Academic Editor: Zhonghua Sun
Atrial fibrillation (AF) can lead to embolic stroke and in subjects with non-valvular AF most of thrombi are sited in the left atrial appendage (LAA). LAA is a structure located in the free wall of heart with a wide variable and complex anatomy. LAA occlusion (LAAO) could be taken in consideration in subjects with non-valvular AF and who cannot have long-term anticoagulant therapy. It is a complex preventive procedure given the high variability of patients characteristics and several LAAO devices available nowadays. Moreover, the ideal postprocedural antithrombotic strategy is still unclear. In this review we aim to describe clinical features of patients committed for LAA occlusion and the function of multimodality imaging in subjects selection, procedural management and follow up.
Atrial fibrillation (AF) is a big risk factor for embolic stroke [1, 2]. In subjects with non-valvular AF, most of thrombi are situated in the left atrial appendage (LAA) (about 90% of clinically significant emboli) [3].
According to 2020 European Society of Cardiology guideline for the diagnosis and
management of atrial fibrillation [4], oral anticoagulant (OAC) therapy is
recommended for prevention of stroke in AF patients with CHA
Two randomized controlled trials, PROTECT AF [5] and PREVAIL [6], demonstrate the positive effect of LAA closure against thromboembolic events.
In this review we aim to describe clinical features of patients referred for LAA occlusion and the role of multimodality imaging in selection of patients for catheter-based LAA occlusion, procedural guidance and follow up.
LAA is a closed pouch from the left atrium (LA) [7]. It originates anteriorly from the left upper pulmonary vein ostium and, in most hearts, represents the upper-anterolateral part of LA. A narrowing at the orifice of the appendage is named the junction. LAA can considerably vary in size and shape, and this could be a problem when interventional procedures are executed.
Its orifice is typically oval-shaped, less frequently can be round-shaped, triangle-shaped, and waterdrop-shaped [8]. Ligament of Marshall divides LAA from the left pulmonary veins, leading to a neck region that opens into the body of the appendage. The LAA may contain more than one lobe, most commonly two, defined as protrusions from its many body. More common anatomic variants of the LAA are four [9]: the chicken wing (48%), the cactus (30%), the windsock (19%) and the cauliflower (3%). The shape of the LAA may influence stroke risk (i.e., the “cauliflower” morphology—Fig. 1) and can increase the technical interventional challenge for LAA closure; moreover, the presence of extensive trabeculations is correlated to higher risk [10, 11, 12, 13].
 Fig. 1.
Fig. 1.More common anatomic variants of the LAA are four: the chicken wing (48%), the cactus (30%), the windsock (19%) and the cauliflower (3%). This is an example of the “cauliflower” morphology (see arrow), which is frequently related to embolic manifestations.
The inner surface of the LAA is characterized by the pectinate muscles. The thicker muscle bundles may be erroneously considered a thrombus.
AF confers a prothrombotic state as result of flow abnormalities or stasis, atrial remodeling and dilatation and abnormalities of the endocardium [14].
AF associated risk stroke is 5% about per year, with a multifactorial pathogenesis, and occurs in non-paroxysmal AF more than paroxysmal AF [4, 10, 15].
Patients with AF and elevated stroke risk (CHA
Moreover, this therapy could also be taken in consideration in the following clinical situations [10]:
• Patients with high-thromboembolic and hemorrhagic risk;
• Patients requiring triple antithrombotic therapy all lifelong;
• Patients in whom OAC has failed;
• Patients with kidney failure or under dialysis;
• Frail patients (old age, mental diseases, bad nutrition state, etc.);
• Patients with difficulty in managing oral therapies.
An individualized risk-benefit assessment should be considered and a decision between LAA occlusion and medication should be made.
There are actually two strategies available for percutaneous LAA closure [18]: LAA occlusion and LAA exclusion. An ideal LAA occluder device must be adaptable to the large variety of LAA anatomy, with a low rate of procedural adverse events (such as thrombus) and it should be effective in exclude the LAA from circulation.
Current catheter-based devices for LAAO are based on three different principles [19]:
(1) Plug [20]: endovascular delivery of a device lobe or umbrella obstructing
the neck of the LAA to prevent blood flow into the body of the LAA
(WATCHMAN
The WATCHMAN FLX device [21, 22, 23], (approved by Boston Scientific in 2015) a second generation of Watchman device, is shorter, has fluoroscopic marker on the distal tip, can be re-utilized, and has a larger range of sizes (from 20 to 35 mm) allowing several possibilities of treatment, has a bigger number of perimeter anchors arranged in two rows, to create a flat surface on the atrial side. Although its use is supported by strong scientific evidences, it requires deep incannulation of LAA [10]. It is easily commercially available worldwide and it is actually the most used.
The WaveCrest device is comparable to the Watchman in size (22–32 mm) and in its endocardially delivering; separation of positioning from anchoring, sealing along the distal margin of the device, and Gore-Tex material [10, 24] are distinctive features. Its material is occlusive and nontrhombogenic and allows for a short landing zone [24].
(2) Pacifier principle [25]: endovascular delivery of a device with a lobe or
umbrella and an additional disc to seal the ostium of the LAA from the left
atrial side (AMPLATZER
AMPLATZER and Amulet devices are supported by trials and aim especially for fluoroscopic approach, with low rate of peri-procedural harmful events and a low rate of stroke and systemic embolism especially for the AMPLATZER. The 2021 new Amulet IDE trial showed the non-inferiority for stroke prevention and superiority for LAA occlusion of Amulet occluder compared with the Watchman device [26].
Ultraseal and LAmbre devices have sealing depending on disc. LAmbre is an innovative, self-expanding LAA occluder that consists of an umbrella and a cover linked by a short central waist. The device is delivered by an 8–10 French sheath and has full re-utilizing proprieties; it has a notable advantage since it allows implantation in a variety of anatomies [10].
(3) Ligation: LARIAT® (SentreHEART) [25] aim to snare and ligate the body of the LAA using both endocardial and epicardial approach. It puts no foreign materials in direct communication with blood flow and leads to a potential electrical isolation of LAA [10]. It showed acute success rate and low incidence of adverse effects [25]. However, it can induce pericardial effusion and has strictly anatomical exclusion criteria [10]: these represent two major limitations to its use.
Generally, anatomical contraindications are unusual for these devices; implantation is technically practicable in most of patients.
Imaging is essential in patients selection, pre-plan implantation strategy, to guide procedural device implantation, and also for device surveillance post implantation. Its importance has already been proved [29, 30].
Noninvasive imaging modalities, including two (2D) and three (3D) dimensional transesophageal echocardiography (TOE), and cardiac computed tomography angiography (CCTA) are both used for preprocedral imaging [31].
TOE is the conventional gold-standard preprocedural imaging for LAA occlusion [32]; it’s widely available, not requires X-ray or contrast medium and it is used for procedural imaging guidance in most centers.
However, CCTA offers superior spatial resolution, high-quality multiplanar and 3D characterization of the LAA anatomy and accurate sizing [33]. Pre-procedural cardiac CCTA can accurately describe LA size, exclude LA thrombi and determine the anatomic feasibility of LAA occlusion procedure [34], other than better provide the ideal device size. Furthermore, utilization of CCTA 3D data may be helpful in avoiding procedure failing and complications (Fig. 2).
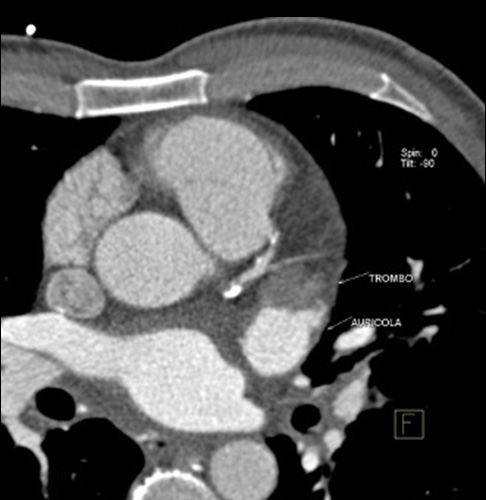 Fig. 2.
Fig. 2.Pre-procedural cardiac CCTA can accurately describe LA size, exclude LA thrombi and determine the anatomic feasibility of LAA occlusion procedure.
Nevertheless, the routine use of CCTA into clinical practice is not so diffused also due to the lack of standardized imaging protocol [35].
The main TOE e/o CCTA exclusion criterion is the existence of thrombus in the LAA [35]. Nowadays, TOE is the most used imaging technique to exclude the presence of thrombi in LAA (Fig. 3).
 Fig. 3.
Fig. 3.The main TOE e/o CCTA exclusion criterion is the existence of thrombus in the LAA (see arrow).
Once the presence of thrombus has been ruled out, TOE is used to assess LAA size and shape, number and location of LAA lobes [3, 36].
A full 0–135
 Fig. 4.
Fig. 4.By use of multiplanar TOE, full 0–135
Finally, the presence of valvular abnormalities, mobile aortic atheroma and anatomic characteristics of interatrial septum has to be described [35]. An atrial septal repair or the presence of closure device are contraindications to LAA occlusion.
Although 2D TOE represents the routinely modality for pre-operative assessment, the use of 3D TOE gives practical additive information in identifying uncommon morphologies [12], and its use is increasing. While its anatomic estimations are superior to 2D TOE, slow temporal resolution of 3D TOE and lacking professional guidelines still represent limitations of this technique.
Detailed characterization of the LAA body and orifice shapes can facilitate device selection (Fig. 5).
 Fig. 5.
Fig. 5.The use of 3D TOE provides useful additive evaluation about unusual morphology or irregular orifices.
LAA measurements can be different depending on devices: for the WATCHMAN [28], the LAA landing zone is set from the peak of the mitral valve annuls to a point 20 mm below the tip of the left upper pulmonary vein. Depth is considered from the plane of the LAA orifice to the LAA apex. For the Amulet [27], the LAA ostial diameter is set as the line from the pulmonary vein to the circumflex artery. The landing zone diameter is then calculated about 10 mm distal to the ostium, perpendicularly to the neck axis. Depth is measured perpendicular to the plane of the LAA orifice.
Since the LAA is thin-walled and distensible, volume status of the patients should be evaluated and a NaCl bolus of 500–1000 mL is recommended before device sizing.
Nowadays, new techniques are rapidly gaining scientific attention, also in LAA
occlusion technique. Simulation technology (3D printing or in silico modelling)
represent interesting preprocedural device size and positioning evaluations.
FEops HEARTguide
Fluoroscopy and TOE are both suitable modalities for procedural imaging for LAA closure. LAA occlusion can be carried out with the guidance of fluoroscopy only without general anesthesia, which has been frequently used for first-generation devices. Fluoroscopic 2D imaging often does not provide sufficient anatomic detail or guidance to visualize the planned structural heart intervention, due to poor characterization of nonradiopaque structure and 2D projections only. Moreover, this practice is limited to not many centers and the use of intraprocedural ultrasound in addition to fluoroscopy have showed better results [38]: therefore, this technique cannot be routinely recommended [10, 35]. Hence, procedural imaging should be performed with either TOE or intracardiac echocardiography (ICE) guide [31].
TOE usually requires anesthesia or sedation and a dedicated team, and is limited by gastrointestinal disease. However, some less invasive modalities to perform TOE are gaining scientific interest, such as the use of pediatric probes (mini- micro- TOE) [39].
In some centers, ICE has become standard method: it requires minimal sedation
but additional femoral venous access and a second transseptal puncture. The main
advantage of the use of TOE is its views ranging from 0 to 180
Once LA thrombus has been ruled out, the size and shape of the LAA should be re-assessed to guide device size selection.
Independently of the device used, the basic steps are common to all percutaneous LAA occlusion/exclusion procedures [18, 40, 41, 42]:
(a) Peripheral venous access: the first step is the peripheral venous access; usually the procedure is performed from the right femoral vein through which the whole transseptal system is passed.
(b) Transseptal puncture: consequently, a transseptal puncture is effected to access the left atrium. With echo guidance, the puncture is not only more precise, but it is also safer. Three standard echo views are used to determine the coordinates of the puncture: the bi-caval view is used to determine the position of the puncture on the superior-inferior axis, the short axis view at the base is used to check the position on the anterior-posterior axis, and the four-chamber view to measure the distance from the mitral valve annulus. Most frequently the puncture location is at a posterior and inferior location. Once an acceptable position has been confirmed, the needle is proceeded until puncture is occurred. A wire is subsequently passed into the left atrium and placed in the left superior pulmonary vein (Fig. 6).
 Fig. 6.
Fig. 6.Intra-operative TOE monitoring. After the transeptal puncture, a wire is passed into the left atrium and typically positioned in the left superior pulmonary vein and after into the LAA.
(a) Device deployment: the implantation system and device must be prepared and moved into the LAA. When the device is open, before device release, a combined check is made by angiography and echocardiography, to verify: (1) position: “the shoulder” of the device should not protrude excessively from the LAA; (2) anchor: slowly pull back then release deployment knob to visualize movement of device and LAA together (“tug test”) is performed; (3) size: the device diameter is evaluated by 2D TOE and is measured “shoulder to shoulder”; (4) seal: ensure all lobes are far to closure device and sealed. These are the so-called PASS (position, anchor, size and seal) criteria, specific for Watchman devices. If the device position is not good, the device can still be recaptured and repositioned (Fig. 7).
 Fig. 7.
Fig. 7.Intra-operative TOE monitoring. When the device is open, before device release, a combined check is performed by angiography and echocardiography, to verify: (1) position; (2) anchor; (3) size; (4) seal.
Pericardial effusion (PEF) is one the worst complication during LAA occlusion
procedure [43]. It can occur as a result of intraprocedural perforation of
cardiac chambers. Therefore, at the end of the process, an additional
echocardiographic evaluation is needed to exclude PEF, device embolization and
para-device leak (PDL) [44]. PDLs
Even if most evidences report high implantation success rate and low procedure complications [45], the implantation procedure can cause serious complications.
Either TOE or CCTA is highly recommended at 45 days (
Reassess the device position and stability; device embolism may be asymptomatic (Fig. 8).
• Assess for any residual or new PDL; PDL
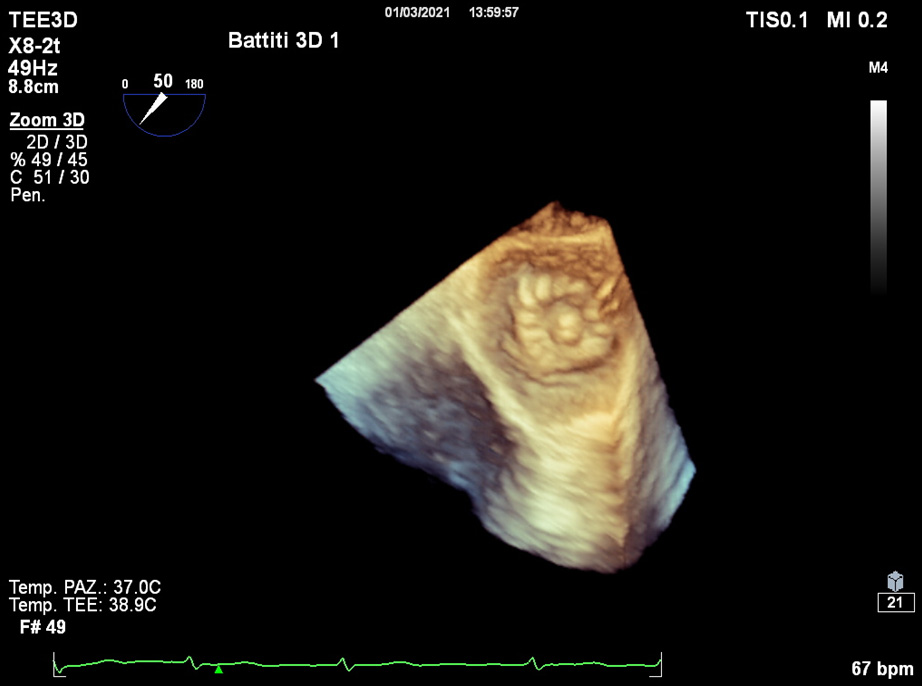 Fig. 8.
Fig. 8.Post-operative 3D TOE. Device visualization within the atrium.
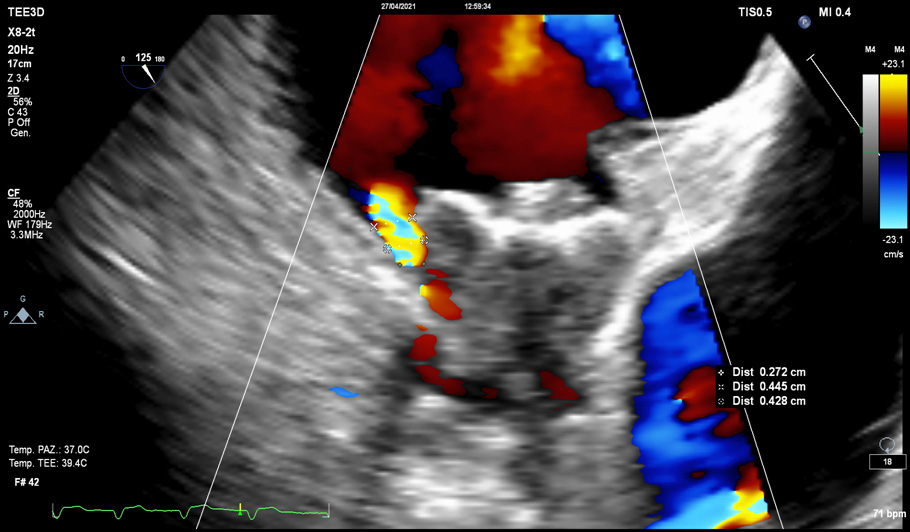 Fig. 9.
Fig. 9.Para-device leak (PDL). PDLs
• Assess for thrombus on the left atrial side of the device.
• Look for residual atrial septum defect (ASD) (Fig. 10).
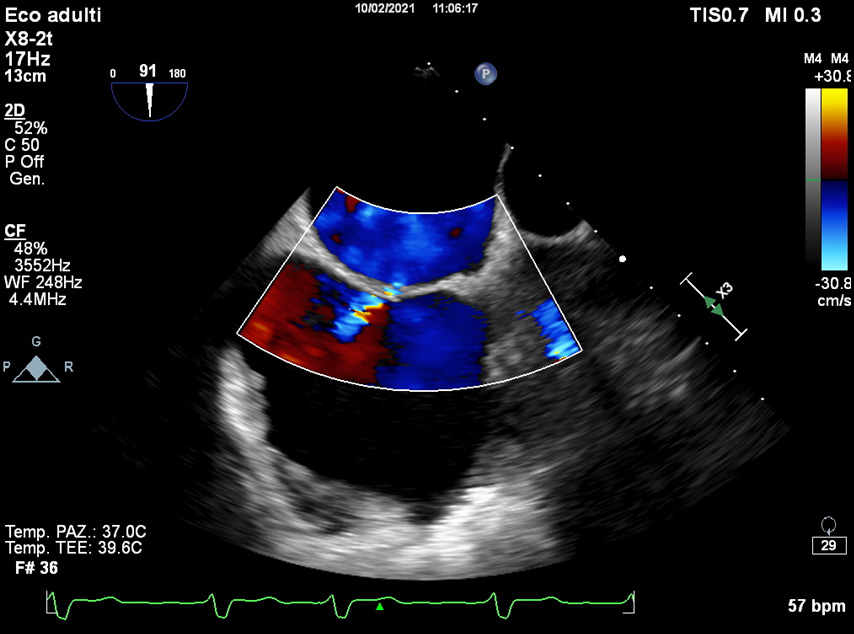 Fig. 10.
Fig. 10.Post-operative TOE. Residual atrial septum defect (ASD): the majority of procedure-related ASD disappear.
• Exclude device erosions or infections: endocarditis prophylaxis for 6 months after closure device implantation is recommended.
Device related thrombus (DRT) have also been reported also 12 months after the procedure [48, 49]: therefore, device surveillance is recommended at 12 months with either TOE or CCTA.
LAAO devices do not remove the short-term need for antithrombotic drugs in the postprocedural period due to the increased risk of DRT before device endothelialization [16, 35, 50]. Thus, the postprocedural management period after LAAO implant remains especially challenging and the optimal postprocedural antithrombotic strategy is uncertain.
Current recommendations for Watchman devices are based on PROTECT AF trial [5]
and PREVAIL trial [6]. For patients without absolute contraindications to OAC,
the postprocedural management protocol consists of aspirin (81–325 mg)
indefinitely and warfarin for 45 days. At 45 days (
In real-world practice multiple alternate antithrombotic strategies post-LAAO are described in registry studies [51, 52], considering differences between devices. For patients with absolute contraindicated to anticoagulation therapy the protocol is different: begin clopidogrel daily and aspirin daily for 6 months post-implant and then continue on aspirin indefinitely.
LAA is a thin cardiac structure with a highly variable, complex and unique anatomy. LAA occlusion is therefore a complex preventive procedure, considering that there are lots of LAAO devices available and that patient characteristics are dissimilar. Training of physicians performing this procedure is essential. New imaging technologies can facilitate device sizing and LAA anatomy assessment, in order to individualize the interventional approach and to reduce procedural complication rate.
LR and SP conceived of the presented idea. RS, RG, FP developed the theory and performed the computations. AL, GM, MR, CS verified the analytical methods. GQ and AD encouraged AM to investigate LAAO and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
The study fulfills the ethical requirements of the Declaration of Helsinki. Written consent was obtained from the patient.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
