recommendations, UF could be used in patients who are diuretic resistant. Though findings so far should be interpreted with caution, it is readily concluded that as every other therapy, benefit obtained from UF will be maximized, if the treatment is tailored to patient’s clinical status and background. Further carefully designed studies are needed to assess short- and long-term outcomes in association with UF and help clinicians drive their approach via the formation of relevant guidelines.
AS, AC, EA, EL designed the research study. AS and AC performed the literature research. AS, AC, EA and EL wrote the first draft of the manuscript. All the authors contributed to editorial changes in the manuscript and read and approved the final manuscript.
Not applicable
We would like to express our gratitude to all those involved in the management of patients with acute heart failure in our Departments. We also would like to thank all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
