- Academic Editor
†These authors contributed equally.
Symptomatic chronic internal carotid artery occlusion (CICAO) may lead to stroke and cognitive decline. Although endovascular recanalization has been proven to reduce the risk of future strokes, the effect on cognition remains controversial and requires further exploration. We explored alterations in functional connectivity (FC) and their associations with cognition in patients with symptomatic CICAO before and after carotid revascularization.
Eighteen patients with unilateral CICAO and fifteen healthy controls (HCs) were enrolled. Resting-state functional magnetic resonance imaging (rs-fMRI) and neuropsychological assessment were performed on all participants, before and after 6 months post-recanalization in the patient group. FC alterations in multiple brain networks and their correlations with cognitive scores were analyzed.
The FC of the CICAO group were markedly lower relative to the HC group for the following: the dorsal attention network (DAN) with the ipsilateral (occlusion side, right) middle frontal gyrus and frontal pole; the default mode network (DMN) with the ipsilateral angular gyrus; the visual network (VN) with the ipsilateral fusiform gyrus; and the frontoparietal network (FPN) with middle temporal gyrus on the side contralateral to the occlusion. The decreased FC of the DAN exhibited a positive association with the total score of the Mini-Mental State Examination (MMSE, r = 0.499, p = 0.049), Montreal Cognitive Assessment (MoCA, r = 0.515, p = 0.041), and Backward Digit Span Test (BDST, r = 0.594, p = 0.015), and negatively correlated with the score of Trail Making Test (TMT)-A (r = –0.563, p = 0.023) and TMT-B (r = –0.602, p = 0.014). The CICAO group exhibited significantly increased FC of the DMN seed region with the middle occipital gyrus ipsilateral to the occlusion. Additionally, the VN seed region demonstrated increased FC with the fusiform gyrus ipsilateral to the occlusion following endovascular recanalization. The preoperative FC values of the DMN exhibited a strong positive association with the improvement in TMT-A score (r = 0.629, p = 0.021).
Our exploratory study found that FC disruption may induce cognitive decline in symptomatic CICAO patients. Endovascular recanalization may improve FC within key brain networks, supporting cognitive improvement. The baseline DMN FC was significantly associated with the postoperative improvement in TMT-A scores, suggesting that preoperative DMN FC could serve as a potential predictor of cognitive recovery.
NCT05292729. Registered 1 December 2021, https://clinicaltrials.gov/study/NCT05292729?intr=NCT05292729&rank=1.
Chronic internal carotid artery occlusion (CICAO) is a prevalent cerebrovascular condition. It is mainly characterized with a high risk of ischemic stroke [1, 2, 3], and cognitive dysfunction [4]. The latter is a common neurological deficit in patients with CICAO affecting multiple cognitive domains, which requires significant attention. The prevalence of cognitive decline in CICAO patients ranges between 50% to 67% [5]. The pathogenesis of cognitive decline in CICAO patients is still poorly understood. Cerebral hypoperfusion is considered to be a key contributing factor [2], with long-term hemodynamic disturbances can lead to neuronal damage or disruption of brain microstructures [6]. In patients with symptomatic CICAO, cognitive impairment is closely linked to stroke etiology, pathogenesis, and lesion location, given the heterogeneous nature of stroke [7, 8, 9]. Neuroinflammation, a key consequence of ischemic brain injury, is also a significant contributor to post-stroke cognitive decline [10]. Supporting this, Glumac et al. [11] demonstrated that corticosteroids, by mitigating the perioperative inflammatory response, can reduce the incidence and severity of cognitive decline, further emphasizing the crucial role of inflammatory pathways in the development of post-stroke cognitive impairment.
The current guidelines do not recommend endovascular revascularization for symptomatic CICAO due to lack of high quality evidence [12, 13]. However, for patients with hemodynamic impairment, endovascular therapy may be considered as an alternative treatment option [14]. Previous studies have demonstrated the applicability and safety of endovascular revascularization in this patient population [15, 16, 17]. Successful recanalization not only reduces ischemic events, but prevents continuous brain function impairment by improving cerebral perfusion as well. Notably, the cognitive improvement of symptomatic CICAO patients following carotid revascularization has been successfully demonstrated [18, 19]. The underlying neuro-mechanism is however not well understood and requires further investigation.
The resting-state functional magnetic resonance imaging (rs-fMRI) is increasingly being utilized to assess brain functional connectivity (FC) and its association with cognitive functions. Several investigations have uncovered brain functional alterations in patients with carotid stenosis and showed disruption in brain connectivity [20, 21, 22]. Increased FC following revascularization have been associated with cognitive benefits [6, 23, 24, 25]. Given that CICAO patients are more vulnerable to compromised hemodynamics than those with carotid stenosis [26], the manifestation of FC disruptions in CICAO may differ. Nevertheless, the changes in brain connectivity in symptomatic CICAO patients following carotid revascularization remain unexplored.
This preliminary exploratory study employed rs-fMRI to investigate alterations in FC within the neural networks of symptomatic CICAO patients before and after endovascular revascularization. We further examined the correlation between these FC alterations and cognitive function. Our primary hypothesis was that changes in brain connectivity mediate cognitive decline in patients with symptomatic CICAO. We also hypothesized that improvements in cognitive function following endovascular therapy would be associated with increased FC within specific brain networks.
Our study has a small-sample exploratory design. Between January 2022 to May
2023, 18 patients with symptomatic unilateral CICAO and who were candidates for
endovascular recanalization were enrolled into the study. Confirmed symptomatic
CICAO due to a diagnosis of transient ischemic attack (TIA) or infarction within
the distribution territory of the affected carotid artery during 6 months
preceding enrolment in the study. The following inclusion criteria were applied:
(1) age
Patients were administered two antiplatelet agents (aspirin and clopidogrel) for a minimum of 5 days prior to undergoing endovascular revascularization. The procedure was performed via the femoral artery access under local anaesthesia and general heparinization, utilizing embolic-protection devices. Technical success was characterised by the successful implantation of stents following the recanalization of the occluded carotid artery, achieving a final residual diameter stenosis of 20% or less and modified Thrombolysis in Cerebral Infarction (mTICI) grade 3 antegrade flow [28]. Post-recanalization systolic blood pressure was maintained between 100 and 140 mmHg. Patients were prescribed with antiplatelet agents (aspirin and clopidogrel) for a duration of three months post-intervention and followed by lifelong monoclonal antiplatelet therapy. The occurrence of neurological sequelae, intracranial haemorrhages, and mortality was documented in the postoperative period. Additionally, computer tomography (CT) angiography (CTA) was scheduled at the 6-month follow-up.
In our study, neuropsychological evaluations were conducted by a neurologist who had undergone specialized training. Mini-Mental State Examination (MMSE) (Chinese version) [29] and Montreal Cognitive Assessment (MoCA) (Beijing Version) [30] were used to assess the global cognition. Trail Making Test (TMT)-A and -B were used to examine visuospatial and executive function [31]. Symbol Digit Test (SDT) [32] was employed to examine visual search, perception, and graphomotor speed. Digit Span Test (DST), including Forward DST (FDST) and Backward DST (BDST) [32], was used to evaluate working memory. Neuropsychological assessments were conducted within 7 days before surgery for patients and at baseline for healthy controls. Identical assessments were repeated for patients at the 6-month follow-up after carotid artery recanalization.
Patients received brain MRI sessions at two time points: 1 week prior to carotid recanalization and 6 months following the procedure. Imaging data were obtained using 3.0-Tesla MR system (SIGNA Architect, General Electric (GE) Medical systems, Waukesha, WI, USA and DISCOVERY MR750, GE). A standard 19-channel head coil was used to receive the signal (GE Healthcare, serial number, SN: H01511, Aurora, OH, USA). The subjects were placed on supine, with their heads supported with foam pads and a belt. They were required to close their eyes, avoid thinking about anything and falling asleep during the MRI.
In the SIGNA Architect System (SN: PG75A1900119SC, GE Medical systems),
T1 parameters were as follows: repetition time (TR) of 7.7 ms; echo
time (TE) of 3.1 ms; field-of-view (FOV) of 256 mm; voxel size of 1.0
The initial pre-processing step included flipping the images of patients with left-sided carotid occlusion, standardizing the affected hemisphere to the right-hand side across all participants. Pre-processing of rs-fMRI and T1 data was conducted using the data processing & analysis for brain imaging (DPABI) package (Version 7.0, The R-fMRI Lab, Institute of Psychology, Chinese Academy of Sciences, Beijing, China) [33]. The first 10 time points were excluded to stabilize the magnetic field and allow participants to adapt. Slice timing was corrected by matching the centre slice, while head motion was corrected by spatial realignment to the mean volume of a series of images. The T1 images were co-registered to the fMRI image, segmented and normalized to a group specific template in Montreal Neurological Institute (MNI) space via the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolkit [34] within DPABI package (http://rfmri.org/DPABI). Detrending was applied to fMRI images to remove signal intensity drift. Nuisance regression was carried out to remove physiological noise from cerebrospinal fluid, white matter, and motion (Friston 24 method). The last pre-processing step included applying band-pass filtering between 0.01 and 0.10 Hz, followed by image smoothening using a 4-mm full-width half-maximum (FWHM) Gaussian kernel to reduce the registration error between subjects and improve signal-to-noise ratio.
After the pre-processing, seed-based voxel-wise functional connection analysis was conducted using DPABI. The following 6 networks were evaluated based on a previous study in patients with carotid artery stenosis [15]: salience network (SN), at the seed of dorsal anterior cingulate cortex (–1, 10, 46); visual network (VN), at the seed of left primary visual cortex (–4, –81, –10); default mode network (DMN), at the seed of posterior cingulate cortex (0, –50, 22); sensorimotor network (SMN), at the seed of left primary motor cortex (–41, –20, 62); frontoparietal network (FPN), at the seed of left middle frontal gyrus (–45, 29, 32); and dorsal attention network (DAN), at the seed of left frontal eye field (–26, 6, 48). All spherical seeds with a 4 mm radius were positioned contralateral to the occlusion side.
R version 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria) was
utilized to analyse data in the two groups. Data normality distribution was
tested by the Shapiro-Wilks normality test. Those that followed a normal
distribution were presented as the mean
At the image level, one-sample t-test were performed for both the
control and the patient groups before and after endovascular recanalization, with
a minimum cluster size of 40 voxels and a threshold of p
To explore the relationship between FC alterations and baseline cognitive
performance, the average of the FC strengths for each patient (N = 18) were
extracted from voxels in each distinct cluster. Pearson correlation analysis was
conducted to associate these FC values with their respective cognitive scores.
The relationship between FC alterations in distinct clusters and the changes in
cognitive assessment pre- and post-operation was analysed using partial
correlation analysis, with education years and age included as covariates. All
Pearson correlation analysis were two-sided, and a p
Prior to rs-fMRI analysis, we established the head movement threshold (3.0 mm and 3.0 degree in max head motion) [33] to exclude subjects with excessive head motion. Ultimately, no patients were excluded based on this criterion. Among the 18 patients, infarct lesions were predominantly located in watershed areas, with 15 cases in the internal watershed, 2 cases involving both anterior and posterior watershed regions, and 1 case in the anterior watershed region. The Circle of Willis was evaluated via DSA. The AcomA was observed in eight patients, the PcomA in six patients, with AcomA and PcomA observed in four patients. All patients with symptomatic CICAO underwent endovascular recanalization procedures. Due to the inability of a micro-guidewire to enter the true lumen, there were two failed cases of endovascular recanalization. Another patient who was successfully recanalized suffered a transient exacerbation of neurological symptoms due to new ischemic infarcts in the hemisphere ipsilateral to occlusion which confirmed by MRI scan. No other patients suffered any perioperative complications. Therefore, after excluding the aforementioned three cases, 15 patients completed the follow-up.
The baseline characteristics and cognitive function evaluations are presented in Table 1. Comparative analysis confirmed that the two groups did not markedly differ in age, sex, education, and vascular risk factors. Marked differences were observed in cognitive performance, with patients with symptomatic CICAO exhibiting lower MMSE, MoCA, SDT, FDST, and BDST scores. Conversely, patients presented with significantly higher TMT-A and -B scores. These findings suggested a decline in general cognition and several specific cognitive domains, including attention, working memory, visual-spatial function and executive function in the symptomatic CICAO patients compared to the HCs.
| HCs (N = 15) | Patients (N = 18) | p values | |
| Sex-male | 12 (80%) | 15 (83.3%) | 1.000 |
| Age | 57.7 |
62.7 |
0.078 |
| Education | 6 (6, 9) | 6 (6, 9) | 0.817 |
| Smoking | 6 (40%) | 11 (61.1%) | 0.303 |
| Drinking | 2 (13.3%) | 6 (33.3%) | 0.242 |
| Hypertension | 10 (66.7%) | 14 (77.8%) | 0.697 |
| Diabetes | 3 (20%) | 6 (33.3%) | 0.458 |
| MMSE | 29 (28.5, 30) | 25 (24, 26) | |
| MoCA | 28 (27, 28) | 23 (20, 23) | |
| SDT | 30 (28, 33.5) | 14.5 (10, 22) | |
| TMT-A | 50 (48, 51) | 109.5 (98, 135) | |
| TMT-B | 87.6 |
178.8 |
|
| Forward DST | 8 (8, 8) | 6 (6, 7) | |
| Backward DST | 7 (7, 7) | 5 (5, 5) |
*: denotes statistical significance. MMSE, Minimum Mental State Examination; MoCA, Montreal Cognitive Assessment; TMT, Trail Making Test; SDT, Symbol Digit Test; DST, Digit Span Test; HC, healthy control; CICAO, chronic internal carotid artery occlusion.
The CTA performed at 6 months in 15 patients and confirmed no restenosis or occlusion. During the 6-month follow-up period, patients who achieved successful recanalization had no recurrent stroke or TIA. The MMSE, MoCA, and FDST scores were higher in the patient group after carotid recanalization (Table 2).
| Cognitive score | Pre-operation | Post-operation | p values |
| MMSE | 25 (24, 26) | 26 (25, 27) | 0.009* |
| MoCA | 23 (20, 23) | 26 (21.5, 26) | 0.019* |
| SDT | 15.4 |
17.7 |
0.424 |
| TMT-A | 114.8 |
104.6 |
0.257 |
| TMT-B | 182.2 |
172.9 |
0.653 |
| Forward DST | 6 (6, 7) | 7 (7, 8) | 0.009* |
| Backward DST | 5 (5, 5) | 5 (5, 6) | 0.217 |
*: denotes statistical significance.
In the patient group before endovascular recanalization, the side with ipsilateral to the occlusion (right) exhibited asymmetrical hypo-connectivity within the 6 selected networks, with seeding performed at the occlusion contralateral side (left; Fig. 1). Hypo-connectivity of a specific network in patients with symptomatic CICAO was defined by abnormally lower connectivity strengths compared to those observed in HCs. At baseline, for the DAN seed, the patients group showed disrupted FC particularly within the ipsilateral (occlusion side, right) frontal pole and middle frontal gyrus compared to the HCs. The DMN seed region revealed reduced FC with the ipsilateral angular gyrus, while the VN seed region showed reduced FC with the ipsilateral fusiform gyrus (Fig. 2, Table 3). Additionally, the FPN seed region displayed reduced FC with the middle temporal gyrus on the side contralateral to the occlusion (Fig. 2, Table 3). Notably, the between-group differences in the SMN and SN were not significant.
 Fig. 1.
Fig. 1.
A within-group examination of six brain networks in patients and healthy controls. The predefined regions of interest (ROIs) for specific networks are indicated by hollow circles. Patients with left-sided of carotid occlusion were flipped to the right side. Color bars indicate T scores. DAN, dorsal attention network; SMN, sensorimotor network; FPN, frontoparietal network; DMN, default mode network; VN, visual network; SN, salience network.
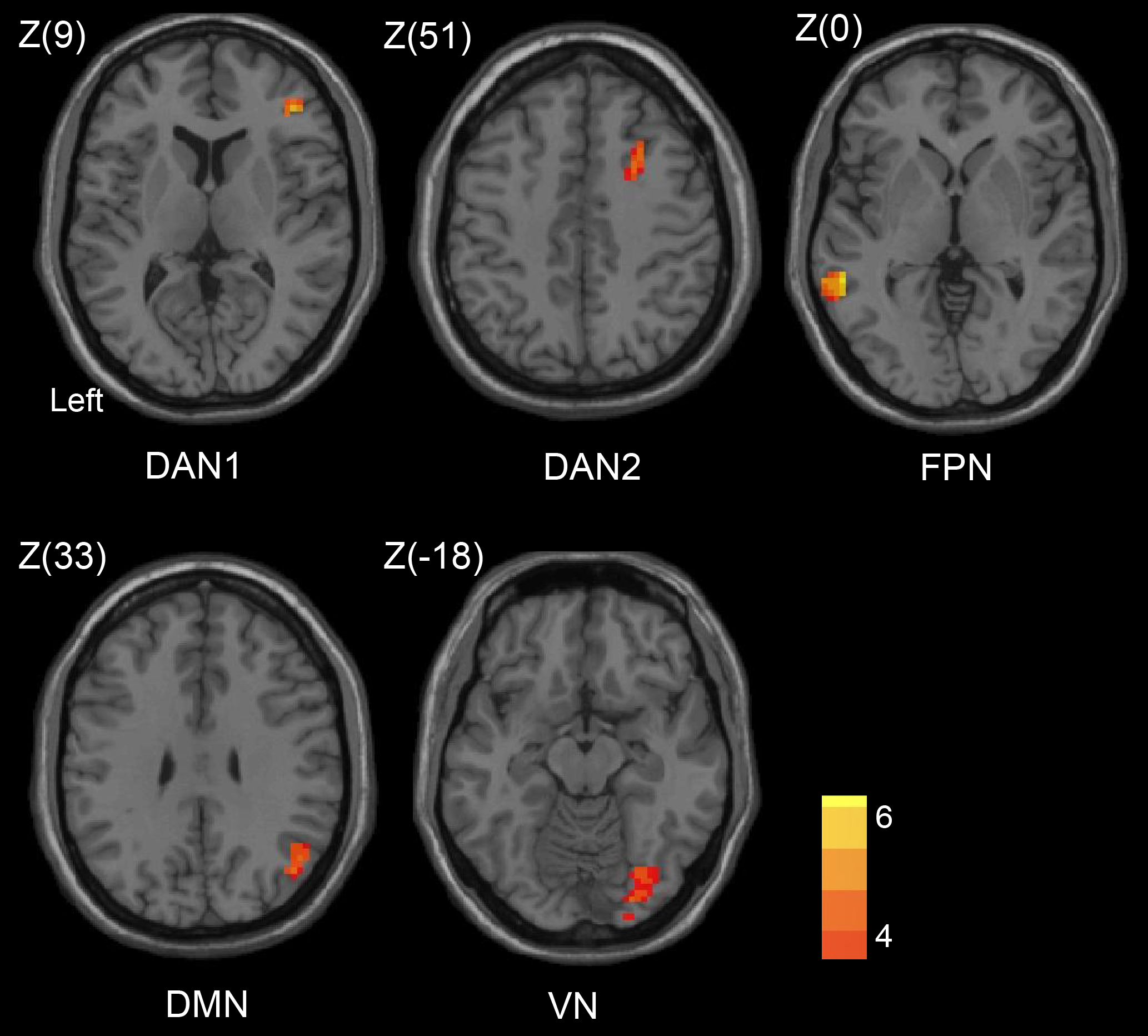 Fig. 2.
Fig. 2.
Group comparisons of six brain networks between patients and
HCs. Clusters with significant increments of functional connectivity are shown
in red. Patients with left-sided of carotid occlusion were flipped to the right
side. Color bars indicate T scores (voxel-level p
| Networks | Coordinates (MNI) | Cluster size | T score | |||
| X | Y | Z | ||||
| Dorsal attention network | ||||||
| Frontal pole (R) | 42 | 39 | 9 | 55 | 4.22 | |
| Middle frontal gyrus (R) | 33 | 27 | 51 | 43 | 4.43 | |
| Frontoparietal network | ||||||
| Middle temporal gyrus (L) | –57 | –42 | 0 | 86 | 5.69 | |
| Default mode network | ||||||
| Angular gyrus (R) | 45 | –57 | 33 | 43 | 3.83 | |
| Visual network | ||||||
| Occipital fusiform gyrus (R) | 27 | –87 | –18 | 96 | 4.53 | |
Patients with left-sided carotid occlusion were flipped to the right side. MNI, Montreal Neurological Institute; R, right; L, left.
We further assessed the correlation between FC changes and cognitive function, revealed: a positively correlation between a reduction in DAN with frontal pole (ipsilateral to occlusion) and the total score of MMSE (r = 0.499, p = 0.049), MoCA (r = 0.515, p = 0.041), BDST (r = 0.594, p = 0.015); and a negative association with TMT-A (r = –0.563, p = 0.023) and TMT-B scores (r = –0.602, p = 0.014) (Fig. 3).
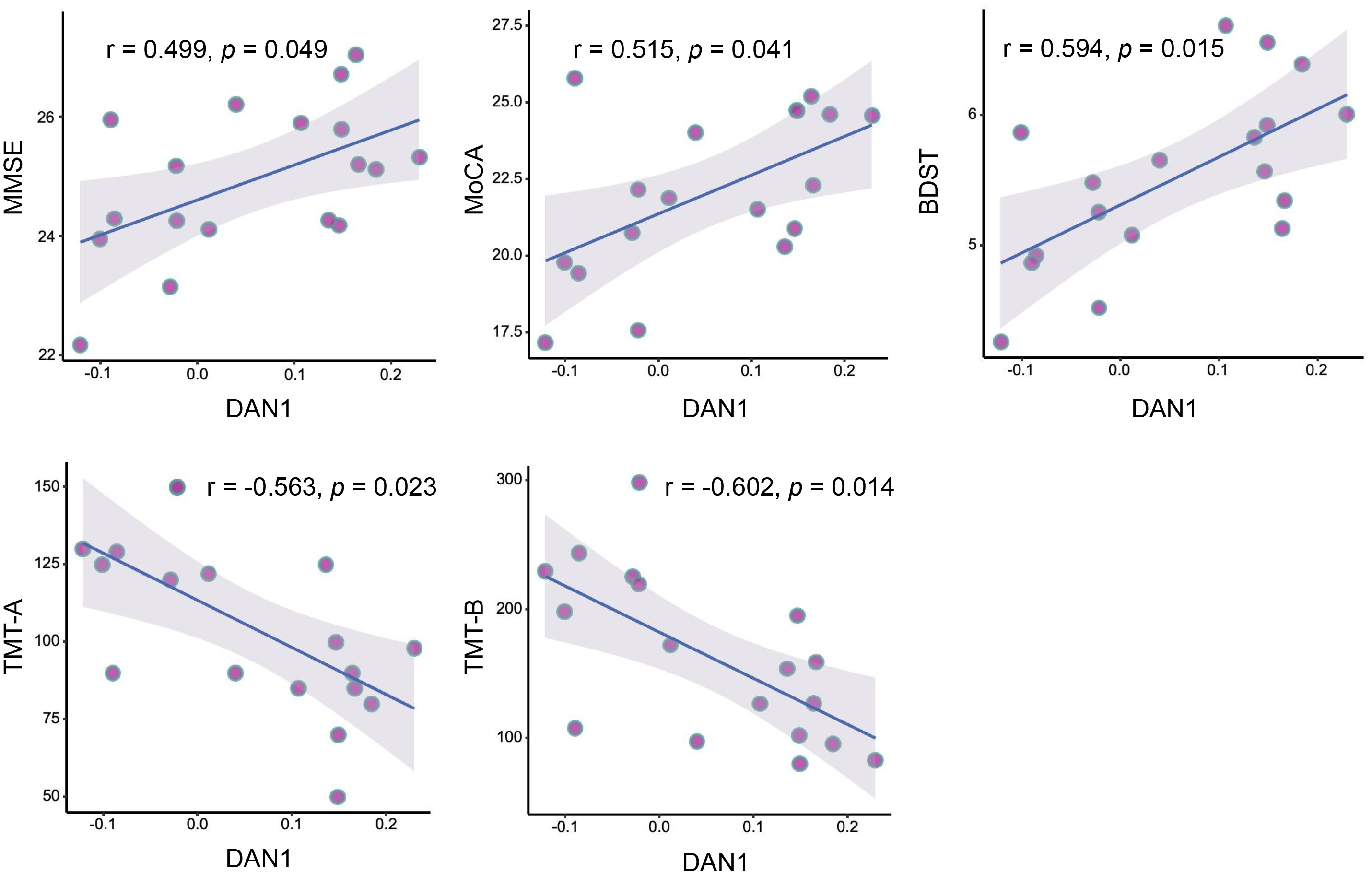 Fig. 3.
Fig. 3.
Pearson correlation analysis between FC strength and neurocognitive performance at baseline. DAN1, dorsal attention network (at the frontal pole ipsilateral to occlusion); BDST, backward Digit Span Test.
Six months after endovascular recanalization, the inter-hemispheric FC exhibited
a symmetrical shape, particularly in the DMN, similar to manifestations observed
in HCs (Fig. 4). The two-sample paired t-test, adjusted for mean FD of
head motion, revealed significant FC increases. Specifically, the DMN seed region
exhibited enhanced FC with the middle occipital gyrus ipsilateral to occlusion,
and the VN seed region showed increased FC with the fusiform gyrus ipsilateral to
occlusion following endovascular recanalization (voxel-level p
 Fig. 4.
Fig. 4.
FC maps at the group-level in the DMN. From left to right:
healthy controls (A), CICAO patients before carotid recanalization (B), 6 months
after carotid recanalization (C). Color bars indicate T scores (voxel-level
p
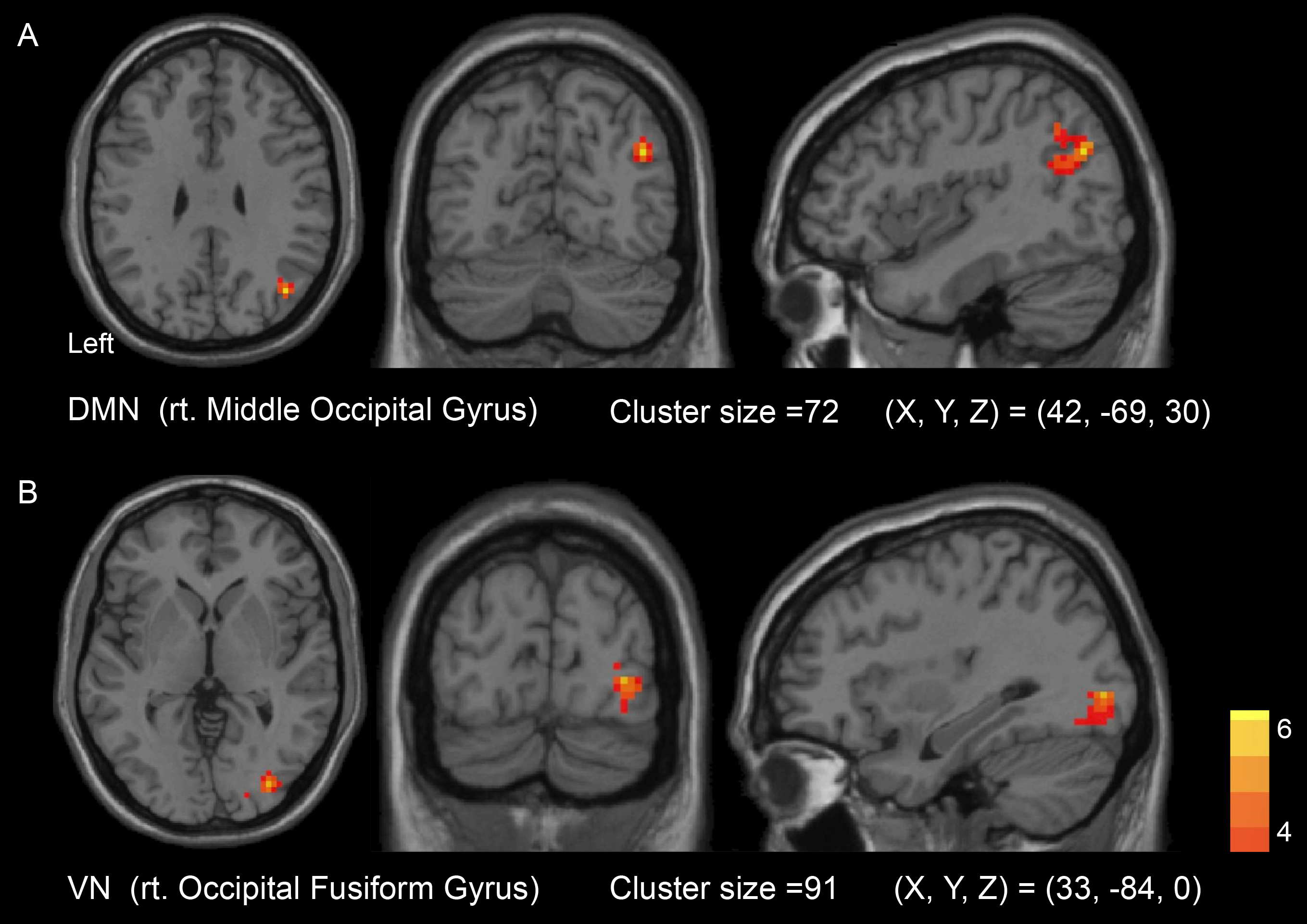 Fig. 5.
Fig. 5.
Seed-to-voxel contrast between post-operation and
pre-operation of carotid recanalization showed significantly increased FC
strengths in DMN with right Middle Occipital Gyrus (A) and VN with right
Occipital Fusiform Gyrus (B). Patients with left-sided carotid occlusion were
flipped to the right side. Color bars indicate T scores (voxel-level p
The improvement scores for the patient group were calculated by subtracting the post-operative MMSE, MoCA, SDT, TMT-A, TMT-B, FDST and BDST, from preoperative the scores, and then taking the absolute values. The mean FC values for preoperative and postoperative FC were extracted for two distinct cluster, DMN and VN, and partial correlation analysis revealed a positive association of the preoperative FC values of the DMN with the improvement in TMT-A score (r = 0.629, p = 0.021) (Fig. 6).
 Fig. 6.
Fig. 6.
Partial correlation analysis uncovered a positive association between the preoperative FC of DMN and improvements in TMT-A score.
The impact of carotid recanalization on symptomatic CICAO patients was explored, focusing on the possibility of enhancing cognition and brain connectivity. There were two main findings in this study. First, at baseline, FC showed significantly decreased networks of DAN, FPN, DMN and VN in the patient group compared to HCs. Furthermore, decreased FC in the DAN between frontal pole ipsilateral to occlusion was significantly correlated with cognitive decline in the patient group. Second, both cognitive scores and brain FC were significantly improved after carotid recanalization in symptomatic CICAO patients. The baseline FC strengths of DMN exhibited a positive relationship with TMT-A score improvement after carotid recanalization. However, increased FC of the DMN with ipsilateral middle occipital gyrus to occlusion and VN with ipsilateral fusiform gyrus to occlusion did not correlate with improvements in cognitive scores. These findings suggested that cognitive decline in symptomatic CICAO patients may attributed to the disruptions of FC. Furthermore, carotid revascularization can increase the FC and improve cognitive function in symptomatic CICAO patients.
Park and Friston [36] revealed that neural activity can be understood as a complex interplay of distinct brain networks [37]. Brain networks exhibit intricate interconnectivity, influencing and modulating each other’s activity. Through coordinated function, these networks contribute to various aspects of brain function, including cognition [36]. Before carotid revascularization, the patients showed diffuse disruption of multiple brain networks, including in the DAN (at the frontal pole ipsilateral to occlusion and ipsilateral middle frontal gyrus), DMN (at the ipsilateral angular gyrus), VN (at the ipsilateral fusiform gyrus), FPN (at the contralateral middle temporal gyrus) and none in the SN or SMN. The DAN, crucial for orienting and maintaining attention, encompasses the intraparietal sulcus and frontal eye fields bilaterally [38, 39]. Disruptions in DAN FC have been implicated in cognitive decline observed in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) [40, 41, 42]. The FPN, comprising the posterior parietal cortex and lateral prefrontal cortex, serves as a critical hub for cognitive regulation, particularly fluid intelligence [43]. Furthermore, frontoparietal regions controls in various cognitive functions, such as mental imagery, episodic memory retrieval and working memory [44]. In our study, the FPN seed region showed reduced FC with the middle temporal gyrus contralateral to occlusion, indicating that the unilateral internal carotid artery occlusion not only affects the brain connectivity of the affected hemisphere but also impacts the contralateral hemisphere. The DMN has been extensively studied [45] and with evidence suggesting its role, including reviewing past knowledge and processing memory [46]. Zhang et al. [47] reported disruption FC in the DMN influenced the disease severity in AD patients. In addition, the DMN is closely related to processes associated with episodic memory [48]. The VN interacts with other brain networks, enabling the integration of complex cognitive processes involving visuospatial functions [49]. Reduced VN FC has been linked to cognitive decline, particularly affecting object recognition and visuospatial orientation [50]. In our study, the observed widespread disruptions of FC within these cognitive-related brain networks likely contribute to cognitive impairment in CICAO patients. While comparing the FC of asymptomatic carotid stenosis patients (CAS) and HCs, Lin et al. [22] observed disruptions of FC in the FPN, SN, DMN and DAN. A comparing the rs-FC data in patients with asymptomatic unilateral carotid stenosis or occlusion with age-matched HCs, reported a decrease in connectivity strength in DAN, FPN and DMN [51]. Our results are congruent with the aforementioned reports. In addition, we found that the regions in the DMN, FPN and DAN predominantly in the territory supplied by the internal carotid artery (ICA) were significantly impacted, suggesting that FC disruption in symptomatic CICAO patients may be related to hypoperfusion and region-specific.
Furthermore, the decreased FC in the DAN (at the frontal pole ipsilateral to occlusion) was related to cognitive decline in the patients. The frontal pole, referred to as the Brodmann area 10, plays a vital role in the higher-order cognitive domains [52]. This, in combination with the above results, suggests that disruptions in FC between the DAN and the frontal pole, play a crucial role which lead to cognitive decline in symptomatic CICAO patients.
This study, observed a marked increase in FC in DMN (at the middle occipital gyrus ipsilateral to occlusion) in patients after carotid recanalization. Cheng et al. [23] observed a modest increase of FC in the DMN ipsilateral to stenosis in asymptomatic CAS patients following stenting. Similarly, Kohta et al. [53] reported an increased FC of the DMN after carotid stenting. To the best of our knowledge, this is the first rs-fMRI study performed in patients with symptomatic CICAO, showing differences in rs-FC after carotid recanalization. This is consistent with the aforementioned studies. The increased FC of the DMN, may attribute to the improvement in cerebral perfusion following successful recanalization of the carotid artery [54]. Considering the strong association between the DMN and cognitive function, the observed cognitive improvements in our patients may be partially explained by enhanced FC of the DMN. Furthermore, partial correlation analysis uncovered a positive correlation between the preoperative FC of DMN and the improvements in TMT-A score. This means that the baseline FC strength of the DMN could serve as a significant predictor for cognitive improvement following carotid artery recanalization.
A marked elevation in FC in the VN was also uncovered in patients following endovascular revascularization. The VN, which includes both the primary visual cortex and several higher-order visual areas distributed across the occipital, parietal, and temporal lobes, interacts with other cognitive networks to support higher-level cognitive functions that rely on visuospatial information processing [49]. The fusiform gyrus is an integral part of the ventral visual stream involved in the modulation of visual cognitive functions [55]. Decreased FC in VN (at the fusiform gyrus ipsilateral to occlusion) suggests a reduced transmission of visuospatial information from the primary to the higher cortex. This may be exhibited with the dysfunction of visual cognitive functions. Though VN is mainly supplied by the vertebrobasilar circulation, the patients in this study presented with decreased FC of VN at baseline probably due to the posterior communicating artery and posterior leptomeningeal branch of the brain providing compensatory blood supply to the carotid artery system. Once the carotid artery is recanalized, this compensatory mechanism is reduced or eliminated, resulting in enhanced perfusion of the VN, which may increase the FC of the VN. However, no significant correlation was observed between the increase FC of VN with the improvement of cognition scores.
The following limitations need to be highlighted. Firstly, due to the exploratory design, a power analysis was not conducted. Besides, owing to the limited sample size, multiple comparison corrections were not applied during the Pearson correlation analysis, potentially increasing the likelihood of Type I errors. Moreover, the small sample size also precluded FC analyses stratified by the side of occlusion and sex differences. Recent research has uncovered sex differences in stroke prognosis [56] and vascular cognitive impairment [57]. Secondly, routine postoperative brain MRI scans to detect silent micro-embolism, which may affect cognitive decline, were not performed in the patient group. However, a comparison of the T1 structural imaging before and 6 months after the surgery, revealed that patients who underwent successful recanalization did not exhibit increased lesions postoperatively. Thirdly, the potential impact of learning effects on the assessment of patients’ cognitive functions during follow-up cannot be ruled out. However, given that the reassessment interval was six months, any learning effects, if present, were likely to be minimal. Fourthly, pinpointing the exact timing of occlusion was a significant challenge. The duration of the occlusion could potentially influence the reversibility of cognitive impairments. Fifth, the follow-up period of our study was relatively short, meaning the long-term outcomes of carotid revascularization on cognition are yet to be determined. Finally, data were acquired from two MRI systems, which could potentially introduce bias. To mitigate this potential impact, data from the two systems were processed separately, and instrument model was incorporated as a covariate in the statistical analysis. Therefore, these findings should be interpreted carefully and deserve validation in larger cohorts.
Disruptions in FC within brain networks may induce cognitive decline in symptomatic CICAO patients. Endovascular recanalization improves FC within brain networks, thereby promoting cognitive improvement. The baseline DMN FC may serve as a potential predictor of cognitive recovery. However, as this was a small-scale exploratory study, these findings should be interpreted with caution. Further investigations enrolling more patients and adopting a longer follow-up are advocated to confirm our findings.
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
RJJ, JZ and BYL contributed to the study conception and design. Material preparation, data collection and analysis were performed by RJJ, SXZ, CLD, HFC and ZQX. The first draft of the manuscript was written by RJJ, SXZ and revised by BYL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was approved by Institutional Review Board of the First Affiliated Hospital of Zhejiang university (Reference number: 2021IIT No.772). Written informed consent was obtained from patients or their families/legal guardians. This study has been performed in accordance with the Declaration of Helsinki.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
