- Academic Editor
Stress is a critical determinant of social behavior, with oxytocin playing a key role in buffering stress effects and facilitating social bonding. However, the relationship between stress-induced fear and oxytocin-associated sociability remains unclear, particularly in contexts reminiscent of prior stress. This study investigates whether acute restraint stress (ARS) alters anxiety-related behaviors and prosocial choices, and whether these effects can be modulated by pharmacological intervention targeting the oxytocin and corticotropin-releasing hormone (CRH) systems.
Sprague-Dawley rats were subjected to ARS and assessed for anxiety-like behavior using the elevated T-maze (ETM) and for prosocial behavior using the social choice test (SCT). The effects of the oxytocin receptor antagonist L-368899 and CRH receptor antagonist antalarmin were evaluated in this paradigm. Plasma corticosterone was checked peripherally and the tissue concentrations of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) were measured in the hippocampus, medial prefrontal cortex (mPFC), and amygdala to assess stress-related neurochemical changes in the fear circuit.
(i) ARS rats showed a significant increase in prosocial preference compared to control, an effect blocked by L-368899 or antalarmin. (ii) ARS rats exhibited reduced corticosterone levels, together with shorter avoidance latency, and longer escape latency in the ETM. (iii) Neurochemically, ARS rats had decreased DA and increased NE levels in the mPFC, both of which were normalized by L-368899 treatment.
Oxytocin modulates stress-induced alterations in monoaminergic activity within the mPFC, influencing social choice behavior. These findings provide new insights into the neurobiological mechanisms underlying stress-related sociability and the context-dependent role of oxytocin in fear memory and social behavior.
Stress plays an important role in humans’ daily lives; it helped our ancestors develop problem-solving abilities, with which they were able to leave the jungle some 200,000 years ago [1]. However, stress can also affect cognitive and emotional functions, including sociability [2, 3]. In general, stress reduces an individual’s willingness to engage in social activities, while in other situations, it can facilitate prosocial behavior [4]. However, the mechanisms underlying these alternating effects remain unclear. From a neurobiological perspective, at least the upstream and downstream profiles of the hypothalamic–pituitary–adrenal axis (HPA) axis and neural substrates projecting to stress-related brain areas (particularly the hippocampus, amygdala, as well as medial prefrontal cortex) are highly involved, representing an incorporated way to manage stress throughout the peripheral and central components [5, 6, 7, 8]. Oxytocin plays a key role in integrating these mechanisms.
Oxytocin is a neuropeptide produced in the hypothalamus and released from the posterior pituitary gland [9]. Initially identified as a hormone produced by expectant mothers to aid in childbirth, it has been recognized for its multiple biological utilities with a connection to the HPA axis, including the involvement of corticotropin-releasing hormone (CRH), the key to bridging stress management and social behavior [10, 11, 12]. For example, increasing evidence has indicated that oxytocin, colloquially known as the ‘love hormone’, enhances feelings of social bonding [13, 14]. However, it has also been reported to enhance emotional distress, in which oxytocin-induced social behavior may be interpreted as an outcome of conflicting or opposing factors [15, 16]. Diversity in the milieu or contextual environment in which oxytocin may operate to differentially modulate stress-induced psychological outcomes, where appropriate, remains unclear [13]. Furthermore, whether stress/anxiety-related fear memory influences oxytocin-driven prosocial behavior may involve the disruption of neurotransmission across fear memory circuits, including the hippocampus, medial prefrontal cortex (mPFC), as well as amygdala, via a highly area-dependent operating mechanism regulated by oxytocin function requires further investigated [17, 18, 19, 20].
In our previous studies using a rat model of post-traumatic stress disorder (PTSD), we demonstrated that oxytocin successively restored traumatic stress-reduced prosocial contact, whereas CRH was more sensitive to the animals’ anxiety/ambivalence state [20, 21]. However, compared to PTSD, a long-term biological adaptation process, it appears that stress may more commonly trigger quick/reactive effects, as modeled by acute restraint stress (ARS). The ARS is a rodent paradigm of movement constraints that elicits unpleasant reaction [22, 23, 24, 25, 26]; thus it has been used to induce acute stress specific to the inability to escape. Therefore, the objective of the present study was to investigate whether oxytocin-involved social choice is influenced by acute stress, and whether it is related to disturbances in monoamine neurotransmitters, such as serotonin (5-HT), dopamine (DA), and norepinephrine (NE), across different fear circuit areas including the hippocampus, mPFC, as well as amygdala.
In this study, we aimed to fill the significant knowledge gap regarding the role of oxytocin in acutely stressed rats experiencing psychological conflict. These findings may provide novel insights into the relationship between stress and oxytocin-associated prosocial behaviors.
Fig. 1 is a schematic diagram of the experimental design for this study. In brief, ARS rats were employed, and their behavioral performance were measured by social choice test (SCT) and elevated-T maze (ETM), referring to prosocial behavior and anxiety activity, respectively. The ARS procedure was employed to mimic real-life stressful psychosocial events [27, 28, 29]. During the ARS, rats were restrained in a ventilated plastic holder with an adjustable top for two hours. Afterward, they were isolated in their home cages for one day. Meanwhile, the rats that did not undergo ARS remained undisturbed in their cages.
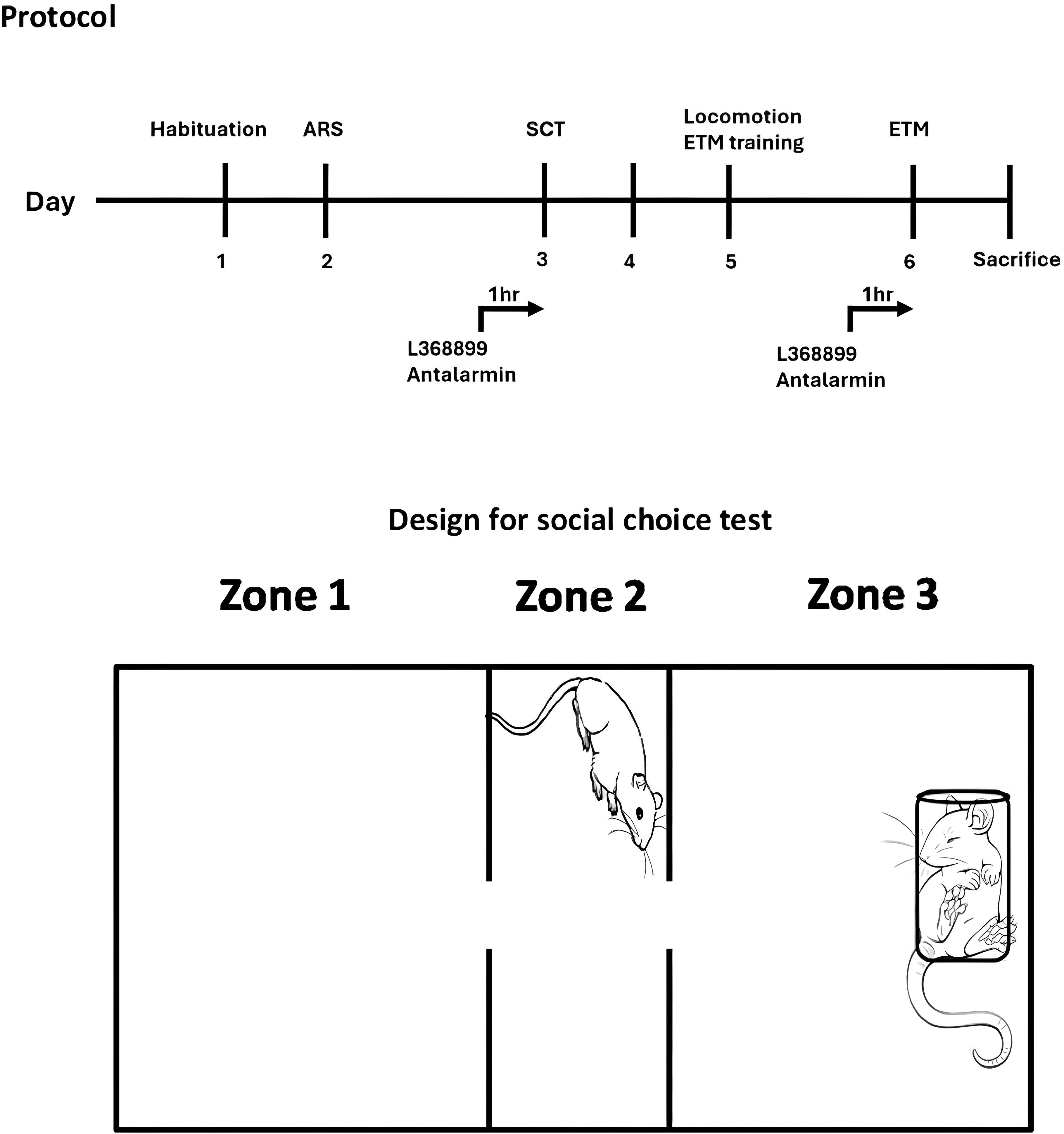 Fig. 1.
Fig. 1.
The experimental procedure (the upper half of the figure) and the design of social choice test (SCT). L-368899 (5 mg per kg, i.p.) or Antalarmin (20 mg per kg, i.p.) was administered 1 hour before SCT and ETM. Tissue levels of 5-HT, DA and NE in the hippocampus, mPFC, as well as amygdala were measured after sacrifice. ARS, acute restraint stress; ETM, elevated T maze; mPFC, medial prefrontal cortex; 5-HT, serotonin; DA, dopamine; NE, norepinephrine.
Our paradigm of SCT is a three-chamber design which was modified from previous social interaction paradigm [30, 31], to reflect not only rats’ social choice for the chamber placing a holder-restrained conspecific rat, it also effectively examines the effect of fear memory as the experimental rats being restrained in the same holder previously when underwent ARS [20]. ETM provides both behavioral profile of conditioned and non-conditioned anxiety, thus is broadly employed in stress paradigm [32, 33]. Neurochemical assessments included measuring plasma corticosterone levels and analyzing monoamines concentrations in the hippocampus, mPFC, as well as amygdala. The endpoint of the experiment was done by decapitating the rats after they were exposed to 5% isoflurane (#1001936040, Baxter International Inc., Deerfield, IL, USA) vapor until death.
Oxytocin antagonist (L-368899 hydrochloride, Tocris Bioscience, Bristol, UK) was obtained from Tocris Bioscience, UK, it was dissolved in saline and administered intraperitoneally (i.p.) at a dose of 5 mg per kg, one hour prior to SCT and ETM. The dose of L-368899 was chosen based on previous studies [34, 35]. Corticotropin releasing hormone receptor 1 (CRHR1) antagonist antalarmin was obtained from Sigma-Aldrich, St. Louis, MO, USA (A8727). It was dissolved in 1:10 ratio of Tween 80 (P4780, Sigma-Aldrich, St. Louis, MO, USA)/saline and intraperitoneally administered at 20 mg per kg 1 hour before SCT and ETM. For all groups, drugs were freshly prepared before administration. Each control group received the corresponding vehicle of the drug being tested (saline).
We used 24 male and aged 8 weeks Sprague–Dawley (SD) rats in this study, these rats were purchased from BioLASCO Co., Ltd. When the rats arrived at the animal center of National Defense Medical Center, they were randomly assigned to four groups (3 rats in one cages), including control-vehicle (CON-veh), ARS-vehicle (ARS-veh), ARS-L368899, ARS-antalarmin (N = 6), respectively.
The rats were housed under controlled temperature and humidity conditions, with house lights on from 7:00 AM to 7:00 PM at the animal center of National Defense Medical Center. Standard laboratory chow (Ralston Purina, St. Louis, MO, USA) and sterile filtered water were provided ad libitum.
The behavioral tests in this study began at 8:00 AM and were completed by no later than 6:00 PM, and the experiment was conducted at the same time for each rat as much as possible. The animal care committee of NDMC approved the experimental procedures and ethical guidelines (IACUC-17-300). Every effort was made to minimize animal use and to alleviate any potential suffering.
The locomotor activity was similar to the protocol used in previous study [36], Two hours prior to ETM, the rat was individually placed in a white box (40 cm in width, 40 cm in depth, as well as 20 cm in height) containing 16 squares (10 cm in width and 10 cm in depth for each) and allowed to explore the environment for 5 min. During this period, the number of rears and total line crossings were recorded as indicators of vertical and horizontal activity, respectively.
The SCT was used to assess social responses and empathy-like behaviors [30, 31].
It was employed in a non-transparent white acrylic box (SCT box, 60 cm in width,
30 cm in depth, as well as 30 cm in height.) with three chambers: zone 1
(designated as the non- social zone), zone 2 (designated as the center), as well
as zone 3 (designated as the social zone). Each SCT trial lasted 300 seconds,
during which a non-familiar conspecific rat was placed in a transparent
plexiglass restrainer (8.75 cm in width
During the initial 3 days, all tested rats were habituated to the SCT box for 10 min daily. On the test day, the tested rat was placed in the center chamber of SCT box (zone 2). The time spent in each zone and the interactions between the two rats were recorded and the social choice-related behavior was evaluated by two senior experimenters who remained blinded to the group assignment throughout the study to ensure unbiased data collection and interpretation. Note that a rat was counted only when all four of its paws were in that zone.
The ETM was performed three days after the SCT, similar to the protocol used in previous studies [33, 37], in which the performance of avoidance latency and escape latency are considered to reflect generalized and panic anxiety in human, respectively [38].
The black ETM apparatus featured a 50-cm high T-shaped platform with three equal arms (12 cm in width and 50 cm in depth), a 40 cm-high black walls surrounded the enclosed middle arm and 1 cm-high black barriers surrounded the two open side arms. For the training stage of ETM, we blocked the enclosed middle arm and then placed the rats on the one of the open side arm for 30 min. The test stage was conducted 24 hours after the training stage of ETM, the rats were cautiously placed along the wall at the enclosed arm’s end, and the experimenter maintained a distance of at least 5 meters until each trial was completed. The time spent to leave the arm was recorded (defined as the moment all four paws exited the arm). This process was repeated three times, corresponding to baseline, avoidance 1 latency, and avoidance 2 latency. After the avoidance 2 trial, the rats were positioned at the open arm’s end with their heads facing the center, and the escape latency was recorded as the time taken for all four paws to enter the middle enclosed arm. The intertrial interval (ITI) was set at 180 seconds, with a maximum duration of 300 seconds for each trial. A trial concluded as soon as the rats moved to a different arm. To minimize potential habituation effects, the rats were carefully returned to their home cages immediately after each trial.
A 0.5 cc blood samples were collected from the rats’ tail veins between 2:00 PM and 3:00 PM to ensure that corticosterone stays at its relative lower level to avoid the influence from circadian change [39]. The blood samples were quickly mixed with 1 cc heparin sodium. After centrifuging the blood samples at 6000 g for 10 minutes at 4 °C, the supernatants (plasma) were collected and the corticosterone concentration was further assessed by using a corticosterone EIA kit (item number: 501320, Cayman Chemical Company, Ann Arbor, MI, USA). The layout and reagent volumes for the corticosterone EIA kit can be found in Supplementary Fig. 2.
Rats were euthanized via rapid decapitation, and their hippocampus, mPFC, as
well as amygdala were rapidly dissected [40]. The process of brain region
dissection was carried out on an icy cold plate. Each brain tissue was carefully
weighed immediately after dissection. The tissues were then homogenized in 0.2 mL
of 7 N perchloric acid (244252, Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at
12,000
In this study, comparisons between the Control-veh and ARS-veh groups were made using an unpaired t-test. Note that if the data failed to normality or equal variance test, the Mann-Whitney rank sum test (MW test) was applied instead. To analyze differences among the ARS-veh, ARS-L368899, and ARS-antalarmin groups, a one-way analysis of variance (ANOVA) was conducted. A p-value of less than 0.05 was considered indicative of statistical significance. The statistical analyses in this study were carried out with SPSS (version 16.0, IBM Corp., Chicago, IL, USA).
For the locomotion activity, unpaired t-test showed no significant difference between Control-veh and ARS-veh (Fig. 2A). Furthermore, one-way ANOVA also revealed no significant difference among the drugs in ARS groups (Fig. 2B).
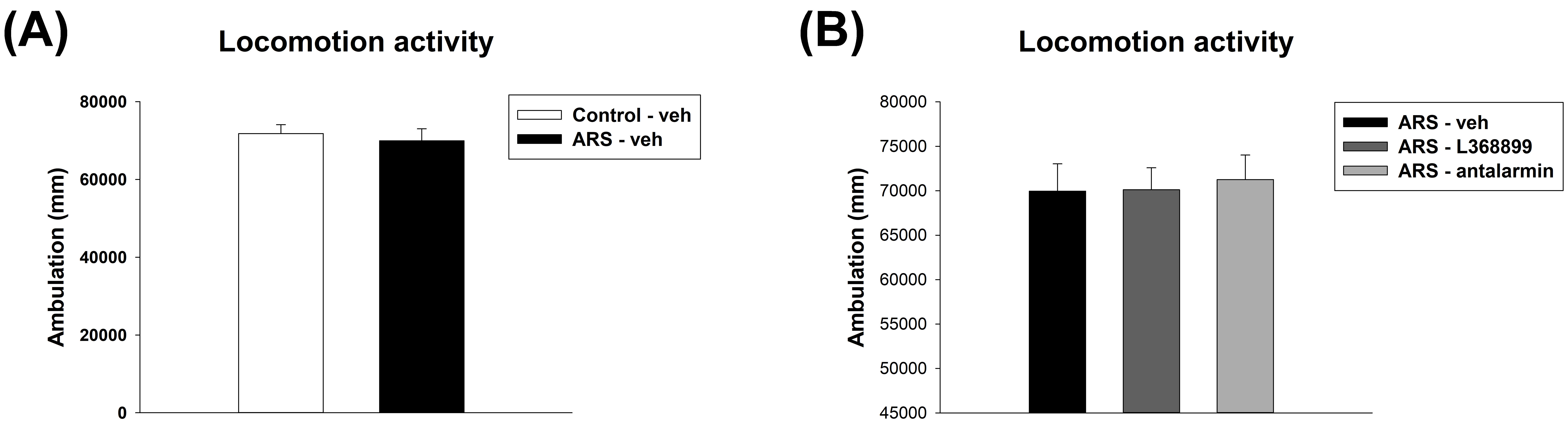 Fig. 2.
Fig. 2.
The locomotor activity. The ARS procedure and drugs (L368899
and antalarmin) administration did not affect locomotor activity. (A) The
locomotor activity between the Control-veh and the ARS-veh groups. (B) The
locomotor activity among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The
data are shown as mean
For the contact time to the social partner, unpaired t-test showed a significant
difference between Control-veh and ARS-veh (t(10) = 7.706, p
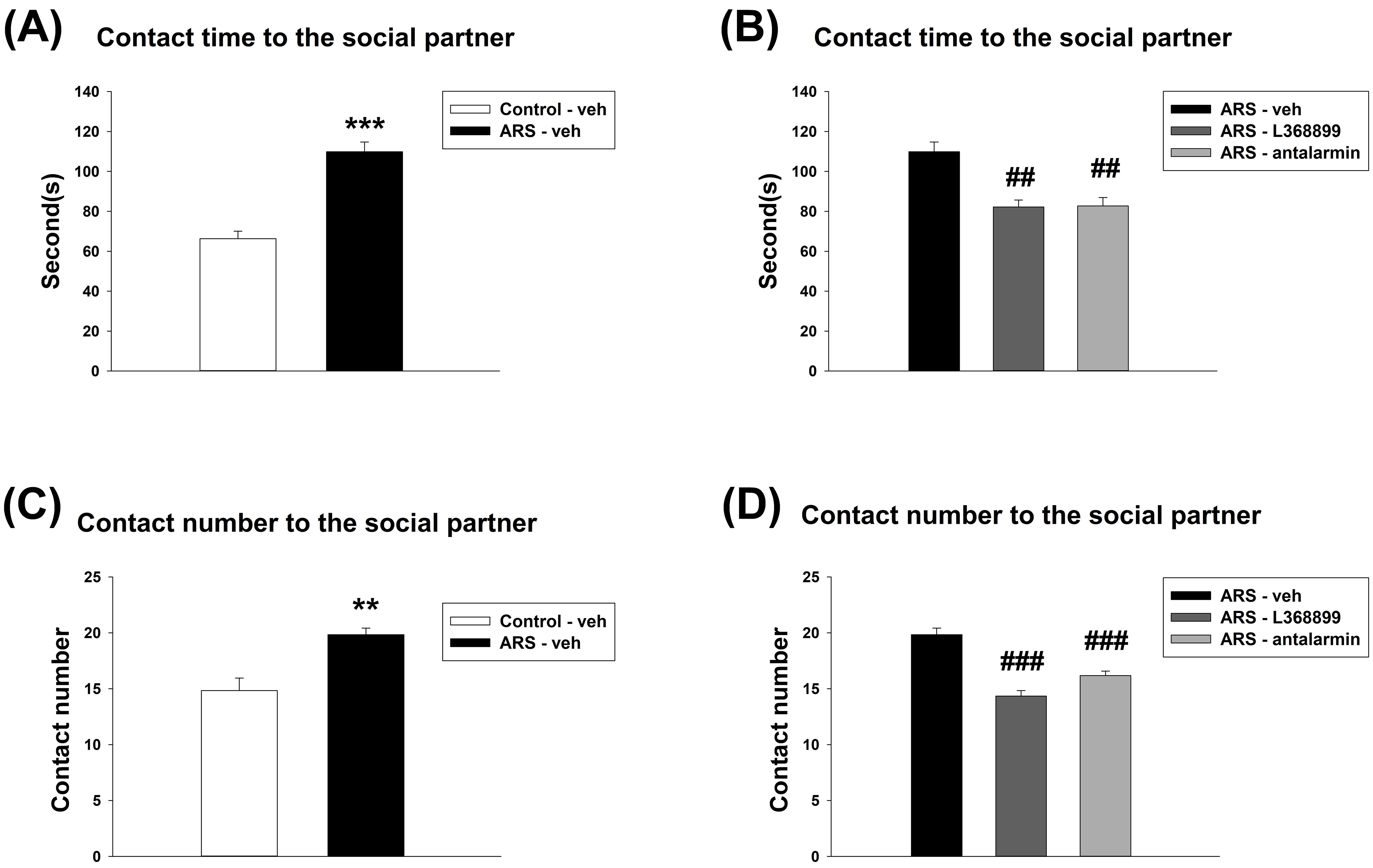 Fig. 3.
Fig. 3.
The contact time and contact number of social choice test. Both
L368899 and antalarmin reduced the enhancement of contact time and contact number
induced by ARS. The contact time (A) between the Control-veh and the ARS-veh
groups and (B) among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The contact
number (C) between the Control-veh and the ARS-veh groups and (D) among ARS-veh,
ARS-L368899, and ARS-antalarmin groups. The data are shown as mean
For the duration spent in Zone 1, unpaired t-test showed no significant difference between Control-veh and ARS-veh (Fig. 4A). Furthermore, one-way ANOVA also revealed no significant difference among the drugs in ARS groups (Fig. 4B). For the duration spent in Zone 2, unpaired t-test showed no significant difference between Control-veh and ARS-veh (Fig. 4C). Furthermore, one-way ANOVA further revealed a significant difference among drugs in the ARS groups (F(2,15) = 4.272, p = 0.034). This difference was driven by the differences observed between ARS-L368899 and ARS- antalarmin (p = 0.041) (Fig. 4D). For the duration spent in Zone 3, unpaired t-test showed no significant difference between Control-veh and ARS-veh (Fig. 4E). Furthermore, one-way ANOVA also revealed no significant difference among the drugs in ARS groups (Fig. 4F).
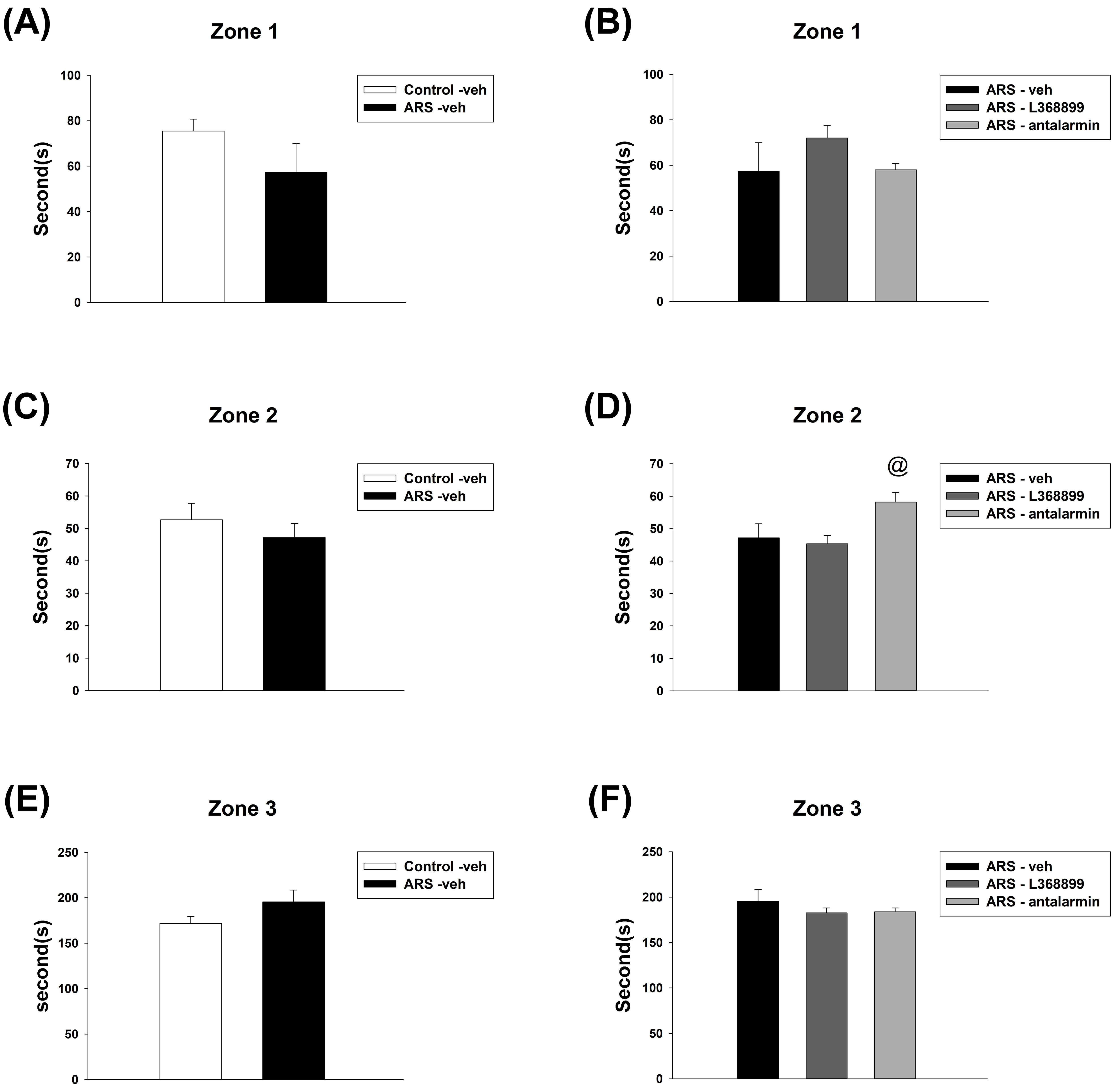 Fig. 4.
Fig. 4.
The time spent in zones 1, 2, and 3 of social choice test. The
ARS procedure and administration of drugs (L368899 and antalarmin) did not
significantly affect the time spent in zones 1, 2, and 3, except for the
ARS-antalarmin group, which spent more time in zone 2 compared to the ARS-L368899
group. The time spent in zones 1 (A) between the Control-veh and the ARS-veh
groups and (B) among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The time
spent in zones 2 (C) between the Control-veh and the ARS-veh groups and (D) among
ARS-veh, ARS-L368899, and ARS-antalarmin groups. The time spent in zones 3 (E)
between the Control-veh and the ARS-veh groups and (F) among ARS-veh,
ARS-L368899, and ARS-antalarmin groups. The data are shown as mean
For the ratio of contact time and contact number to the social partner, unpaired
t-test showed no significant difference between Control-veh and ARS-veh
(Fig. 5A). Furthermore, one-way ANOVA also revealed no significant difference
among the drugs in ARS groups (Fig. 5B). For the ratio of contact time to the
social partner and time spent in Zone 3, unpaired t-test showed a
significant difference between Control-veh and ARS-veh (t(10) = 6.606,
p
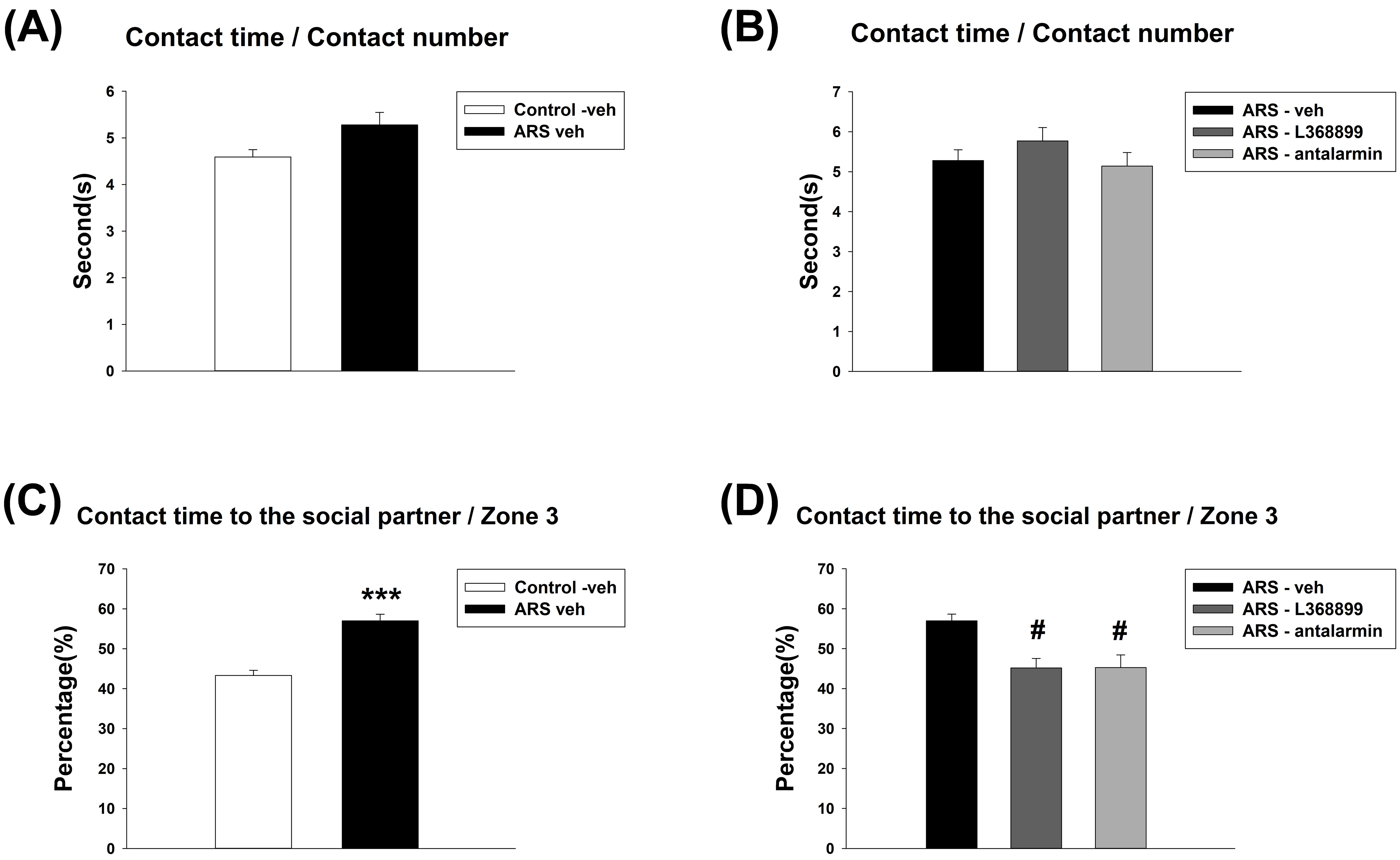 Fig. 5.
Fig. 5.
The ratio of contact time to contact number and the ratio of
contact time with the social partner to time spent in zone 3 of social choice
test. There was no effect on the ratio of contact time to contact number after
the ARS procedure and administration of drugs (L368899 and antalarmin). However,
both L368899 and antalarmin reduced the enhancement of the ratio of contact time
with the social partner to time spent in zone 3 induced by ARS. The ratio of
contact time to contact number (A) between the Control-veh and the ARS-veh groups
and (B) among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The ratio of
contact time with the social partner to time spent in zone 3 (C) between the
Control-veh and the ARS-veh groups and (D) among ARS-veh, ARS-L368899, and
ARS-antalarmin groups. The data are shown as mean
For the avoidance 1 latency of ETM, unpaired t-test showed a significant
difference between Control-veh and ARS-veh (t(10) = 3.413, p
= 0.007) (Fig. 6A). Furthermore, one-way ANOVA further revealed no
significant difference among the drugs in ARS groups (Fig. 6B). For the escape
latency of ETM, MW test showed a significant difference between Control-veh and
ARS-veh (T = 25, p = 0.026) (Fig. 6C). Furthermore, one-way ANOVA
revealed a significant difference among the drugs in ARS groups (H(3) =
6.99, p = 0.03). This difference was driven by a difference observed
between ARS-veh and ARS-L368899 (p
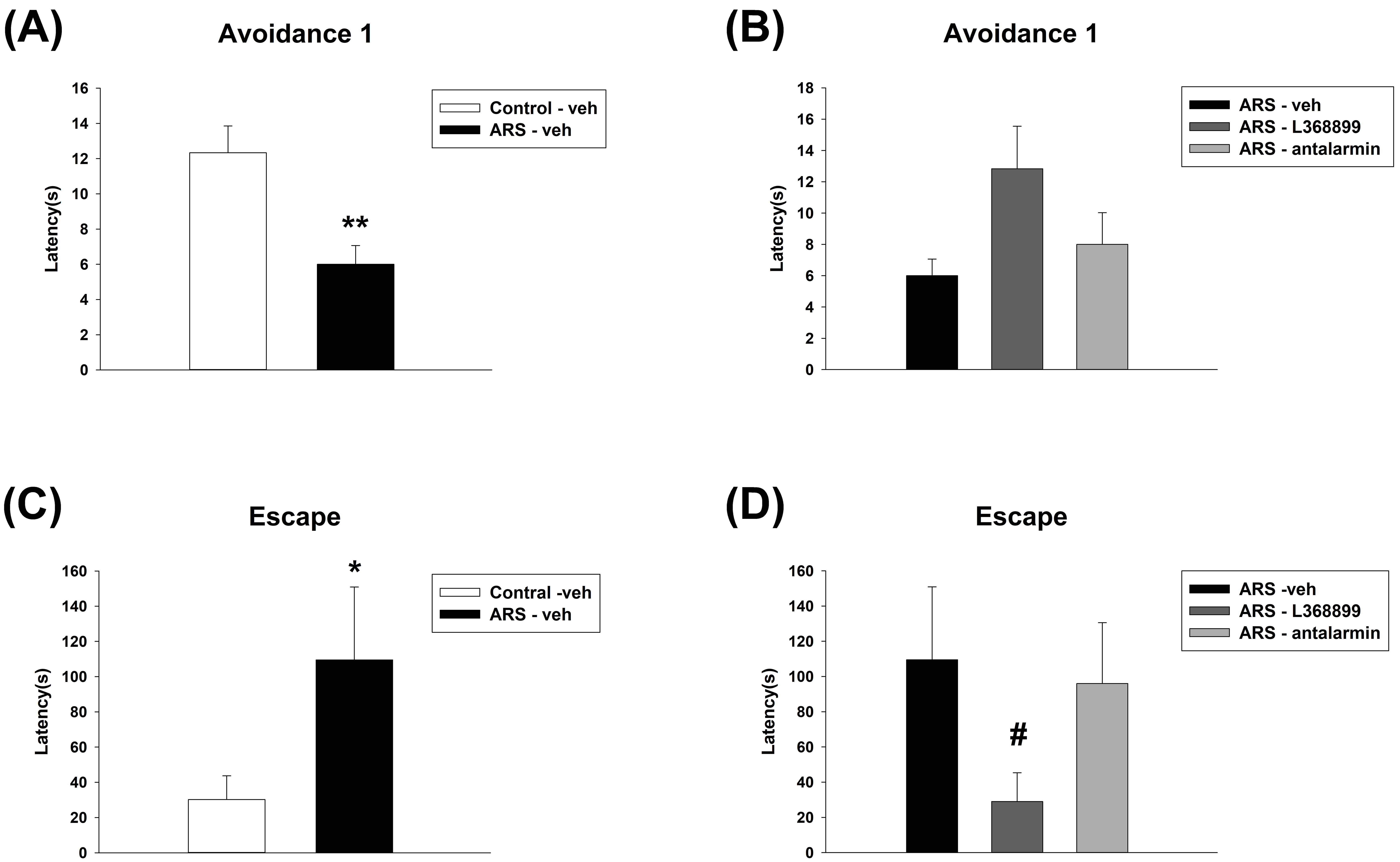 Fig. 6.
Fig. 6.
The avoidance 1 latency and escape latency of ETM test. ARS
reduced avoidance 1 latency and increased escape latency, and only L368899
inhibited the increased escape latency induced by ARS. The avoidance 1 latency
(A) between the Control-veh and the ARS-veh groups and (B) among ARS-veh,
ARS-L368899, and ARS-antalarmin groups. The escape latency (C) between the
Control-veh and the ARS-veh groups and (D) among ARS-veh, ARS-L368899, and
ARS-antalarmin groups. The data are shown as mean
For the concentration of plasma corticosterone, unpaired t-test showed a significant difference between Control-veh and ARS-veh (t(8) = 3.286, p = 0.011) (Fig. 7A). However, one-way ANOVA revealed no significant difference among the drugs in ARS groups (Fig. 7B).
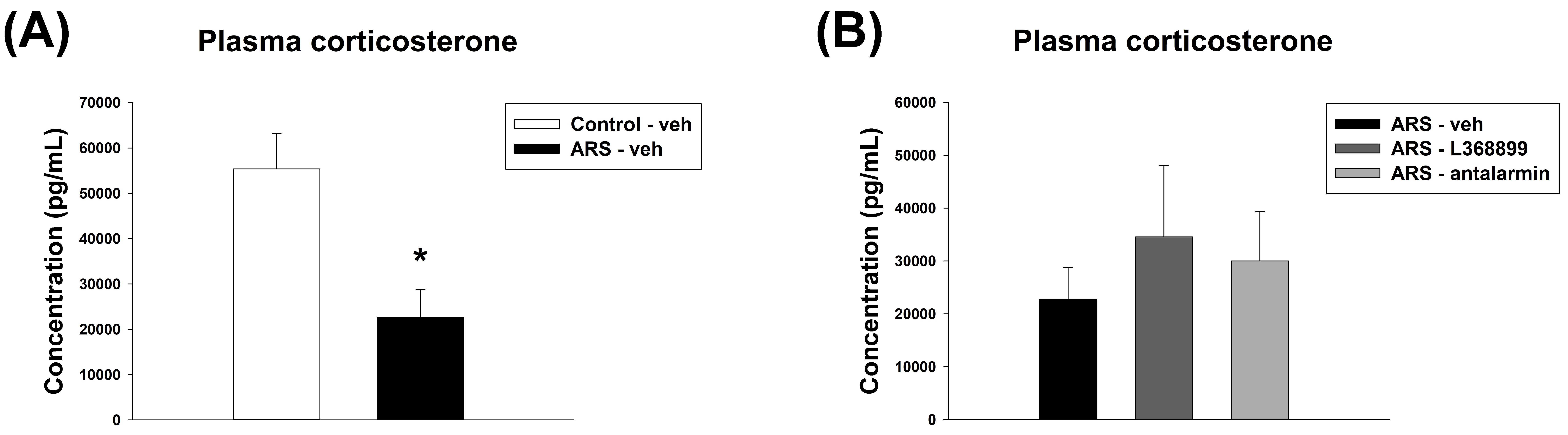 Fig. 7.
Fig. 7.
The plasma corticosterone level. Both L368899 and antalarmin did
not reverse the reduction of plasma corticosterone levels induced by ARS. The
plasma corticosterone level (A) between the Control-veh and the ARS-veh groups
and (B) among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The data are shown
as mean
For the Hippocampus 5-HT level, unpaired t-test showed no significant difference
between Control-veh and ARS-veh (Fig. 8A). Furthermore, one-way ANOVA further
revealed a significant difference among the drugs in ARS groups (F(2,12) =
13.333, p
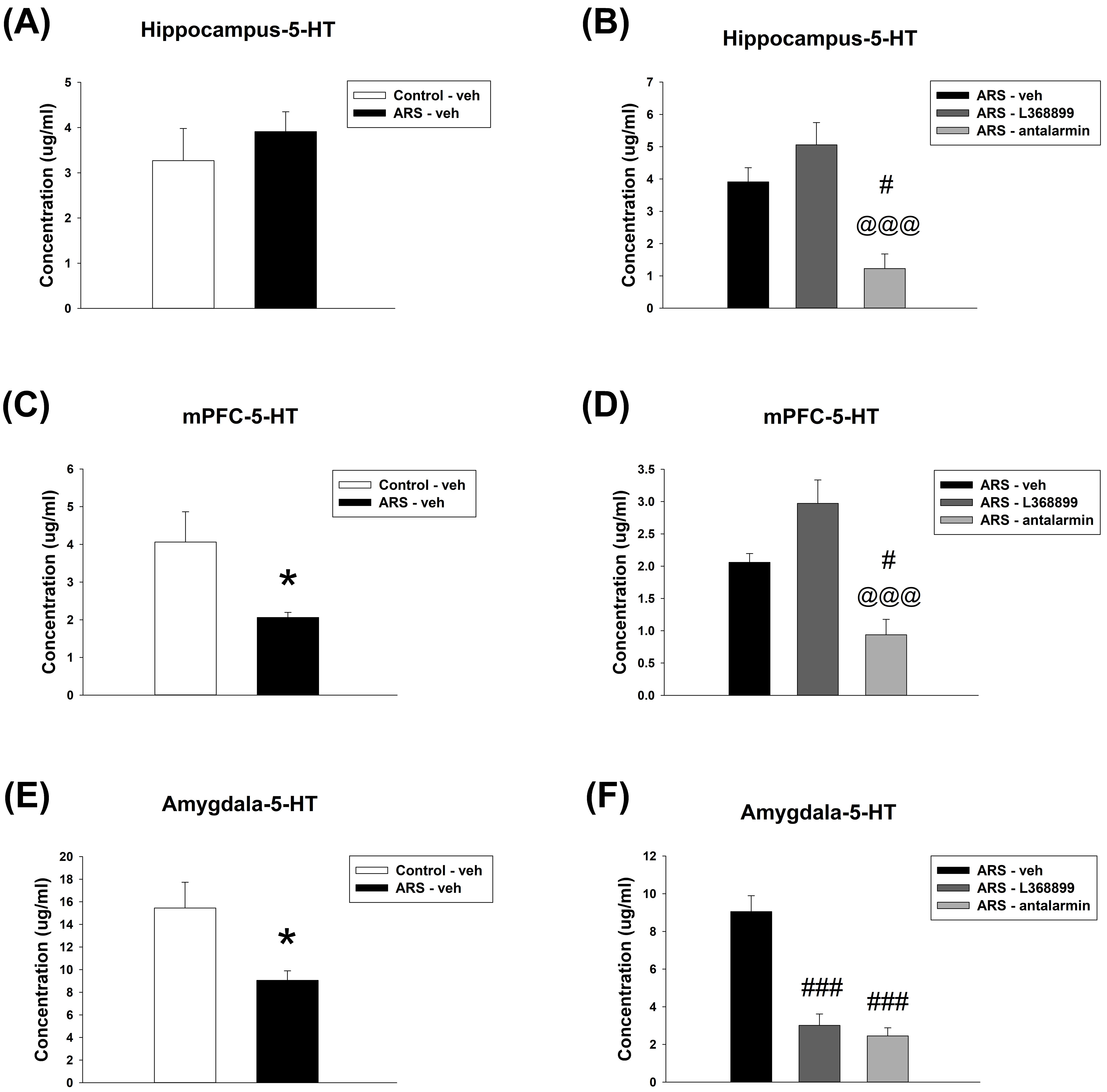 Fig. 8.
Fig. 8.
The 5-HT level in the hippocampus, mPFC, as well as amygdala.
ARS reduced the 5-HT level in the mPFC and amygdala, and antalarmin reduced the
5-HT level in the hippocampus, mPFC, as well as amygdala after ARS. Furthermore,
L368899 also decrease the 5-HT in the amygdala after ARS. The hippocampal 5-HT
level (A) between the Control-veh and the ARS-veh groups and (B) among ARS-veh,
ARS-L368899, and ARS-antalarmin groups. The mPFC 5-HT level (C) between the
Control-veh and the ARS-veh groups and (D) among ARS-veh, ARS-L368899, and
ARS-antalarmin groups. The amygdala 5-HT level (E) between the Control-veh and
the ARS-veh groups and (F) among ARS-veh, ARS-L368899, and ARS-antalarmin groups.
The data are shown as mean
For the Hippocampus DA level, unpaired t-test showed a significant difference
between Control-veh and ARS-veh (t(8) = 7.216, p
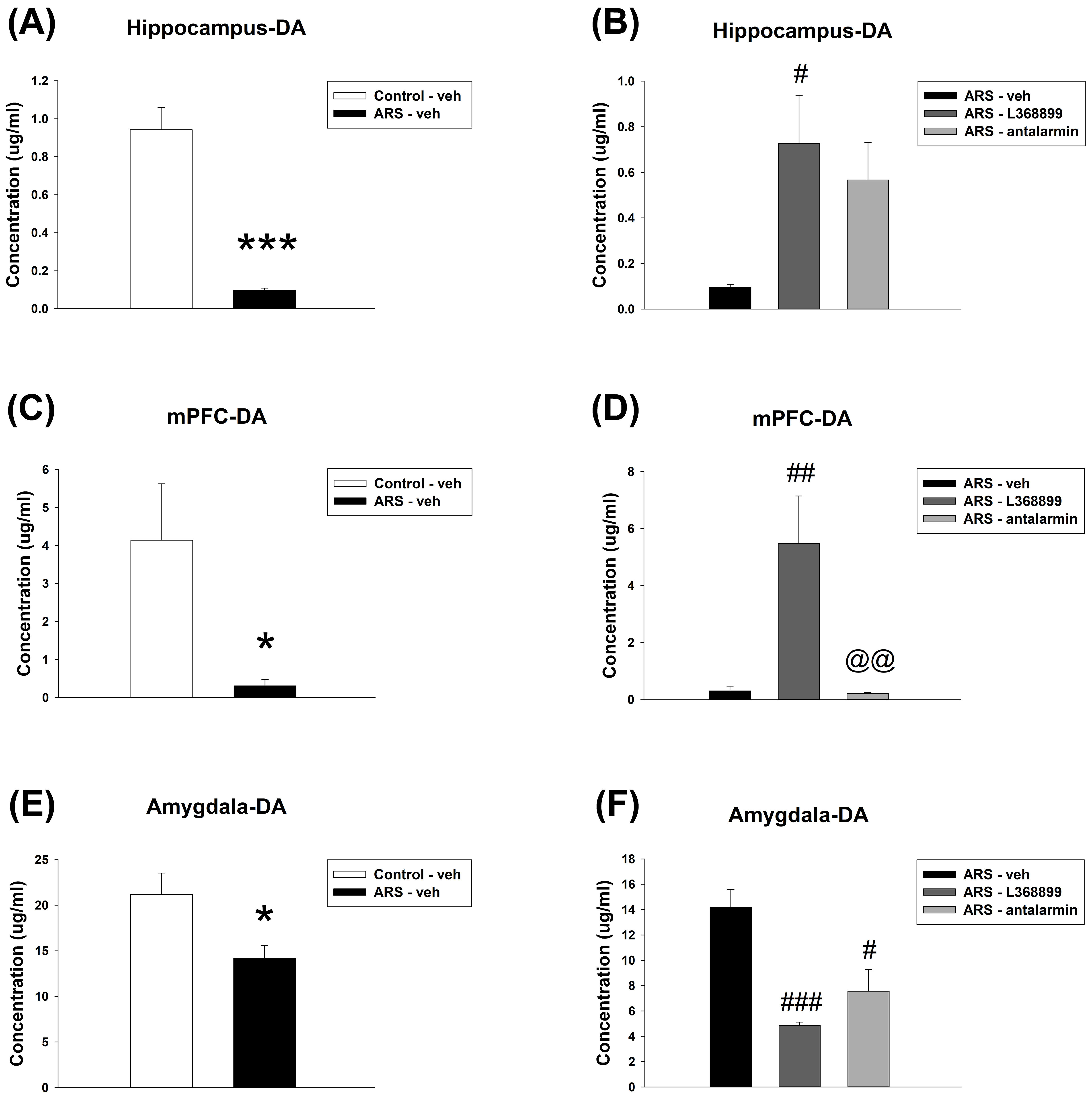 Fig. 9.
Fig. 9.
The DA level in the hippocampus, mPFC, as well as amygdala. ARS
reduced the DA level in the hippocampus, mPFC and amygdala. L368899 reversed the
reduction of DA levels in the hippocampus and mPFC induced by ARS. However, both
the ARS-L368899 and ARS-antalarmin groups had lower DA levels in the amygdala
compared to the ARS-veh group. The hippocampal DA level (A) between the
Control-veh and the ARS-veh groups and (B) among ARS-veh, ARS-L368899, and
ARS-antalarmin groups. The mPFC DA level (C) between the Control-veh and the
ARS-veh groups and (D) among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The
amygdala DA level (E) between the Control-veh and the ARS-veh groups and (F)
among ARS-veh, ARS-L368899, and ARS-antalarmin groups. The data are shown as mean
For the hippocampus NE level, unpaired t-test showed a significant
difference between Control-veh and ARS-veh (t(8) = 2.345, p
= 0.047) (Fig. 10A). Furthermore, one-way ANOVA further revealed no
significant difference among the drugs in ARS groups (Fig. 10B). For the mPFC NE
level, unpaired t-test showed a significant difference between
Control-veh and ARS-veh (t(8) = 7.414, p
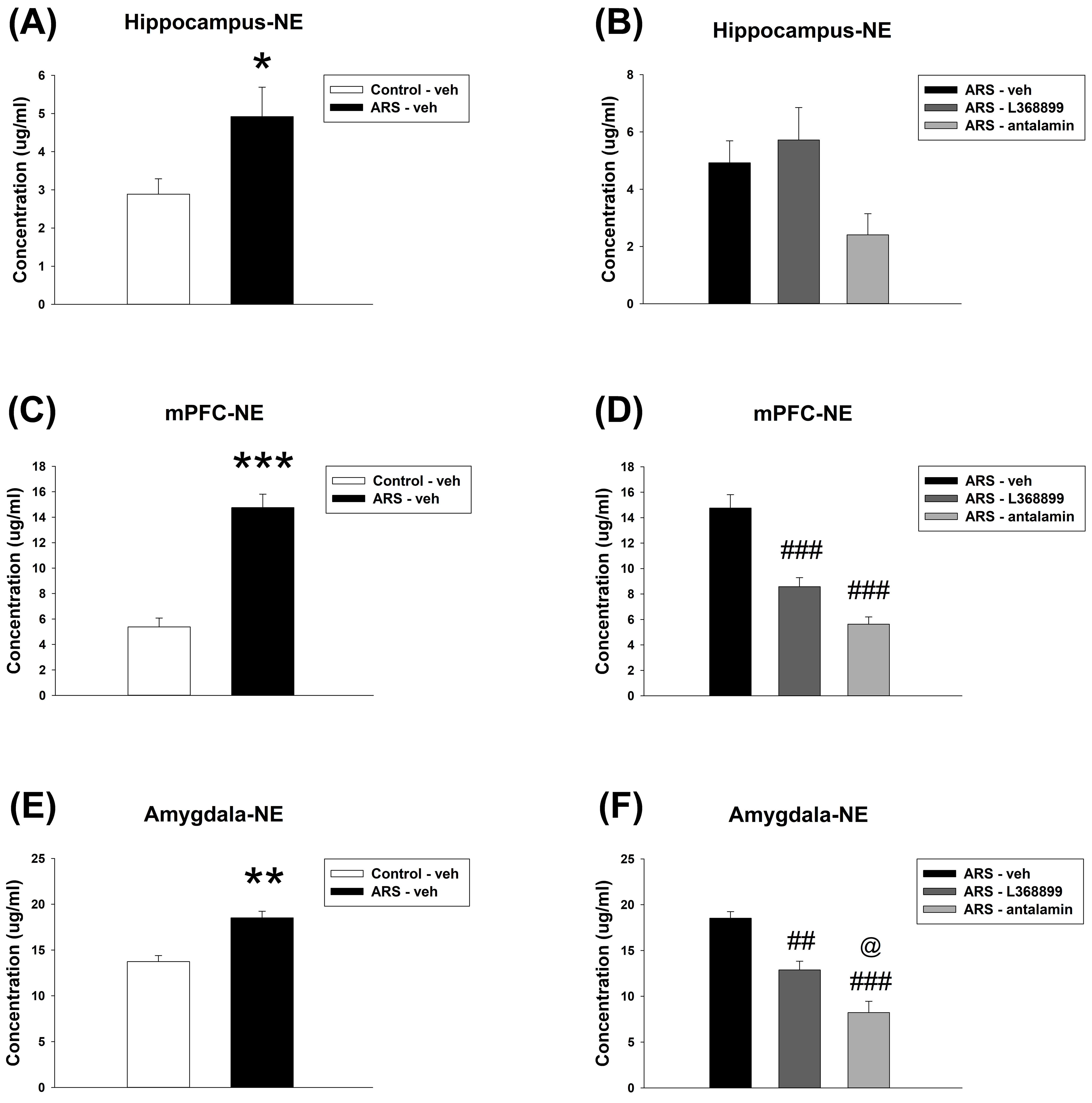 Fig. 10.
Fig. 10.
The NE level in the hippocampus, mPFC, as well as amygdala. ARS
increased the NE level in the hippocampus, mPFC and amygdala. L368899 and
antalarmin can reverse the enhancement of NE levels in the mPFC and amygdala
induced by ARS. The data are presented as the mean
Social behaviors extend far beyond simply presenting with positive or negative feelings towards others; they may include ambivalent and emotionally mixed feelings in the decision-making process. In the present study, we designed a psychological dilemma for rats that had previously undergone restraint stress (i.e., being placed in a physical no-escape holder), in which they had to decide whether to engage in a prosocial opportunity involving an explicit cue (i.e., the no-escape holder) associated with their fear memories. Using this design, we examined the roles of oxytocin (the so-called ‘love hormone’) and CRH (an anxiety-triggering hormone) in tasks targeting prosocial behavior and anxiety activity, together with the corresponding changes in central monoamines within fear circuits. Overall, our results demonstrated the following: (i) ARS rats chose the chamber with prosocial opportunities more frequently, and this phenomenon was reversed by L-368899 and antalarmin. (ii) ARS rats exhibited lower corticosterone levels, together with shorter avoidance latency and longer escape latency in the ETM, indicating reduced anxiety. (iii) Compared to controls, ARS rats had lower DA levels, but higher NE levels in the mPFC, both of which were reversed by L-368899. The findings of this study are discussed below.
ARS rats showed a higher contact time/number with their partner indicates a prosocial tendency. However, as these rats were previously restrained in the same no-escape holder, the restraint impact on fear memory should also be considered. In other words, choosing a chamber with a social partner represents the outcome of the emotional conflict between fear memory (i.e., being far away from the chamber) and prosocial engagement (i.e., approaching the chamber). Regarding this type of social choice, Ben-Ami Bartal et al. [41] successfully demonstrated the strong urge of rats to free a cage mate trapped in a restrainer using an innovative cage-liberating task design. They considered this an evidence of “empathic concern” in rats. The neural basis of empathy-like behavior involves individuals’ previous experiences of an affective state that the empathy-associated neural network shares (or co-activates), specifically in the context of environmental similarity [42]. This hypothesis is further supported by the corresponding changes in ultrasonic vocalization, an animal communication mechanism, in rats previously experiencing electrical foot shocks while witnessing their conspecific cage undergoing the same shocks [43].
The above argument indicates a complicated relationship between stress and social behavior, specifically regarding how stressed rats express their social needs when fear memory is retrieved. Our oxytocin data may be helpful for interpretation. Oxytocin has been consistently acknowledged for its effect to enhance the feelings of social bonding [13, 14]. Hormones have also been reported to exert a fear-enhancing effect that increases emotional distress [15]. The twofold role of oxytocin appears to be strongly associated with its context-dependent mechanism, particularly when stress is combined with social interactions [42, 44, 45]. In the present study, the oxytocin antagonist L-368899 noticeably reversed the ARS-induced prosocial effect. Together with our previous findings that intranasal oxytocin amended the prosocial deficit in a pharmacologically specific manner in a rodent model of PTSD, indicating a mediating role of oxytocin to prompt social behavior when required [46], these results suggest that oxytocin-strengthened fear memory benefits the prosocial effect in ARS rats, as the required neuronal network is co-activated for contextual similarity with fear memory [42].
The observed anxiety profile in the present study highlights the role of anxiety in terms of its implications for prosocial behavior following acute stress. Overall, we found a lower level of corticosterone in ARS rats, together with a shorter avoidance latency and longer escape latency in the ETM. This raises the possibility that stress does not always trigger individual anxiety; rather, it may function in a positive way to soothe one’s mood or even promote defensive capacity to deal with incoming distress [47]. An alternative explanation could be that re-exposure to the traumatic analog itself may be considered a process for treating stress-induced disturbances and fear memory-associated cortisol abnormalities [48]. In this regard, it is possible that SCT (performed on day 3, one day following ARS) serves as an exposure therapy to soothe the rats’ anxiety tone (tested by ETM on Day 5) and reduce the level of plasma corticosterone (examined on day 6). These effects were reversed by L-368899, supporting the contribution of oxytocin to the anxiolytic effect once the rats were placed again into the context of trauma. In line with our previous finding that oxytocin helps fear extinction, oxytocin antagonism reduced escape latency in the ETM (i.e., heightened panic anxiety) in the present study, supporting the therapeutic role of oxytocin in anxiety disorders and PTSD [49, 50].
Monoaminergic neurotransmitters appeared to exert different effects across fear circuit areas (i.e., the hippocampus, mPFC, as well as amygdala) following ARS. In the present study, 5-HT and DA levels in these brain regions were reduced compared to those of the NE, which increased. In particular, the opposite changes in DA and NE levels in the mPFC were similar to those observed in the PTSD paradigm [8]. This indicates that catecholamines are crucial to regulate expression/suppression of fear. In the mPFC, DA downregulation and NE upregulation could represent a shared yet dissociable stressed neuronal process in both the acute reaction (as in the ARS paradigm) and long-term adaptation (as in the PTSD paradigm) [8, 51]. Overall, these results suggest that ARS-induced DA/NE changes in the mPFC are related to oxytocin-mediated social effects that mediate fear memory [52]. Neurochemical abnormalities can be reversed by the oxytocin antagonist L-368899, which reversed the ARS-induced prosocial effect, supporting the mediating role of oxytocin on mPFC DA transmission, potentially in a DA 1 receptor-dependent manner [53]. In other words, the mediating/buffering role of oxytocin may play a key role in explaining why fear memory facilitates prosocial behavior.
Overall, these results raise an interesting debate regarding whether oxytocin-dependent social behavior is delivered with ease or unease. Antalarmin negated ARS-induced prosocial inclinations, indicating that social needs are enhanced when individuals feel stressed or anxious. However, ARS rats also showed less anxiety, as indicated by lower plasma corticosterone levels, shorter avoidance latency, and longer escape latency in the ETM. This discrepancy highlights a complicated mechanism in which individuals are in conflict between prosocial tendencies and fear memory-associated anxiety, in which time-dependent neuroadaptation may be a key. For example, anxiety levels were increased three weeks after traumatic stress, yet the lower anxiety level found in the present study reflected incomplete neuroadaptation, as it was measured only 4 days after stress. These results have significant clinical implications. Specifically, our L-368899 data suggested that immediate oxytocin administration after stress restores the impaired socioemotional function [54], possibly via a fear circuit DA normalization mechanism, while conversely contributing to maintaining low plasma corticosterone levels, similar to that in PTSD [55]. These findings suggest a dual effect of oxytocin, supporting its potential therapeutic utility in individuals with socioemotional problems, while necessitating caution in those diagnosed with PTSD.
Some limitations must be addressed when interpreting our data. First, the number of rats in each group appeared to be insufficient, and increasing the number of animals should be beneficial for validating the scientific significance. Second, as this study had a single-dose design for L-368899 and antalarmin, the negative findings of their effects on corticosterone should be conservative, as different dosing and timing arrangement were found to disturb the corticosterone level [34]. Finally, we were unable to quantify fear memory, as a conditioned fear memory test was not performed. These concerns should be addressed in a future study involving pharmacologically fully scaling the dose profile of oxytocin and CRH receptors and behaviorally incorporating the Pavlovian fear conditioning test.
The present study demonstrated that acute restraint stress mediates anxiety and facilitates prosocial contact in a context-dependent manner involving signaling collaboration between oxytocin and CRH. Our results not only fill the knowledge gap regarding the relationship between stress, anxiety, and sociability, but also highlight the role of oxytocin in fear-related contexts.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
SCW and YPL designed the study and wrote the protocol. CCL and CCC performed the experiment. SCW and YPL worked for the clinical interpretation. CCL and CCC helped the neurochemical analysis. SCW, CCL, CCC and YPL worked for writing drafts of the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Ethics approval had been granted by the Laboratory Animal Center from the National Defense Medical Center (IACUC-17-300). All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Not applicable.
This study was supported by grants from National Science and Technology Council (NSTC 112-2410-H-350-001), Ministry of Science and Technology (MOST 111-2410-H-350-001) and Cheng Hsin General Hospital and National Defense Medical Center (CH-NDMC-110-9 and CH-NDMC-111-09).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/JIN33400.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
